Abstract
Background
The Sec-dependent protein export apparatus of Escherichia coli is very efficient at correctly identifying proteins to be exported from the cytoplasm. Even bacterial strains that carry prl mutations, which allow export of signal sequence-defective precursors, accurately differentiate between cytoplasmic and mutant secretory proteins. It was proposed previously that the basis for this precise discrimination is the slow folding rate of secretory proteins, resulting in binding by the secretory chaperone, SecB, and subsequent targeting to translocase. Based on this proposal, we hypothesized that a cytoplasmic protein containing a mutation that slows its rate of folding would be recognized by SecB and therefore targeted to the Sec pathway. In a Prl suppressor strain the mutant protein would be exported to the periplasm due to loss of ability to reject non-secretory proteins from the pathway.
Results
In the current work, we tested this hypothesis using a mutant form of λ repressor that folds slowly. No export of the mutant protein was observed, even in a prl strain. We then examined binding of the mutant λ repressor to SecB. We did not observe interaction by either of two assays, indicating that slow folding is not sufficient for SecB binding and targeting to translocase.
Conclusions
These results strongly suggest that to be targeted to the export pathway, secretory proteins contain signals in addition to the canonical signal sequence and the rate of folding.
Background
The Sec-dependent protein export pathway of Escherichia coli is responsible for translocation of secretory proteins across the inner membrane to final destinations in the periplasm or outer membrane. Secretory proteins, also called preproteins, are synthesized with a cleavable amino terminal signal sequence that functions both to slow folding of the preprotein and to aid in recognition of the secretory protein by export factors. Export of many, but not all, secretory proteins is dependent on interaction with SecB, a cytoplasmic chaperone that maintains the preprotein in a loosely folded conformation competent for translocation. Both SecB and the preprotein provide binding sites for SecA, a peripheral membrane ATPase. SecA targets the preprotein to the membranous translocase complex composed of SecY, SecE, SecG, SecD, SecF, and YajC. Formation of the complete translocase complex promotes an ATP binding and hydrolysis cycle by SecA that results in segmental translocation of the secretory protein across the membrane [1-4].
The signal sequence is crucial for efficient translocation; mutations in the signal sequence significantly reduce export of the preprotein and complete deletions of the signal sequence eliminate essentially all export [5-8]. Selection for extragenic suppressors of such export defective preproteins led to the identification of the prl alleles of secY (prlA), secE (prlG), secA (prlD), and more recently secG (prlH) [6,9-11]. Early hypotheses predicted that the Prl suppressors function by expanded or altered interactions with signal sequences, facilitating recognition of mutant as well as wild type signal sequences [6].
More recent observations support an alternative mechanism of action for the PrlA/SecY and PrlG/SecE suppressors [8,12]. First, PrlA/G-mediated suppression does not exhibit allele specificity; all prlA and all prlG alleles that have been examined suppress all signal sequence mutations, implying that suppression is not due to a specific altered interaction that allows recognition of a mutant signal sequence [8,12]. Furthermore, all of the prlA and prlG alleles suppress complete deletions of the signal sequence, suggesting that an interaction between the signal sequence and the Prl suppressor is not necessary for suppression or even for export [5,8,13]. Based on these observations, it was proposed that the PrlA and PrlG suppressors do not function through altered interactions with signal sequences, but rather by loss of recognition [8,12]. The wild type SecE and SecY proteins are thought to function in concert to proofread the signal sequence of secretory proteins, rejecting defective precursors from the export pathway. The PrlA (SecY) and PrlG (SecE) suppressors are compromised in their ability to proofread, allowing export of proteins with mutations, or even complete deletions, of the signal sequence.
This proofreading model predicts that the critical step for translocation of a signal sequence-defective secretory protein is recognition by SecB and subsequent presentation to SecE/Y. SecB binds portions of the mature secretory protein [14-18], and SecA would bind to the SecB-precursor complex by virtue of its ability to bind SecB [19,20] as well as the preprotein [21-23]. Thus, SecA-mediated targeting of the preprotein to translocase would occur. In a wild type cell, the mutant precursor would be rejected at this point by SecY/E, but in a PrlA or PrlG suppressor strain, rejection would not occur and the mutant secretory protein would be exported.
In fact, all of the PrlA and PrlG suppressors are completely dependent on functional SecB for manifestation of suppressor activity [5,8]. Even certain proteins that are not normally SecB-dependent for translocation become SecB-dependent when they contain a signal sequence mutation and are exported via the suppressor pathway. For example, PhoA is normally exported independent of SecB. Deletion of the signal sequence from phoA severely compromises export; export is restored in a prlA suppressor strain. However, this suppression is dependent on SecB [5]. Therefore, there are characteristics of the mature portion of PhoA that promote recognition by SecB, and this recognition is essential for PrlA-mediated suppression.
Although prlA and prlG strains export secretory proteins that completely lack a signal sequence, there is no evidence of export of any cytoplasmic proteins in a prl suppressor strain [13]. That is, proteins that are supposed to remain in the cytoplasm are not mislocalized to the periplasm. It has been suggested that perhaps cytoplasmic proteins are not exported in prl suppressor strains because they fold rapidly and escape recognition by SecB, while secretory proteins, even those lacking a signal sequence, fold more slowly, allowing time for SecB recognition and binding [5,13]. SecB binds to a variety of unfolded proteins in vitro [24,25], although binding appears to be more selective in vivo [26]. This selectivity may be based on the slower folding characteristics of secretory proteins.
Therefore, we proposed previously that if a cytoplasmic protein contains a mutation that slows its rate of folding, SecB will recognize and bind to that mutant protein. Binding of SecB would result in targeting of the unfolded protein to the translocation apparatus. According to the proofreading model, in a wild type strain, SecE and SecY would reject the unfolded protein due to lack of a signal sequence. In a Prl suppressor strain, however, the proofreading function would be compromised and the mutant protein would be exported [8]. In the experiments described here, we tested this hypothesis by examining the export of such a cytoplasmic protein with a folding mutation. The results indicate that slow folding is not sufficient for SecB binding and subsequent targeting to translocase and further, that secretory proteins contain targeting information in addition to the signal sequence and the folding kinetics.
Results and Discussion
To test the hypothesis that a cytoplasmic protein containing a mutation that slowed its folding would be exported in a Prl suppressor strain, a well characterized cytoplasmic protein was obtained. The protein of choice was the amino terminal portion of the λ repressor protein, a 92 residue domain that folds into a stable, predominantly α-helical structure [27]. Folding studies were performed at a pH and ionic strength similar to physiological conditions and the folding parameters were predicted to approximate the in vivo situation. The wild type protein (N102LT) is very stable at 37°C, with a Tm for unfolding of about 55°C. The mutant version that we used (LA57-N102LT) contains a Leu to Ala alteration of amino acid 57 that dramatically reduces the stability of the folded structure. The LA57-N102LT protein has a Tm of approximately 25°C, and is about 80% unfolded at 37°C and 60% unfolded at 30°C [27]. Further, the mutant protein has been shown to have a half-life of about 40 minutes in wild type E. coli strains at 37°C [27].
Localization of N102LT and LA57-N102LT
Plasmids pKL106 and pKL107 encoding LA57-N102LT-FLAG or N102LT-FLAG, respectively, were transformed into strains IM104 (wild type secY) or IM105 (prlA4). These strains contained deletion mutations in the lon and degP genes to limit proteolysis in both the cytoplasmic and periplasmic spaces. Following induction of the λ repressor fragment, whole cell lysates, spheroplasts, and periplasmic fractions were prepared from each strain. Samples were analyzed by Western blotting using antibodies directed against the FLAG epitope present at the carboxyl terminus of the λ repressor fragments.
According to our hypothesis, the largely unfolded nature of LA57-N102LT should allow SecB to bind, resulting in targeting of LA57-N102LT to translocase. In the secY wild type strain, proofreading by SecE/Y would block export of LA57-N102LT, while in the prlA4 strain, the PrlA4 suppressor would allow export of a portion of LA57-N102LT to the periplasm. This was not the result we observed (Fig. 1). No LA57-N102LT was detected in the periplasm, even in the prlA4 strain, indicating that export did not occur to any significant extent. To ensure that all periplasmic contents were released during the spheroplast preparation, the blots were stripped of FLAG antibody and reprobed with antibody directed against MalE, a periplasmic binding protein. All detectable MalE was present in the periplasmic fractions (data not shown), demonstrating that complete release of the periplasmic contents had been attained. Therefore, there was no detectable export of LA57-N102LT in the prlA4 strain.
Figure 1.

Immunodetection of λ repressor fragments in bacterial fractions LA57 indicates the slow folding λ repressor, LA57-N102LT, while wt refers to the wild type λ repressor, N102LT. The strains used were either wild type secY/prlA or prlA4 as indicated. The individual lanes are as follows: M – purified N102LT used as a molecular weight marker, W – whole cell lysate, S – spheroplast fraction (containing cytoplasmic contents and membranes), P – periplasmic fraction.
There were two reasonable explanations for this unexpected finding; 1) an unfolded state is not sufficient for SecB binding, or 2) SecB binding is not sufficient to promote export in a prl suppressor strain. To distinguish between these possibilities, we examined whether SecB was able to bind LA57-N102LT using two different methods to identify interactions. The first approach was co-immunoprecipitation of LA57-N102LT-SecB complexes, the second was an in vitro protease protection assay.
Co-immunoprecipitation of SecB complexes
Co-immunoprecipitations were performed using whole cell lysates of strains induced for expression of λ repressor. Immunoprecipitations were performed as described using antibodies directed either against SecB or against the His tag on the λ repressor. Following the immunoprecipitation reaction, samples were analyzed by Western blotting using the opposite antibody, that is, samples that had been immunoprecipitated with anti-SecB were examined with anti-His while those that had been precipitated with anti-His were probed with anti-SecB. In addition, as a positive control, the interaction of SecB with the secretory protein LamB was examined using anti-LamB antibodies. While an interaction between LamB and SecB was clearly seen (Fig. 2c), no interaction was observed between SecB and either N102LT or LA57-N102LT (Fig. 2a and 2b). This was true regardless of whether the precipitating antibody was directed against SecB or the His tag. The wild type N102LT was not expected to interact with SecB as it would fold very rapidly and stably; it was more surprising that no interaction was detected between SecB and the destabilized LA57-N102LT. This result implied that the unfolded nature of LA57-N102LT was not sufficient for SecB binding.
Figure 2.
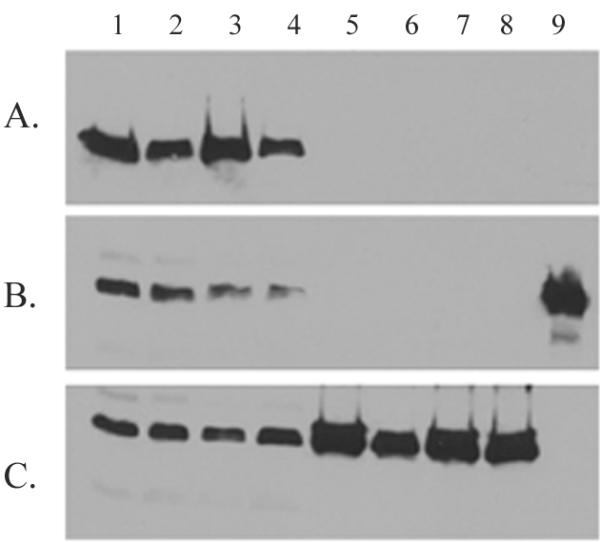
Immunoprecipitations of SecB complexes A. Precipitation was performed with antibody directed against SecB, followed by immunoblotting using antibody against the His tag on the λ repressor fragments. B. Precipitating antibody was directed against the His tag, immunoblotting utilized the anti-SecB antibody. C. Precipitation was carried out with antibody against LamB, anti-SecB was used for immunoblotting. In all gels, samples were as follows: lanes 1–4 – whole cell lysates prior to immunoprecipitation, lanes 5–8 – immunoprecipitated material; lanes 1 and 5 – N102LT/prlA4, lanes 2 and 6 – LA57-N102LT/prlA4, lanes 3 and 7 – N102LT/prlA+, lanes 4 and 8 – LA57-N102LT/prlA+, lane 9 in gel B – purified SecB.
Protease protection assays
To substantiate the results observed by co-immunoprecipitation, an in vitro protease protection assay was employed. SecB has a proteolytic-sensitive domain that is cleaved by proteinase K in a limited proteolysis reaction. When substrate is bound to SecB, the conformation is altered such that the site is no longer protease accessible, rendering SecB protease resistant [28]. Thus, this assay can be used to detect binding of SecB to a substrate. Poly-L-lysine and small peptides are routinely used as substrates for this analysis as demonstrated in Fig. 3 (lanes 3 and 4). To determine whether a larger protein was also able to protect SecB, we used purified, urea-denatured proOmpA as a substrate. The proOmpA also was able to bind to and protect SecB (Fig. 3, lanes 9 and 10) although a much higher concentration of substrate was required to achieve 50% protection. To ensure that the observed protection was not due simply to the high protein concentration in the reaction, a non-secretory protein was used as a negative control. BSA was added to the same concentration or higher as proOmpA with no resultant protection of SecB (data not shown). Therefore, this assay reliably serves as an additional method to observe SecB-substrate interactions.
Figure 3.
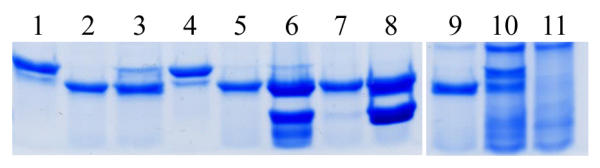
Protease protection assays Lane 1 – undigested SecB, lane 2 – SecB digested with proteinase K; lanes 3 through 10 – SecB was incubated with potential substrates at the indicated concentrations prior to proteinase K digestion; lane 3 – 20 nM poly-L-lysine, lane 4 – 150 nM poly-L-lysine; lane 5 – 150 nM N102LT (wild type), lane 6 – 4 μM N102LT; lane 7 – 150 nM LA57-N102LT (slow folding mutant), lane 8 – 4 μM LA57-N102LT; lane 9 – 150 nM proOmpA, lane 10 – 1.4 μM proOmpA; lane 11 – proOmpA alone digested with proteinase K. Lower molecular weight band in lanes 6 and 8 is the λ repressor fragment.
The λ repressor proteins were purified on a nickel affinity chromatography column by virtue of the His tag at the carboxyl terminus of the proteins. Each was assayed for protection of SecB at concentrations up to and exceeding that used for proOmpA. Neither N102LT nor LA57-N102LT protected SecB from proteolysis, indicating that no binding occurred (Fig. 3, lanes 5–8). Although the mutant LA57-N102LT is predicted to be in an unfolded conformation in the bacterial cell, the protease protection assay was performed at 4°C. To verify that refolding of LA57-N102LT at this temperature was not interfering with interpretation of the results, we further tested the SecB binding activity by incubating LA57-N102LT in 8 M urea, then diluting it into the protection reaction, similar to the conditions used for proOmpA. Again, no protection was detected (data not shown). These results demonstrated that even when fully unfolded, LA57-N102LT was not a substrate for SecB binding.
Conclusions
The hypothesis driving the present studies was premised on the observation that prl suppressor strains are able to correctly identify secretory proteins, even in the absence of a signal sequence. It has been widely thought that this recognition was due to slow folding of secretory proteins in the cytoplasm, resulting in binding by SecB and subsequent targeting to translocase. While slow folding may be a necessary criterion for export, the data presented here indicate that an unfolded nature is not sufficient for SecB binding and targeting. This conclusion is based on failure of the LA57-N102LT protein to bind SecB either in vivo or in vitro. The in vivo results are founded both on the co-immunoprecipitation experiments and on the fact that no export of the protein was detected in a prlA4 strain, while the in vitro results are based on the protease protection assays. Therefore, we conclude that secretory proteins must contain information, in addition to the signal sequence and slow folding characteristics, that targets them to the secretory pathway or that cytoplasmic proteins contain information that prevents export even if the folding parameters are altered. Further studies are underway to identify such targeting sequences.
Methods
Bacterial strains and plasmids
All bacterial strains used were derivatives of E. coli K12 strain MC4100 [29]. IM104 is MC4100 lamB14D lon::Tn10 degP::kan and IM105 is IM104 prlA4. Plasmids pKL106 and pKL107 are pET11c (Novagen) derivatives carrying tandem copies of the λ repressor fragment (derived from plasmids pAD103 and pAD105, generous gifts from Alan Davidson, University of Toronto and Robert Sauer, MIT). In pKL106, the wild type λ repressor is tagged with the myc epitope and the LA57 λ repressor has the FLAG epitope. In pKL107, the wild type λ repressor contains the FLAG eiptope and the LA57 mutant has the myc epitope. In all experiments, only antibodies to the FLAG epitope were used, therefore pKL106 resulted in detection of the mutant λ repressor and pKL107 was used to observe the wild type. Plasmids pIM101 and pIM102 were used in the co-immunoprecipitation assays and for purification of the λ repressor fragments. These plasmids are derivatives of pET11c that contain a single copy of the λ repressor, either wild type (pIM102) or LA57 (pIM101). The λ repressor inserts contain a FLAG tag at the carboxyl terminus followed by a hexahistidine motif.
Bacterial growth, induction and fractionations
Plasmid-containing bacteria were grown in LB [30] with ampicillin (100 μg ml-1) at 30°C. Cells were induced for expression of the λ repressors at an OD600 = 0.4–0.5 by addition of 1 mM IPTG either for 30 minutes (spheroplast procedures) or for 3 hours (immunoprecipitation experiments).
Spheroplasts were prepared similarly to published protocols [31]. Briefly, 1 ml of bacterial cells was pelleted for 3 min at 16,000 rpm in an Eppendorf centrifuge. All subsequent steps were performed on ice. The cell pellet was resuspended in 0.5 ml spheroplast buffer [1 M sucrose, 25 mM TrisHCl (pH 8.0), 5 mM EDTA, and lysozyme (50 μg ml-1)]. After incubation on ice for 12 min, MgCl2 was added to a final concentration of 3 mM and incubation continued for an additional 5 min. The spheroplasts were pelleted by centrifugation at 16,000 rpm for 10 min at 4°C. The resultant supernatant contained the periplasmic contents. The pellet, which consisted of the membranes and cytoplasmic contents, was resuspended in 0.5 ml of spheroplast buffer. A parallel sample was prepared without the final centrifugation step, resulting in a whole cell lysate. All samples were precipitated with TCA and resuspended in gel loading buffer preparatory to electrophoresis.
Immunoblotting
Fractions were electrophoresed on 17% polyacrylamide gels and transferred to nitrocellulose. Immunoblotting was performed as described [32] using monoclonal antibody M2 directed against the FLAG epitope (Eastman Kodak) at a 1:600 dilution, or monoclonal antibody against MalE (Sigma) at a 1:500 dilution. When necessary, antibodies were stripped from the nitrocellulose by incubation in stripping buffer [1 M glycine (pH 2.2), 20 mM MgAc, 50 mM KCl] followed by immunoblot blocking solution.
Immunoprecipitations
Bacterial cells were induced for 3 hours for λ repressor expression as described above and harvested by French Press lysis. Lysates were immunoprecipitated with minor alterations to previous protocols [8]. Specifically, the IP buffer contained only 50 mM NaCl and incubation with the primary antibody was performed overnight at 4°C. Antibodies used were directed against the His epitope (monoclonal, Boehringer Mannheim), SecB (polyclonal, gift from William Wickner), or LamB (polyclonal, gift of Thomas Silhavy). Immune complexes were collected with Protein A agarose (Pierce), samples were electrophoresed on 15% polyacrylamide gels, and immunoblotting performed as described above. Following immunodetection, all blots were stripped of primary antibody and reprobed with the precipitating antibody to verify that precipitation had occurred.
Protease protection assays
SecB protease protection assays were performed as described [28], followed by electrophoresis on 15% polyacrylamide gels and detection by Coomassie Blue staining. The λ repressor fragments were purified by nickel NTA affinity chromatography (Qiagen).
Authors' contributions
Localization studies were performed by IM, immunoprecipitations and protease protection assays were carried out by MAS. AMF conceived of the study and participated in its design and coordination. The manuscript was written by AMF and edited jointly by all authors. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We are grateful to Kathy LaVoi for construction of pKL106 and pKL107. We thank Alan Davidson and Kevin Young for helpful discussion. This material is based upon work supported by, or in part by, the U.S. Army Research Office under contract/grant number DAAH04-96-1-0426 (G).
Contributor Information
Ipsita Mallik, Email: ipsita123@yahoo.com.
Margaret A Smith, Email: masmith@medicine.nodak.edu.
Ann M Flower, Email: aflower@medicine.nodak.edu.
References
- Danese PN, Silhavy TJ. Targeting and assembly ofperiplasmic and outer-membrane proteins in Escherichia coli. Annu Rev Genet. 1998;32:59–94. doi: 10.1146/annurev.genet.32.1.59. [DOI] [PubMed] [Google Scholar]
- Duong F, Eichler J, Price A, Leonard MR, Wickner W. Biogenesis of the gram-negative bacterial envelope. Cell. 1997;91:567–573. doi: 10.1016/s0092-8674(00)80444-4. [DOI] [PubMed] [Google Scholar]
- Fekkes P, Driessen AJ. Protein targeting to the bacterial cytoplasmic membrane. Microbiol Mol Biol Rev. 1999;63:161–173. doi: 10.1128/mmbr.63.1.161-173.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manting EH, Driessen AJM. Escherichia coli translocase: the unravelling of a molecular machine. Mol Microbiol. 2000;37:226–238. doi: 10.1046/j.1365-2958.2000.01980.x. [DOI] [PubMed] [Google Scholar]
- Derman AI, Puziss JW, Bassford PJ, Jr, Beckwith J. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 1993;12:879–888. doi: 10.1002/j.1460-2075.1993.tb05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr SD, Hanley-Way S, Silhavy TJ. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981;23:79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Fikes JD, Bassford PJ., Jr Novel secA alleles improve export of maltose-binding protein synthesized with a defective signal peptide. J Bacteriol. 1989;171:402–409. doi: 10.1128/jb.171.1.402-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower AM, Doebele RC, Silhavy TJ. PrlA and PrlG suppressors reduce the requirement for signal sequence recognition. J Bacteriol. 1994;176:5607–5614. doi: 10.1128/jb.176.18.5607-5614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieker KL, Silhavy TJ. The genetics of protein secretion in E. coli. Trends Genet. 1990;6:329–334. doi: 10.1016/0168-9525(90)90254-4. [DOI] [PubMed] [Google Scholar]
- Schatz PJ, Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Bost S, Belin D. prl mutations in the Escherichia coli secG gene. J Biol Chem. 1997;272:4087–4093. doi: 10.1074/jbc.272.52.33422. [DOI] [PubMed] [Google Scholar]
- Osborne RS, Silhavy TJ. PrlA suppressor mutations cluster in regions corresponding to three distinct topological domains. EMBO J. 1993;12:3391–3398. doi: 10.1002/j.1460-2075.1993.tb06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz WA, Spiess C, Ehrmann M, Schierle C, Beckwith J. Targeting of signal sequenceless proteins for export in Escherichia coli with altered protein translocase. EMBO J. 1996;15:5209–5217. [PMC free article] [PubMed] [Google Scholar]
- Khisty VJ, Munske GR, Randall LL. Mapping of the binding frame for the chaperone SecB within a natural ligand, galactose-binding protein. J Biol Chem. 1995;270:25920–25927. doi: 10.1074/jbc.270.43.25920. [DOI] [PubMed] [Google Scholar]
- de Cock H, Overeem W, Tommassen J. Biogenesis of outer membrane protein PhoE of Escherichia coli evidence for multiple SecB-binding sites in the mature portion of the PhoE protein. J Mol Biol. 1992;224:369–379. doi: 10.1016/0022-2836(92)91001-6. [DOI] [PubMed] [Google Scholar]
- Gannon PM, Li P, Kumamoto CA. The mature portion of Escherichia coli maltose-binding protein (MBP) determines the dependence of MBP on SecB for export. J Bacteriol. 1989;171:813–818. doi: 10.1128/jb.171.2.813-818.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall LL, Topping TB, Hardy SJS. No specific recognition of leader peptide by SecB, a chaperone involved in protein export. Science. 1990;248:860–863. doi: 10.1126/science.2188362. [DOI] [PubMed] [Google Scholar]
- Topping TB, Randall LL. Determination of the binding frame within a physiological ligand for the chaperone SecB. Protein Sci. 1994;3:730–736. doi: 10.1002/pro.5560030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F-U, Lecker S, Schiebel E, Hendrick JP, Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990;63:269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- Breukink E, Nouwen N, van Raalte A, Mizushima S, Tommassen J, de Kruijff B. The C terminus of SecA is involved in both lipid binding and SecB binding. J Biol Chem. 1995;270:7902–7907. doi: 10.1074/jbc.270.14.7902. [DOI] [PubMed] [Google Scholar]
- Cunningham K, Wickner W. Specific recognition of the leader region of precursor proteins is required for the activation of translocation ATPase of Escherichia coli. Proc Natl Acad Sci USA. 1989;86:8630–8634. doi: 10.1073/pnas.86.22.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura E, Akita M, Matsuyama S, Mizushima S. Determination of a region in SecA that interacts with presecretory proteins in Escherichia coli. J Biol Chem. 1991;266:6600–6606. [PubMed] [Google Scholar]
- Lill R, Dowhan W, Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature portions of precursor proteins. Cell. 1990;60:271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- Hardy SJS, Randall LL. A kinetic partitioning model of selective binding of nonnative proteins by the bacterial chaperone SecB. Science. 1991;251:439–443. doi: 10.1126/science.1989077. [DOI] [PubMed] [Google Scholar]
- Fekkes P, den Blaauwen T, Driessen AJM. Diffusion-limited interaction between unfolded polypeptides and the Escherichia coli chaperone SecB. Biochemistry. 1995;34:10078–10085. doi: 10.1021/bi00031a032. [DOI] [PubMed] [Google Scholar]
- Kumamoto CA, Francetic O. Highly selective binding of nascent polypeptides by an Escherichia coli chaperone protein in vivo. J Bacteriol. 1993;175:2184–2188. doi: 10.1128/jb.175.8.2184-2188.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell DA, Sauer RT. The structural stability of a protein is an important determinant of its proteolytic susceptibility in Escherichia coli. J Biol Chem. 1989;264:7590–7595. [PubMed] [Google Scholar]
- Randall LL. Peptide binding by chaperone SecB: implications for recognition of nonnative structure. Science. 1992;257:241–245. doi: 10.1126/science.1631545. [DOI] [PubMed] [Google Scholar]
- Casadaban MJ. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Silhavy TJ, Berman ML, Enquist LW. Experiments with gene fusions. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. 1984.
- Thorstenson YR, Zhang Y, Olson PS, Mascarenhas D. Leaderless polypeptides efficiently extracted from whole cells by osmotic shock. J Bacteriol. 1997;179:5333–5339. doi: 10.1128/jb.179.17.5333-5339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies A laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory. 1988.


