About 40–60 million years before the advent of human agriculture, three insect lineages, termites, ants, and beetles, independently evolved the ability to grow fungi for food. Like humans, the insect farmers became dependent on cultivated crops for food and developed task-partitioned societies cooperating in gigantic agricultural enterprises. Agricultural life ultimately enabled all of these insect farmers to rise to major ecological importance. Indeed, the fungus-growing termites of the Old World, the fungus-growing ants of the New World, and the cosmopolitan, fungus-growing beetles are not only dominant players in natural ecosystems, but they are also major agriculture, forestry, and household pests (1). Not surprising, much is known about the extermination of these pest insects, but only recently have genetic techniques been applied to elucidate the evolutionary histories of these unique nonhuman agricultural systems.
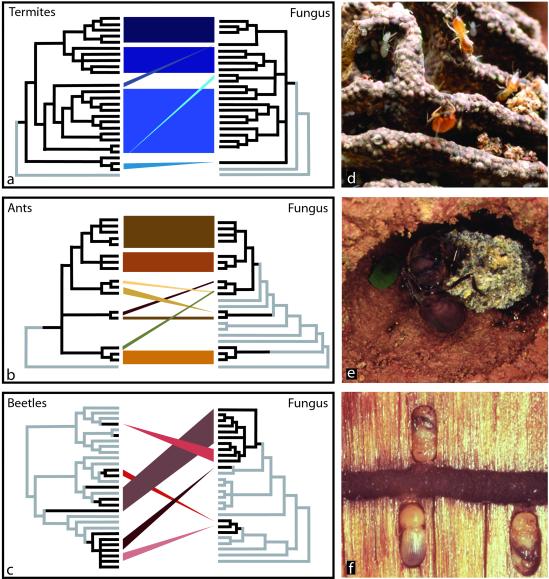
In a recent issue of PNAS, Aanen et al. (2) present such an evolutionary analysis for fungus-growing termites and their cultivated fungal crops, complementing similar analyses recently completed for fungus-growing ants (3–6) and fungus-growing beetles (7–9) (Fig. 1 a–c). Aanen et al.'s methodology followed the same two-part analysis anthropologists have taken to unravel the histories of human agricultural societies and their various crops. First, they assessed the patterns of relatedness between cultivated crops and wild, undomesticated varieties; patterns revealed which crops were derived from common ancestral stocks and thus the number of independent domestication events. Second, they determined the relationships between independent farmer societies to infer common origins of agricultural practices. Even in the absence of a fossil (or archaeological) record, a juxtaposition of these two phylogenetic histories can reveal surprising details of agricultural evolution.
Fig 1.
Evolutionary histories of fungiculture in termites, ants, and beetles. (a–c) Comparison of the patterns of evolutionary diversification in the insect farmers (left cladograms) and their cultivated fungi (right cladograms). In the left cladograms, farmer lineages are shown in black; nonfarmer relatives are shown in gray. In the right cladograms, fungal cultivar lineages are shown in black; noncultivated feral fungi are shown in gray. The number of independent origins of fungicultural behavior appears as independent farmer lineages in the left cladograms. The number of independently domesticated fungal lineages appears as separate cultivar lineages in the right cladograms. Cladograms synthesize pertinent information from published evolutionary reconstructions, but the number of lineages per group has been reduced because of space constraints. Similarly reduced is the number of nonfarmer lineages and noncultivated fungi interpositioned between, respectively, the farmer lineages and the cultivated fungi. The history of successive diversification therefore is preserved in the cladograms, but evolutionary distances between lineages are reduced in many cases. Information is taken from ref. 2 for the termites, from refs. 3 and 5 for the ants, and from refs. 7–9 for the beetles. (d) Garden of the fungus-growing termite Macrotermes bellicosus (photo by K. Machielsen). The fungus is grown on fecal pellets that are stacked into walls of the fungus garden (comb). (e) Incipient garden of the fungus-growing ant Atta cephalotes (photo by U.G.M.). The queen is shown resting on the incipient garden that is tended by her first cohort of workers. (f) Gallery of the ambrosia beetle Trypodendron lineatum (photo by S. Kühnholz). The ambrosia fungus appears as the black lining of the gallery. Developing beetle brood is shown in niches off the gallery. Fungal growth is monitored constantly by adult beetles (not shown) patrolling the galleries.
Termite Fungiculture.
About 330 of the >2,600 known termite species are obligately dependent on the cultivation of a specialized fungus, Termitomyces, for food (10, 11). Termitomyces is grown on termite feces in subterranean combs that the termites construct within the heart of nest mounds (11). Combs are supplied with feces of myriads of workers that forage on wood, grass, or leaves (Fig. 1d). Spores of consumed fungus are mixed with the plant forage in the termite gut and survive the intestinal passage (11–14). The addition of a fecal pellet to the comb therefore is functionally equivalent to the sowing of a new fungal crop. This unique fungicultural practice enabled Aanen et al. to obtain genetic material of the cultivated fungi directly from termite guts, circumventing laborious nest excavation during collection. Overall, 32 termite species and their cultivars were sampled, covering the diversity of termites throughout their range in Africa and Asia. For the two-part historical reconstruction mentioned above, Aanen et al. generated DNA sequence information for, first, the Termitomyces cultivars and undomesticated, fungal relatives; and second, the termite farmers and nonfarmer relatives. These two evolutionary reconstructions reveal a remarkably complex fabric of fungicultural evolution in termites (Fig. 1a).
Fungus-growing behavior originated only once in termites, involving a single ancestral Termitomyces lineage that diversified into several defined cultivar groups, each associated almost exclusively with a similarly defined group of termite farmers. Within each of these termite groups, however, cultivars are exchanged frequently between termite lineages. This is analogous to a situation where distinct human lineages would be specialized on particular crops, for example corn, and corn farmers could switch between cultivation of any kind of corn variety but could not easily switch to cultivation of wheat or potato. Termite farmers therefore appear to have evolved adaptations to certain cultivar groups (e.g., specific fertilizing regimes), or cultivars evolved adaptations suitable only for certain farmers (e.g., nutrients benefiting only certain termites; ref. 12), or both.
The juxtaposition of termite and Termitomyces evolution informed Aanen et al. about historical details that would have been impossible to infer from the sparse fossil record of the fungus-growing termites (10). An even richer picture emerges when comparing termite fungiculture to two other known fungus-farming insects, attine ants and ambrosia beetles, which show remarkable evolutionary parallels with fungus-growing termites (Fig. 1 a–c).
Ant and Beetle Fungiculture.
In ants, the ability to cultivate fungi for food has arisen only once, dating back ≈50–60 million years ago (15) and giving rise to roughly 200 known species of fungus-growing (attine) ants (4). Attine colonies typically are founded by a mated female who takes a fungal inoculum from her mother's colony to start her new garden (5, 15) (Fig. 1e). Attine ants grow their fungi in subterranean chambers, manuring the gardens with dead vegetable debris, or in the case of the leafcutter ants, with leaf fragments cut from live plants. Attine ants are obligately dependent on their fungi; their brood is raised on an exclusively fungal diet. Nests of most attine species number only a few dozen workers, but nest sizes can reach millions in leafcutter ants. Leafcutter ants are prodigious consumers of leaves and are among the most damaging agricultural pests in South and Central America.
Many, but not all of the weevils species in the subfamilies Scolytinae and Platypodinae burrow extensive gallery systems into trees as adults for feeding and oviposition. Some of these beetles, collectively called ambrosia beetles (3,400 species), grow fungi on the walls of their galleries for food (Fig. 1f). The largely clonal ambrosia fungi are only known from the beetles and their galleries, suggesting an obligate association with the beetle farmers (9). The fungi serve as the beetle's primary food source and are essential for the completion of the beetle life cycle (16). Like fungus-growing termites and ants, ambrosia beetles protect the fungal garden from harmful contaminants and raise their brood on a fungal diet (16, 17). Fungus-growing beetles are major forestry pests, particularly those burrowing into live trees and infecting them with their fungi.
Fungicultural Origins.
Termite, ant, and beetle farmers appear to have made the transition to fungiculture via different evolutionary avenues. In the termites, fungi probably were an important food source before true cultivation, and fungiculture arose when the termites secondarily developed an ability to manipulate fungal growth in their nests. Many nonfarming termite species are attracted to feed on fungus-infested wood (11, 12), and termite fungiculture therefore may represent an elaboration of such ancestral feeding habits. In contrast, the ancestral insect-fungus association may have been one in which the fungi used insect hosts for dispersal of spores (similar to flowering plants using bees as pollen vectors), and fungus-feeding and fungiculture arose secondarily out of such an ancestral vectoring system. This most likely was the case in the beetles because many nonfungus feeding relatives of the ambrosia beetles are important vectors of fungal spores and because the ambrosia fungi are derived from free-living fungi that depend on arthropods for dispersal (18). It is unclear whether fungiculture in attine ants arose from ancestral mycophagy or from a system of fungal vectoring by ants (15).
Whereas ant and termite fungiculture originated only once in each group (Fig. 1 a and b), fungus-growing by ambrosia beetles has arisen at least seven times (Fig. 1c). Multiple origins of fungiculture in beetles is perhaps not surprising, given the sheer diversity of beetle species and given the importance of feeding specializations in beetle diversification (19). Multiple origins, however, do not preclude beetle-fungus coevolution within each independently derived system. Indeed, in each of the independently evolved farmer beetle lineages, entire groups of species are specialized on particular groups of cultivars, paralleling not only the specializations already discussed above for termites, but also a series of specializations known for different ant groups each associated with its own cultivar group (3, 5, 6). In general, switching of farmers to novel cultivars is possible, but limited almost exclusively to cultivars from within the same cultivar group as the farmers' typical cultivar.
Evolutionary reversal back to a non-fungus-farming lifestyle has apparently not occurred in any of the nine known, independently evolved farmer lineages (one termite, one ant, and seven beetle lineages). This supports the view, formulated first for humans (ref. 20 and references therein), that the transition to agricultural existence is a drastic and possibly irreversible change that greatly constrains subsequent evolution.
Cultivar Exchange and Transmission Between Generations.
Aanen et al.'s analyses indicate that fungal cultivars are exchanged frequently between termite species. Fungal exchange had been suspected for a long time because most fungus-growing termites import cultivar spores from external sources during nest initiation (13, 14). Acquisition of fungal starter material from external sources represents a primitive (ancestral) condition that arose at the origin of termite fungiculture, and it is still practiced by the great majority of fungus-growing termite lineages. The exceptions are two derived lineages that arose late in termite evolution, the genus Microtermes, in which new queens carry asexual spores in their guts as starter inoculum for their new nests, and the single species Macrotermes bellicosus, in which the new king is the sole carrier of spores (13, 14).
Spore acquisition at the nest founding stage generally occurs during the rainy season, a time when Termitomyces fungi produce fruiting structures (mushrooms) (11). During fruiting, Termitomyces sends out mycelial growths toward the nest surface and forms mushrooms for spore production. These spores are produced sexually (meiotically), and they are thus different from the asexual spores produced in combs. Sexual spores may be brought back into the nest by the termites for inoculation of new combs, or they may disperse to recombine with other fungi. It is these recombinants that are thought to be picked up at the nest-founding stage by termite farmers (13, 14), thus explaining the observed high levels of lateral cultivar transfer. As mentioned before, the exceptions to this rule are the vertically inherited fungi cultivated by the genus Microtermes and by Macrotermes bellicosus. Interestingly, these termite lineages are also exceptional in that fungal fruiting structures have never been observed on their nests (13, 14), suggesting that evolution of inheritance of fungal clones from parent to offspring nest led to the abandonment of sexual fruiting as an integral part of the termite-fungus symbiosis.
Ant and beetle fungiculture is most similar to that of Microtermes and Macrotermes bellicosus. Each generation, reproductive adults actively disperse their own fungus when they found new colonies. In ambrosia beetles, the beetles either ingest fungal spores before dispersal from their natal nest, or more commonly, gather spores in specialized storage structures (mycangia) for fungal transport. Whereas predispersal acquisition of fungi is relatively unspecific, only certain fungal species appear to survive in the mycangia, selectively eliminating unwanted fungi (17). As Aanen et al. argue, similar selection must also occur at the nest founding stage in the termites: either the first workers actively select a cultivar for the new combs (e.g., by filtering out appropriate spores from ingested forage in their guts), or the specific growth conditions provided by the termites select indirectly by favoring certain cultivars only (13). No such selection occurs at the nest-founding stage in the ants, but queens may select between different cultivars in their natal nest before their mating flight, provided that multiple cultivar genotypes coexist within single ant colonies. At present, however, there exists no support for such within-nest cultivar diversity in attine gardens (21, 22).
Garden Parasites, Pesticides, and Antimicrobial Defenses.
All three insect farmers are proficient gardeners. They control cultivar growth and remove weedy fungal competitors to maintain healthy gardens. If the farmers are removed, the gardens quickly deteriorate as fungal growth either congests the galleries (16) or the gardens are devastated by contaminants (11, 13, 23, 24). Farming insects also prevent mites and nematodes, common invaders of fungus-gardens, from feeding on the fungus and from contaminating gardens with alien spores. Yet despite the farmer's weeding efforts, the stable conditions of gardens, optimized for fungal growth, occasionally attract specialized fungal garden parasites. This has been documented in detail for ant gardens, where the fungal parasite Escovopsis can infect gardens to the detriment of crop productivity and colony growth (23–26). A similarly parasitic association has been hypothesized for Xylaria fungi in termite combs (11, 13), because Xylaria overgrows the comb when the termites abandon it (11, 13), resembling Escovopsis outbreaks in ant gardens. Specialist fungal parasites of beetle fungiculture have yet to be identified.
To combat Escovopsis infections of gardens, attine ants use antimicrobial “pesticides” that they derive from bacteria grown on specialized regions of their own bodies (24, 27, 28). The bacteria belong to the genus Streptomyces, a well-known genus of soil bacteria that has been used by pharmaceutical industry for the discovery of novel antibiotics (e.g., streptomycin). Interestingly, Streptomyces bacteria and close relatives have also been isolated from termite guts (10, 11). It is unclear whether the gut Streptomyces function as sources of “pesticides” in termite fungiculture, and whether bacteria known to be associated with ambrosia beetles (17) could serve similar antimicrobial functions in beetle fungiculture.
If there is an important message to be learned from the insect agriculturists, it may be that long-term evolutionary success as farmers depends on effective strategies for agricultural disease management. For example, the exceedingly successful termite and ant agriculturists rely on the propagation of cultivar clones in huge monocultures, and the ants are known to suppress crop diseases with antibiotics (24, 27, 28). Management strategies thought to minimize the evolution of specialized crop diseases and the emergence of antibiotic resistance in human agriculture, strategies such as intercropping, sexually recombining crops, and low-pesticide farming, are possibly implemented by some termites (sexual crops) and some beetles (sexual crops, intercropping), but clearly not by all insect farmers, particularly the ants. The ant farmers' disease resilience may lie in the combination of several adaptations, for example the reliance on a live source of pesticides (e.g., antibiotic-producing Streptomyces bacteria) that can coevolve with the pathogens; or more importantly, the intense monitoring of gardens to eradicate immediately resistant pathogen mutants before an epidemiological outbreak. Perhaps, apart from providing us with a glimpse at possible evolutionary outcomes of human agriculture millions of years from now, the insect farmers can teach us more imminently about epidemiological principles and disease management of agricultural pathogens. After all, they have been farmers for millions of years, and maybe they have figured out a trick or two that lead to their remarkable agricultural success.
Acknowledgments
We thank D. Aanen, S. Kühnholz, and K. Machielsen for the insect images in Fig. 1 and D. Aanen, B. Farrell, and S. Kühnholz for comments. N.G. is funded by a Graduate Fellowship from the University of Texas at Austin. The Mueller laboratory is funded by National Science Foundation Faculty Early Career Development Program and Integrated Research Challenges in Environmental Biology grants.
See companion article on page 14887 in issue 23 of volume 99.
References
- 1.Wilson E. O., (1971) The Insect Societies (Belknap, Cambridge, MA).
- 2.Aanen D. K., Eggleton, P., Rouland-Lefèvre, C., Guldberg-Frøslev, T., Rosendahl, S. & Boomsma, J. J. (2002) Proc. Natl. Acad. Sci. USA 99, 14887-14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapela I. H., Rehner, S. A., Schultz, T. R. & Mueller, U. G. (1994) Science 266, 1691-1694. [DOI] [PubMed] [Google Scholar]
- 4.Schultz T. R. & Meier, R. (1995) Syst. Entomol. 20, 337-370. [Google Scholar]
- 5.Mueller U. G., Rehner, S. A. & Schultz, T. R. (1998) Science 281, 2034-2038. [DOI] [PubMed] [Google Scholar]
- 6.Green A. M., Mueller, U. G. & Adams, R. M. M. (2002) Mol. Ecol. 11, 191-195. [DOI] [PubMed] [Google Scholar]
- 7.Cassar S. & Blackwell, M. (1996) Mycologia 88, 596-601. [Google Scholar]
- 8.Jones K. G. & Blackwell, M. (1998) Mycol. Res. 102, 661-665. [Google Scholar]
- 9.Farrell B. D., Sequeira, A. S., O'Meara, B. C., Normark, B. B., Chung, J. H. & Jordal, B. H. (2001) Evolution (Lawrence, Kans.) 55, 2011-2027. [DOI] [PubMed] [Google Scholar]
- 10.Abe T., Bignell, D. E. & Higashi, M., (2000) Termites: Evolution, Sociality, Symbioses, Ecology (Kluwer Academic, Dordrecht, The Netherlands).
- 11.Batra L. R. & Batra, S. W. T. (1979) in Insect-Fungus Symbiosis, ed. Batra, L. R. (Allanheld and Osmun, Montclair, NJ), pp. 117–163.
- 12.Rouland-Lefèvre C. (2000) in Termites: Evolution, Sociality, Symbioses, Ecology, eds. Abe, T., Bignell, D. E. & Higashi, M. (Kluwer Academic, Dordrecht, The Netherlands), pp. 289–306.
- 13.Wood T. G. & Thomas, R. J. (1989) in Insect-Fungus Interactions, eds. Wilding, N., Collins, N. M., Hammond, P. M. & Webber, J. (Academic, New York), pp. 69–92.
- 14.Darlington J. E. C. P. (1994) in Nourishment and Evolution in Insect Societies, eds. Hunt, J. H. & Nalepa, C. A. (Westview, Boulder, CO), pp. 105–130.
- 15.Mueller U. G., Schultz, T. R., Currie, C. R., Adams, R. M. M. & Malloch, D. (2001) Q. Rev. Biol. 76, 169-197. [DOI] [PubMed] [Google Scholar]
- 16.Batra L. R. (1966) Science 153, 193-195. [DOI] [PubMed] [Google Scholar]
- 17.Beaver R. A. (1989) in Insect–Fungus Interactions, eds. Wilding, N., Collins, N. M., Hammond, P. M. & Webber, J. F. (Academic, New York), pp. 121–143.
- 18.Malloch D. & Blackwell, M. (1993) in Ceratocystis and Ophiostoma: Taxonomy, Ecology and Pathogenicity, eds. Wingfield, M. J., Seifert, K. A. & Webber, J. (Am. Phytopathol. Soc., St. Paul), pp. 195–206.
- 19.Farrell B. D. (1998) Science 281, 555-559. [DOI] [PubMed] [Google Scholar]
- 20.Diamond J., (1997) Guns, Germs, and Steel: The Fates of Human Societies (Norton, New York).
- 21.Bot A., Rehner, S. A. & Boomsma, J. J. (2001) Evolution (Lawrence, Kans.) 55, 1980-1991. [DOI] [PubMed] [Google Scholar]
- 22.Mueller U. G. (2002) Am. Nat. 160,(Suppl.), S67-S98. [DOI] [PubMed] [Google Scholar]
- 23.Currie C. R., Mueller, U. G. & Malloch, D. (1999) Proc. Natl. Acad. Sci. USA 96, 7998-8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Currie C. R. (2001) Annu. Rev. Microbiol. 55, 357-380. [DOI] [PubMed] [Google Scholar]
- 25.Currie C. R. (2001) Oecologia 128, 99-106. [DOI] [PubMed] [Google Scholar]
- 26.Currie, C. R., Bot, A. & Boomsma, J. J. (2003) Oikos, in press.
- 27.Currie C. R., Scott, J. A., Summerbell, R. & Malloch, D. (1999) Nature 398, 701-704. [Google Scholar]
- 28.Poulsen M., Bot, A., Currie, C. R. & Boomsma, J. J. (2002) Insectes Sociaux 49, 15-19. [Google Scholar]