Abstract
Shifts in the Mg/Ca ratio of seawater driven by changes in midocean ridge spreading rates have produced oscillations in the mineralogy of nonskeletal carbonate precipitates from seawater on time scales of 108 years. Since Cambrian time, skeletal mineralogies of anatomically simple organisms functioning as major reef builders or producers of shallow marine limestones have generally corresponded in mineral composition to nonskeletal precipitates. Here we report on experiments showing that the ambient Mg/Ca ratio actually governs the skeletal mineralogy of some simple organisms. In modern seas, coralline algae produce skeletons of high-Mg calcite (>4 mol % MgCO3). We grew three species of these algae in artificial seawaters having three different Mg/Ca ratios. All of the species incorporated amounts of Mg into their skeletons in proportion to the ambient Mg/Ca ratio, mimicking the pattern for nonskeletal precipitation. Thus, the algae calcified as if they were simply inducing precipitation from seawater through their consumption of CO2 for photosynthesis; presumably organic templates specify the calcite crystal structure of their skeletons. In artificial seawater with the low Mg/Ca ratio of Late Cretaceous seas, the algae in our experiments produced low-Mg calcite (<4 mol % MgCO3), the carbonate mineral formed by nonskeletal precipitation in those ancient seas. Our results suggest that many taxa that produce high-Mg calcite today produced low-Mg calcite in Late Cretaceous seas.
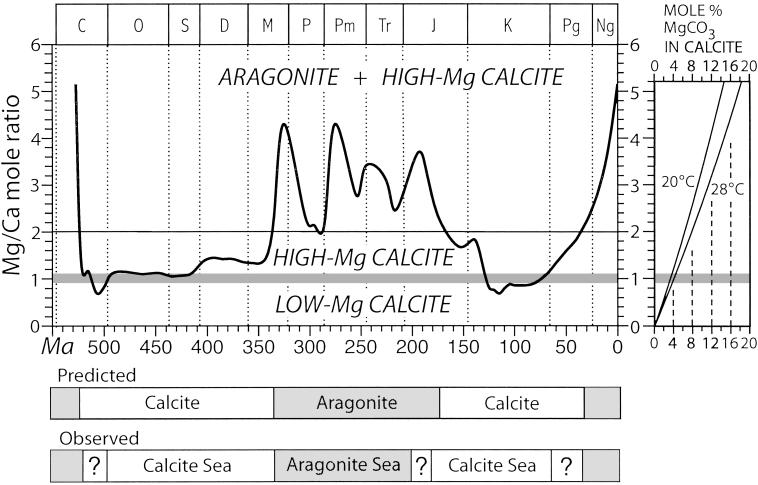
Since the beginning of the Paleozoic Era, there have been two intervals of “calcite seas,” in which nonskeletal carbonate precipitates from seawater have consisted of low-Mg calcite and three intervals of “aragonite seas,” in which these precipitates have consisted of high-Mg calcite or of aragonite, the orthorhombic polymorph of CaCO3 (1) (Fig. 1). Fossils reveal the same temporal patterns for the skeletal mineralogies of anatomically simple organisms that have functioned as major producers of carbonate reefs or bioclastic sediments on shallow sea floors; such organisms exert less control than more complex organisms over the chemical milieu in which they build their skeletons (2, 3). Calcareous skeletons of many anatomically simple organisms share two traits with nonskeletal precipitates: first, they consist of needle-shaped crystals, and second, these crystals are commonly arrayed radially to form spherulites (semiround bundles) (4).
Fig 1.
Effects of the Mg/Ca ratio of seawater on the mineralogy of nonskeletal carbonate precipitates. Secular oscillations in the Mg/Ca mole ratio of seawater during the Phanerozoic Eon, estimated by Hardie (6), predict intervals of calcite and aragonite seas that closely match the occurrence of nonskeletal precipitates in the rock record (1). (Right) The magnesium content of nonskeletal marine calcite precipitated in the laboratory is plotted as a function of the ambient Mg/Ca mole ratio of seawater†‡ (the horizontal band separating high- and low-Mg calcite is bounded by values of ambient Mg/Ca ratios for 20°C and 28°C).
Laboratory experiments show that the mineralogy of nonskeletal carbonate precipitates varies with the Mg/Ca ratio of seawater.†,‡ In water at the ionic strength of modern seawater (0.7) and temperatures typical of tropical seas (25–30°C), low-Mg calcite precipitates when the ambient Mg/Ca mole ratio is below ≈1. When the ambient mole ratio is above ≈1, high-Mg calcite precipitates and the percentage of magnesium carbonate incorporated into a crystal increases with this ratio, reaching ≈6–8 at a ratio of 2, depending on temperature (Fig. 2). At ambient mole ratios between ≈2 and slightly above 5, aragonite precipitates along with high-Mg calcite, but at ratios substantially above 5 only aragonite precipitates.†‡ An analysis of fluid inclusions in marine halite (NaCl) crystals showed that aragonite seas have indeed been within this range throughout Phanerozoic time (5).
Fig 2.
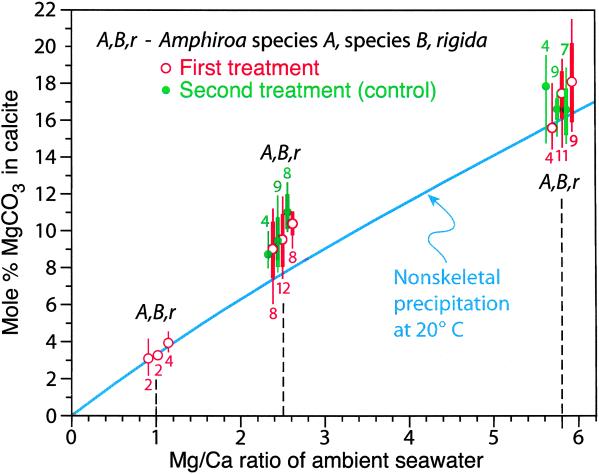
Mol % magnesium substituting for calcium in three Amphiroa species grown in artificial seawater having Mg/Ca mole ratios of 1.0, 2.5, and 5.8. Narrow vertical bars depict ranges for values and heavy vertical bars, 1σ; the number above or below a bar is n. In the first treatment, specimens were moved from an aquarium containing modern seawater to one containing seawater with a Mg/Ca mole ratio of 1.0, 2.5, or 5.8; in the second (control) treatment, specimens were switched between the aquaria with ratios of 2.5 and 5.8. Amphiroa growth was at 20°C (±1). The curve for nonskeletal precipitates at 20°C was interpolated from laboratory experiments at 13.5°C and 28°C.†‡ Specimens for all species have been placed in the U.S. National Museum of Natural History (USNM) for future reference: Amphiroa species A, provided by Walter Adey, USNM (USNM D&ML 59147), Amphiroa species B, from Old Dan Bank, Florida Bay (USNM D&ML 59148), Amphiroa rigida, from off Tavernier Key, Florida (USNM D&ML 59149).
Seawater-basalt interactions along midocean ridges at high temperatures extract magnesium from seawater and release calcium to it. A mixing model incorporating river water and midocean ridge hydrothermal brines shows that changes in the global rate of seafloor spreading should have caused the Mg/Ca mole ratio to oscillate between 1 and 5, a range that can account for transitions between calcite and aragonite seas (6).
In the modern aragonite sea, most anatomically simple skeletal organisms, such as macrophytic algae, sponges, corals, and bryozoans, that are major reef builders or suppliers of carbonate sediment to shallow sea floors secrete either aragonite and/or high-Mg calcite (2, 3). Paralleling the pattern for nonskeletal precipitation,†‡ the percentage of magnesium in skeletal calcite decreases with decreasing temperature. This effect is strongest for simple organisms (7), which suggests that reduction of the Mg/Ca mole ratio from that of modern seawater (5.2) might also cause such organisms to incorporate less magnesium into their skeletons than they do in modern seawater. Skeletons of most extant species of coralline algae are formed with little biological control, by impregnation of cell walls with high-Mg calcite; photosynthesis by the algae induces precipitation of carbonate by extracting CO2 from precipitating fluids (8–12). In at least some species, a small amount of skeletal material is dissolved at night, in the absence of photosynthesis (11).
Although petrographic techniques can indicate original high magnesium content for fossilized skeletal calcite (4, 13–15), diagenetic loss of magnesium from the lattice of high-Mg calcite has impeded quantitative analysis of the geologic history of this mineral's production by organisms. Faced with this limitation, we undertook experiments on the effect of seawater chemistry on the skeletal mineralogy of three extant species of coralline algae that produce high-Mg calcite in modern seas. We varied the ambient Mg/Ca ratio while holding temperature constant.
Methods
We photographed 12 multibranched individuals of each of three species of articulated coralline algae of the genus Amphiroa and positioned them in cylindrical depressions in sterilized tissue culture plates located in three aquaria containing artificial seawater. The sum of the molal concentrations of Mg and Ca in each aquarium was equal to that of modern seawater. The chemical recipe used to prepare artificial seawater, except for variation of the Mg/Ca ratio, was formula 124 of Bidwell and Spotte (16). Additions of MgSO4 and CaCl2 maintained these components within 5% of their initial values for all experiments. In one aquarium the Mg/Ca mole ratio was slightly above that of modern seawater (5.8); in a second it was equivalent to that of Permian seawater, low in the aragonite domain (2.5); and in the third, as in Late Cretaceous seas, it was in the low-Mg calcite domain (1.0). The aquaria were maintained at 20 ± 1°C and provided with identical lighting. After 51 days, we photographed surviving algae again, snipped off samples of new growth, and then switched those in the aquarium for which the Mg/Ca mole ratio was 5.8 with those in the aquarium for which it was 2.5. We harvested both sets of surviving algae after 51 days at their second location and snipped off samples of new growth to conduct controlled experiments. Algae initially placed in the “Late Cretaceous” aquarium died after 4 days and could not be relocated, but grew enough for sampling of new growth. We are considering whether poisoning from sudden exposure to the high concentration of calcium was responsible for this mortality.
We embedded each roughly cylindrical sample of algal stalk in epoxy resin, sectioned it parallel to the longitudinal axis, and, using an electron microprobe (JEOL 8600 Superprobe), measured the mole percentage of magnesium substituting for calcium in the apical region and adjacent hypothalus. Measurements were made at the junctures of four cells, where calcification is heaviest. The mean range for four sets of five replicate measurements of the percentage of MgCO3 for single sites was 2.16.
Results
For each species in each of our experimental treatments, the mean percentage of MgCO3 was close to that for calcite precipitated inorganically from water of the same chemistry, but was slightly above the latter for all three species in seawater with Mg/Ca mole ratios of 2.5 and 5.8 (Fig. 2). This discrepancy might represent a weak vital effect, but we hypothesize that it instead reflects a slight elevation of the Mg/Ca ratio in the confined spaces close to loci of calcification, where precipitation would have removed calcium at a higher rate than magnesium.
At 5.2, the Mg/Ca mole ratio of modern seawater is close to the point above which nonskeletal precipitation produces only aragonite instead of either this mineral or high-Mg calcite.†‡ The fact that all three species of Amphiroa secreted only high-Mg calcite at an ambient ratio of 5.8 supports other evidence that organic compounds in the cell walls of coralline algae serve as templates for construction of a calcite crystal structure (10).
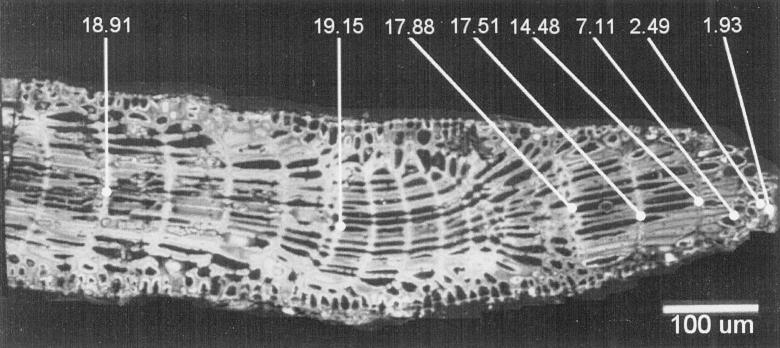
Skeletal magnesium percentages displayed in Fig. 2 for ambient Mg/Ca mole ratios of 5.8 and 2.5 are for skeletal segments grown many days after algae were placed in a new setting, and these segments were analyzed a few days after growth. Algae introduced to an ambient Mg/Ca mole ratio of 2.5 from either modern seawater or seawater with a ratio of 5.8 incorporated progressively less Mg for several days until stabilizing at percentages close to those produced by nonskeletal precipitation. An algal branch grew one row of cells at its tip every day, which permitted us to monitor the rate of decline in the Mg content of calcite formed during the initial period of growth (Fig. 3). Amphiroa is known to add a small amount of skeletal material far from the tip for some time after initial calcification (9). However, the relatively consistent Mg content that we documented for portions of segments formed shortly before transplantation indicates that little calcite was added to the analyzed cell walls after apical calcification.
Fig 3.
Scanning electron micrograph of a longitudinal section of a stalk of Amphiroa species A grown in seawater with the estimated Late Cretaceous Mg/Ca ratio of 1.0. Numbers are mole percentages of magnesium in substitution for calcium in cell wall carbonate. Measurements are at junctures of four cells along cell rows, one of which was added daily. Percentages in the 17.51–19.15 range are for calcification in modern seawater. During 4 days of growth in the Late Cretaceous treatment, the percentage declined progressively, reaching 1.93 before the specimen died.
Eight of the specimens in the aquarium with a Mg/Ca mole ratio of 1 survived about 4 days, and all produced low-Mg calcite before dying (Fig. 2). Thus, they mimicked nonskeletal precipitation for Late Cretaceous time, when according to petrographic evidence, lime mud consisted of low-Mg calcite rhombs.§
Discussion
The progressive daily decline in the Mg content of skeletal calcite in specimens grown in water having a relatively low Mg/Ca mole ratio suggests that the internal aqueous fluid of a branch shifted gradually toward the composition of ambient seawater. The similarity between the magnesium content of the newest skeletal material grown in our experiments and that of nonskeletal calcite precipitated from waters of the same Mg/Ca mole ratio suggests that, although organic templates in cell walls may specify calcite mineralogy in Amphiroa (10), the magnesium content of the skeletal calcite is governed by the Mg/Ca ratio of ambient seawater. By extracting CO2, photosynthesis may have induced precipitation of this material directly from little-modified seawater without the pumping of calcium ions through cell walls.
Our results support the hypothesis that the percentage of magnesium in skeletal calcite of anatomically simple taxa has been positively correlated with the Mg/Ca ratio of seawater over geologic time (2, 3). We conclude that many such organisms whose skeletons consist of high-Mg calcite today probably produced low-Mg calcite in Late Cretaceous seas; these include alcyonarians and some groups of foraminiferans and sponges (7). The percentage of magnesium decreases with decreasing temperature even in the skeletons of anatomically complex taxa that now secrete high-magnesium calcite: serpulid worms, crustaceans, echinoderms (7), and calcitic bryozoans (17), Thus, at past times when the Mg/Ca ratio of seawater was relatively low, these taxa may also have incorporated less magnesium into their skeletons than they do today.
Acknowledgments
We thank Tom Frankovich, Jim Fourqurean, and Laurel Collins for aid in field work, Walter Adey for providing specimens of Amphiroa sp. A, Diane Littler for taxonomic assistance, and Kenneth Livi for aid with mineralogical analyses. This work was supported by the James B. Knapp Endowment of The Johns Hopkins University, National Science Foundation Grant EAR-0202849, and Petroleum Research Fund Grant 38468-AC2.
Füchtbauer, H. & Hardie, L. A. (1976) Geol. Soc. Am. Abstr. Progr. 8, 877 (abstr.).
Füchtbauer, H. & Hardie, L. A., International Society of Sedimentologists Meeting, 1980, Bochum, Germany, pp. 167–169 (abstr.).
Lasemi, Z. (2000) Geol. Soc. Am. Abstr. Progr. 32, A68 (abstr.).
References
- 1.Sandberg P. A. (1985) Am. Geophys. Union Monogr. 32 585-594. [Google Scholar]
- 2.Stanley S. M. & Hardie, L. A. (1998) Palaeogeogr. Palaeoclimatol. Palaeoecol. 144 3-7. [Google Scholar]
- 3.Stanley S. M. & Hardie, L. A. (1999) GSA Today 9 1-19. [Google Scholar]
- 4.Sandberg P. A. (1975) J. Paleontol. 49 587-606. [Google Scholar]
- 5.Lowenstein T. K., Timofeeff, M. N., Brennan, S. T., Hardie, L. A. & Demicco, R. V. (2001) Science 294 1086-1088. [DOI] [PubMed] [Google Scholar]
- 6.Hardie L. A. (1996) Geology 24 279-283. [Google Scholar]
- 7.Chave K. E. (1954) J. Geol. 62 266-183. [Google Scholar]
- 8.Pearse V. B. (1972) J. Phycol. 8 88-97. [Google Scholar]
- 9.Digby P. S. B. (1977) J. Mar. Biol. Assoc. U.K. 57 1111-1124. [Google Scholar]
- 10.Borowitzka M. A. (1981) Mar. Biol. 62 17-23. [Google Scholar]
- 11.Chisolm J. R. M. (2000) Limnol. Oceanogr. 45 1476-1484. [Google Scholar]
- 12.Chisolm J. R. M., Collingwood, J.-C. E. & Gill, F. (1990) J. Exp. Mar. Biol. Ecol. 141 15-29. [Google Scholar]
- 13.Lohmann K. C. & Meyers, W. J. (1977) J. Sedim. Petrol. 47 1078-1088. [Google Scholar]
- 14.Richter D. K. & Füchtbauer, H. (1978) Sedimentology 25 843-860. [Google Scholar]
- 15.Webb G. E. & Sorauf, J. E. (2002) Geology 30 415-418. [Google Scholar]
- 16.Bidwell J. P. & Spotte, S., (1985) Artificial Seawaters: Formulas and Methods (Jones and Bartlett, Boston).
- 17.Rucker J. B. & Carver, R. E. (1969) J. Paleontol. 43 781-799. [Google Scholar]