Abstract
Current models for the role of the SWI/SNF chromatin remodeling complex in gene regulation are focused on promoters, where the most obvious changes in chromatin structure occur. Here we present evidence that the SWI/SNF complex is involved in the remodeling of the chromatin structure of an entire gene in vivo. We compared the native chromatin structures of a small yeast plasmid containing the HIS3 gene purified from uninduced and induced cells. Relative to uninduced chromatin, induced chromatin displayed a large reduction in negative supercoiling, a large reduction in sedimentation rate, and increased accessibility to restriction enzymes with sites located both near and far from the HIS3 promoter. These observations indicate that the entire plasmid was remodeled as a result of induction. Loss of supercoiling required the presence of the SWI/SNF remodeling complex and the activator Gcn4p in vivo. The TATA boxes were not required, suggesting that remodeling was not the result of transcription. The induction-dependent loss of negative supercoiling was not apparent in cells, indicating that the supercoils were lost preferentially from induced chromatin during purification. Thus, induced HIS3 chromatin has a highly labile structure that is revealed as a result of purification. It is concluded that induction of HIS3 creates a domain of labile chromatin structure that extends far beyond the promoter to include the entire gene. We propose that the SWI/SNF complex is recruited to the HIS3 promoter by Gcn4p and then directs remodeling of a chromatin domain, with important implications for transcription.
Eukaryotic DNA is packaged into chromatin, the basic structural repeat unit of which is the nucleosome. The nucleosome core contains 147 bp of DNA wrapped in ≈1.75 negative superhelical turns around a central core histone octamer composed of two each of the four core histones, H2A, H2B, H3, and H4 (1). It is now clear that chromatin is not just a DNA-packaging system, but also an integral component of the gene regulatory apparatus. This finding has become obvious as a result of the discoveries of chromatin remodeling complexes and histone-modification enzymes, with their direct links to proteins involved in transcriptional control (2–4).
Many chromatin remodeling complexes have now been characterized (5). Remodeling complexes use the energy of ATP hydrolysis to effect transitions in chromatin structure. An example is the SWI/SNF remodeling complex, which possesses several ATP-dependent chromatin remodeling activities: it can slide nucleosomes along DNA and transfer histone octamers from one DNA molecule to another, cause mononucleosomes to coalesce, and induce nucleosome-conformational changes (6, 7). Remodeling results in a loss of the DNase I repeat pattern typical of positioned nucleosomes and in increased accessibility of nucleosomal DNA to restriction enzymes (6). However, in most of the experiments conducted in vitro, the SWI/SNF complex did not distinguish one nucleosome from another, prompting the question of how the SWI/SNF complex might be targeted to particular genes in vivo. This problem has been clarified by experiments in vitro demonstrating that the SWI/SNF complex can be recruited to promoters by the activation domain of transcriptional activators, resulting in the disruption of neighboring nucleosomes and facilitating transcription (8, 9).
Nucleosome conformational changes induced by the SWI/SNF complex have been most clearly observed by using a topological assay, making use of the fact that the classical nucleosome core protects one negative supercoil from relaxation by topoisomerases (10). The addition of purified SWI/SNF complex (or the related RSC complex) to nucleosomes reconstituted on circular DNA converts the nucleosomes to an altered conformation, the nature of which is still unclear. It is characterized by a large reduction in the negative supercoiling contained within the nucleosome (11–13) and by the loss of ≈30 bp of DNA from the nucleosome (14). To observe the loss of supercoiling occurring on remodeling, topoisomerase must be added to remove the negative supercoils released from nucleosomes in the remodeling reaction. The mechanism of remodeling by the SWI/SNF complex is unclear, but there is evidence showing that SWI/SNF-generated changes in DNA twist are important in the reaction (15, 16).
Current models for the role of the SWI/SNF complex in gene regulation are focused on promoters, where the most obvious changes in chromatin structure occur. Examples include the yeast SUC2 (17) and PHO8 (18) genes, and the human IFN-β gene (19). However, nuclease sensitivity assays provide evidence of more extensive SWI/SNF-dependent changes in chromatin structure at the yeast FLO1 gene, involving nucleosomes relatively far upstream from the FLO1 promoter (20). Our own recent work on the SWI/SNF-independent yeast CUP1 gene indicates that induction results in movement of nucleosomes not just at the promoter but over the entire gene and flanking regions (21). The latter examples indicate that remodeling is not always confined to promoter chromatin and can involve larger chromatin regions.
Here, we have investigated the possibility that the SWI/SNF complex can direct large-scale changes in chromatin structure in vivo by probing for topological changes. A plasmid-based system is appropriate for such a study, because topological changes can be detected only in circular DNA and thus cannot be observed in the chromosome. We made use of a method we had developed previously for purifying small amounts of plasmid chromatin from the cells of budding yeast (21). We investigated the remodeling events occurring in a small episome containing the HIS3 gene. HIS3 encodes an enzyme required for the biosynthesis of histidine: it is regulated by the Gcn4p activator (22), the SWI/SNF complex (23), and the histone acetylases Gcn5p (24) and Esa1p (25).
Three lines of evidence are presented to show that induction of HIS3 results in remodeling of a chromatin domain that extends far beyond the HIS3 promoter. Induction resulted in: (i) A large-scale loss of negative supercoils from purified chromatin, requiring the SWI/SNF complex and the Gcn4p activator in vivo. The scale of the topological change indicated that most or all of the nucleosomes on the plasmid were affected. (ii) A large reduction in the sedimentation rate, suggesting decompaction of the chromatin. (iii) A large increase in accessibility to restriction enzymes at sites within and far from the HIS3 promoter. Our results suggest a model in which the SWI/SNF complex is recruited to the HIS3 promoter by Gcn4p and then directs long-range remodeling of a chromatin domain. The implications for transcription are discussed.
Materials and Methods
Plasmids.
HIS3 was obtained as a 982-bp PCR fragment with EcoRI ends, corresponding to coordinates −168 to +808 with respect to the HIS3 start codon (equal to the entire HIS3 insert from pRS403; Stratagene), into the EcoRI site of pSP64 (Promega) to obtain pSP-HIS3B, and the sequence was verified. The EcoRI fragment was transferred to pGEM-TRP1ARS1(RV) to obtain pGEM-TAHIS3B. pGEM-TRP1ARS1(RV) contains TRP1ARS1 inserted as an EcoRV fragment at the EcoRV site of pGEMzf5(+) (Promega). pSP-HIS3ΔCUP1B: A 908-bp BsaAI-Ecl136II CUP1 fragment from pCP2 (21) was used to replace the BstBI fragment of HIS3 in pSP-HIS3B. Versions of pGEM-TAHIS3B carrying a TCTRΔ deletion (from −83 to −11 in the HIS3 promoter) or a deletion of the T residue at −99 (ATGACTC) in the Gcn4p binding site were constructed by PCR mutagenesis.
Yeast Strains.
TA-HIS3 was obtained from pGEM-TAHIS3B as a 2,435-bp EcoRV fragment, circularized with ligase, and used to transform YCShis3Δ. YCShis3Δ was derived from YDCcup1Δ2 (21) (MATa cir0 ura3-52 trp1 lys2-801 leu2Δ1 his3Δ200 pep4:HIS3 prb1Δ1.6R can1 cup1Δ:LEU2) by disruption of the HIS3 gene at the pep4 locus with CUP1: YCShis3Δ was transformed with an EcoRI digest of pSP-HIS3ΔCUP1B, and his3Δ:CUP1 integrands were selected by using resistance to 0.1 mM copper (II) sulfate. The integration was confirmed by Southern blot hybridization. BLY33 (MATαsnf2Δ2:URA3 his3Δ200 trp1Δ63 ura3-52) was the gift of B. Laurent (unpublished data). H2060 (MATα gcn4Δ1 leu2-3,-112 his3-609 ura3-52 trp1Δ63) was the gift of A. Hinnebusch (26).
Chromatin Purification.
Chromatin was purified as described (21) with some modifications: cells were grown to late log phase in synthetic complete medium lacking tryptophan (uninduced) or both tryptophan and histidine (induced) and stored at −80°C. Cells from 1 liter of culture were thawed in 50 ml of spheroplasting medium (SM) [the appropriate growth medium with 1 M d-sorbitol/50 mM Tris⋅HCl (pH 8.0)/20 mM 2-mercaptoethanol added] and incubated at 30°C for 15 min. Lytic enzyme (800 mg at 77 units/mg; ICN 152270) was dissolved in SM and added to the cells. Spheroplasting was measured as described (21). Spheroplasts were collected (7,500 rpm, 5 min, Sorvall SS34 rotor, 4°C), washed once with 50 ml of cold 1 M d-sorbitol/50 mM Tris⋅HCl (pH 8.0), and lysed by resuspension in 40 ml of 18% (wt/vol) Ficoll 400/40 mM potassium phosphate/1 mM MgCl2/2 mM Na-EDTA (pH 6.5, adjusted with phosphoric acid), with 5 mM 2-mercaptoethanol/0.1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride/5 μg/ml leupeptin/15 μg/ml pepstatin A added. Two step gradients were used: 20 ml of lysate was layered over 15 ml of 7% (wt/vol) Ficoll/20% (vol/vol) glycerol/40 mM potassium phosphate/1 mM MgCl2/2 mM Na-EDTA (pH 6.5, adjusted with phosphoric acid), with additions as above, and spun (14,000 rpm, 30 min, SS34 rotor, 4°C). Each nuclear pellet was resuspended in 4 ml of 50 mM Tris⋅HCl (pH 8.0)/5 mM Na-EDTA, with additions as above. RNase (DNase-free; Qiagen, Valencia, CA) was added to 1.3 mg/ml, and the suspensions were left on ice for 30 min and spun (10,000 rpm, 5 min, SS34, 4°C). The supernatants, containing the plasmid chromatin, were applied to 850 μl of 30% sucrose cushions in TAE buffer [40 mM Tris-acetate, 2 mM Na-EDTA (adjusted to pH 7.9 with acetic acid), 10 μg/ml BSA (protease- and nuclease-free; Calbiochem) with additions as above] in SW55 tubes and spun (55,000 rpm, 2.5 h, SW55Ti rotor, 4°C). The 30% cushions were pooled, syringe-filtered (0.45-μm pore diameter, low protein binding), divided between two prewashed Centricon-50 filtration units (Millipore), concentrated until the volume was ≈100 μl (6,000 rpm, SS34 rotor, 4°C), and washed twice with 500 μl of TAE buffer to a final volume of ≈100 μl. For TA-HIS3 DNA samples in vivo, washed spheroplasts were extracted directly with 2% SDS/50 mM Tris⋅HCl (pH 8.0)/5 mM Na-EDTA. Potassium acetate (5 M) was added to 1 M, and genomic DNA was purified by two extractions with an equal volume of chloroform followed by precipitation with ethanol. For sucrose gradients, chromatin was loaded on 5–30% (wt/vol) linear sucrose density gradients in PB (125 mM KCl/2.5 mM MgCl2/10 mM Hepes-Na (pH 7.5)/1 mM DTT with protease inhibitors as above). Sedimentation was for 14 h at 23,500 rpm and 4°C in a Beckman Coulter SW41Ti rotor.
Topological Analysis.
Two-dimensional gels were 180 ml of 15 × 15 cm 1.2% agarose in 40 mM Tris/30 mM NaH2PO4/1 mM Na-EDTA, pH 8.2 (TPE) buffer (27). First dimension: 35 V, 16 h with 10 μg/ml chloroquine diphosphate (to resolve higher topoisomers; see Figs. 1 and 5), or in the absence of chloroquine (to resolve weakly supercoiled topoisomers; see Fig. 4). The gel was soaked in three changes of second dimension buffer (20 or 5 μg/ml chloroquine to resolve higher and lower topoisomers, respectively) for 8 h, rotated 90°, and electrophoresed in second dimension buffer at 22 V for 15 h. Southern blots were hybridized with radiolabeled pGEM-TAHIS3B. Topoisomer spots were quantified by using a phosphorimager and expressed as a percentage of the total of all topoisomers (nicked circle was excluded).
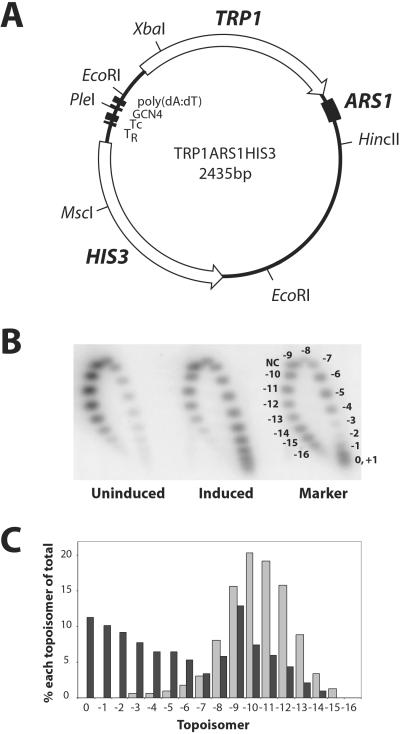
Fig 1.
Induction correlates with a large-scale loss of negative supercoils from purified native HIS3 chromatin. (A) Map of the TA-HIS3 episome (2,435 bp). TA-HIS3 is based on TRP1ARS1, a circularized yeast genomic EcoRI fragment capable of autonomous replication. TRP1 was used as a selection marker; ARS1 is an origin of replication. The HIS3 promoter contains a poly(dA⋅dT) element, a Gcn4p binding site, a consensus TATA box (TR), and a nonconsensus TATA box (TC) (22). (B) Two-dimensional topological analysis of purified TA-HIS3 chromatin. First dimension: 10 μg/ml chloroquine; second dimension: 20 μg/ml chloroquine. The phosphorimage of a Southern blot of a gel probed with labeled pGEM-TAHIS3B is shown. Marker: topoisomers of pSP72 (2,462). NC, nicked circle. (C) Topoisomer distributions of the samples in B: dark bars, induced; light bars, uninduced. Each topoisomer spot was quantified and expressed as a percentage of the total of all topoisomers (excluding nicked circle).
Fig 5.
Induction does not result in loss of negative supercoils from the HIS3 plasmid in cells. (A) Two-dimensional topological analysis of TA-HIS3 DNA extracted directly from cells, without purification of the chromatin. First dimension: 10 μg/ml chloroquine; second dimension: 20 μg/ml chloroquine. The phosphorimage of a Southern blot of a gel probed with labeled pGEM-TAHIS3B is shown. Marker: topoisomers of pSP72 (2,462). NC, nicked circle. (B) Topoisomer distributions of the samples in B: dark bars, induced; light bars, uninduced. Each topoisomer spot was quantified and expressed as a percentage of the total of all topoisomers (excluding nicked circle).
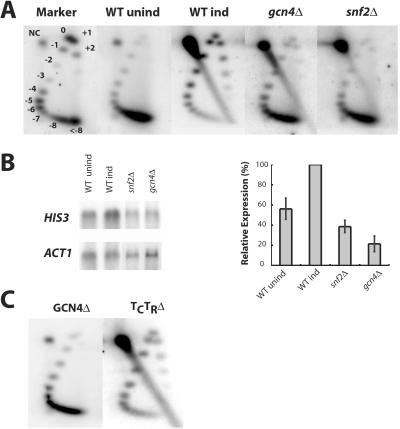
Fig 4.
Remodeling of HIS3 plasmid chromatin requires the presence of the SWI/SNF complex and the Gcn4p activator in vivo, but not the HIS3 TATA boxes. (A) Effects of snf2Δ and gcn4Δ mutations on the topology of purified TA-HIS3 chromatin. Topology of TA-HIS3 chromatin purified from uninduced and induced wild-type (WT) cells and induced snf2Δ and gcn4Δ cells. First dimension: no chloroquine; second dimension: 5 μg/ml chloroquine. Phosphorimages of Southern blots probed with labeled pGEM-TAHIS3B are shown. Marker: topoisomers of pSP72. NC, nicked circle. (B) Effects of snf2Δ and gcn4Δ mutations on the expression of HIS3. Northern blot analysis of HIS3 expression relative to ACT1 in uninduced and induced wild-type (WT) cells and induced snf2Δ and gcn4Δ cells. (C) Effects of HIS3 promoter mutations on the topology of purified TA-HIS3 chromatin. Topological analysis of TA-HIS3 chromatin purified from induced cells, with both TATA boxes deleted (TCTRΔ), or with a single base deletion in the Gcn4p-binding site (GCN4Δ).
Accessibility Assays.
Approximately 5 ng of gradient-purified TA-HIS3 chromatin was mixed with 5 ng of an internal control (pSP-HIS3B for MscI and XbaI, pBR322 for HincII and PleI) in 200 μl of PB and digested at 30°C with 0.2 units of restriction enzyme per μl. Aliquots (40 μl) were removed at different times, and extracted DNA was digested with a second enzyme (XbaI for MscI digests, MscI for XbaI digests, EcoRI for HincII digests, and XbaI+DraIII for PleI digests). Southern blots were hybridized by using the 244-bp HIS3 DraI fragment as probe. (The 237-bp TRP1 HindIII–BglII fragment was used for HincII digests.) For HincII and PleI digests, control DNA was detected in a second hybridization by using the 653-bp pBR322 EcoRI–HincII fragment as probe.
Results
Our experiments used yeast strains harboring the TRP1ARS1HIS3 (TA-HIS3) plasmid (Fig. 1A). This plasmid is based on the well-studied TRP1ARS1 plasmid (28), which is a circularized genomic EcoRI fragment capable of autonomous replication with the ARS1 origin. TRP1 is used as a selection marker for growth in the absence of tryptophan. The TRP1 promoter in TRP1ARS1 is truncated and so lacks upstream activating sequences (29). The entire HIS3 gene was inserted into TRP1ARS1 as an EcoRI fragment. (Care was taken to avoid introducing promoters derived from adjacent genes.) The chromosomal HIS3 gene in this strain was disrupted before transformation with TA-HIS3.
Induction Correlates with a Large-Scale Loss of Negative Supercoils from HIS3 Chromatin.
Cells were grown to late log phase under inducing conditions for HIS3 (synthetic medium lacking tryptophan and histidine) or under noninducing conditions (medium lacking tryptophan). Native TA-HIS3 plasmid chromatin was purified from induced and uninduced cells by using the method of Shen et al. (21). In brief, nuclei were prepared from spheroplasts and lysed in a low-ionic-strength buffer. TA-HIS3 chromatin in the supernatant was spun into a sucrose cushion and washed thoroughly by using a centrifugal filter. The result was a ribosome-free preparation containing TA-HIS3 chromatin and some much larger genomic chromatin.
To compare the topologies of TA-HIS3 chromatin purified from uninduced and induced cells, DNA was extracted from chromatin preparations and the topoisomers were resolved in two-dimensional chloroquine gels. Uninduced TA-HIS3 DNA exhibited a Gaussian distribution of topoisomers centered on 10–11 negative supercoils (Fig. 1 B and C), suggesting the presence of 10–11 nucleosomes, because the nucleosome core protects one negative supercoil from relaxation by topoisomerases (10). In contrast, induced TA-HIS3 exhibited a very different topoisomer distribution (Fig. 1 B and C), suggesting the presence of two populations of TA-HIS3 chromatin, one with an average of 10–11 negative supercoils and therefore resembling uninduced chromatin, and the other with an average of only two to three negative supercoils. Considerable variability existed in the proportions of the two populations from preparation to preparation: induced chromatin ranged from about half to almost all topoisomers in the weakly supercoiled fraction (e.g., see Fig. 4A), and uninduced chromatin often contained a minor fraction of weakly supercoiled chromatin. This finding probably reflected variability in the extent of HIS3 induction (see below). In conclusion, induction of HIS3 correlated with a dramatic loss of negative supercoils from a large fraction of TA-HIS3 chromatin. The loss of so many negative supercoils indicates that a large-scale remodeling event had occurred, involving most or all of the nucleosomes in induced chromatin.
Induced HIS3 Chromatin Sediments More Slowly than Uninduced Chromatin.
Large-scale remodeling might be expected to result in a less compact chromatin structure. This possibility was addressed by sedimentation of chromatin in sucrose density gradients containing buffer at approximately physiological ionic strength. The distribution and topology of TA-HIS3 DNA in the gradients were determined by examining the DNA in each fraction in gels lacking chloroquine (Fig. 2). Uninduced chromatin sedimented considerably further than induced TA-HIS3 chromatin, and, unlike induced chromatin, a significant fraction of uninduced chromatin was pelleted, indicating some aggregation. Both chromatin samples sedimented faster than relaxed protein-free plasmid, indicating that they both retained significant amounts of protein. Because the sedimentation of a particle is governed by its molecular mass and its hydrodynamic shape (i.e., its degree of compaction), the decreased sedimentation of induced chromatin relative to uninduced chromatin indicates that it was much less compact and/or contained much less bound protein than uninduced chromatin. It seems likely that a change in compaction was involved, because a very large difference in amounts of protein bound would be required to account for the observed difference in sedimentation rate. In either case, it may be concluded that induction correlated with a substantially altered chromatin structure.
Fig 2.
Induced HIS3 plasmid chromatin sediments more slowly than uninduced HIS3 plasmid chromatin. Purified uninduced (A) and induced (B) TA-HIS3 chromatin and relaxed pSP72 DNA (C) (a marker for protein-free DNA) were sedimented in 5–30% linear sucrose density gradients. DNA extracted from the gradient fractions and the pellet (P) was analyzed in agarose gels lacking chloroquine. Phosphorimages of Southern blots probed with labeled pGEM-TAHIS3B are shown. Markers: relaxed pSP72 (0) and pSP72 with an average of 10 negative supercoils (−10).
Induced HIS3 Chromatin Is More Accessible to Restriction Enzymes.
Accessibility of TA-HIS3 chromatin to various restriction enzymes was used as an independent test to confirm that induced chromatin had been extensively remodeled (Fig. 3). The accessibilities of restriction sites located both within and far from the HIS3 promoter were probed. Uninduced and induced chromatin were prepared by using sucrose gradients (as in Fig. 2) mixed with a protein-free plasmid as internal control (21) and subjected to time courses of digestion with different restriction enzymes. The extents of digestion of the chromatin and the control were measured by using an indirect end-labeling approach (21). The sites probed were the MscI site located inside the HIS3 ORF, the XbaI site located inside the TRP1 ORF, the HincII site located next to ARS1, and the PleI site at the Gcn4p binding site in the HIS3 promoter. In all cases, the control DNA was completely digested, as expected. Digestion of both uninduced and induced chromatin approached a plateau, indicating the presence of a relatively resistant fraction. The fraction of chromatin cleaved by each enzyme was very reproducible (see Fig. 3), with small standard errors for two or three independent experiments. In all cases, the extent of cleavage of induced chromatin was much higher than that of uninduced chromatin. With the exception of PleI, the accessibilities of uninduced chromatin were all about the same (31–36%), which is consistent with what would be expected from the topological estimate of 10–11 nucleosomes (10–11 nucleosomes each occupying 147 bp would protect ≈65% of the 2,435-bp plasmid). Accessibility to PleI was relatively high, indicating that the HIS3 promoter was more exposed than the rest of the gene, consistent with previous observations (30, 31). Thus, induced chromatin was more accessible to restriction enzymes than uninduced chromatin both at the HIS3 promoter and far away from it. This observation also indicates that induction resulted in a large-scale change in chromatin structure involving the entire plasmid.
Fig 3.
Induced HIS3 plasmid chromatin is more accessible to restriction enzymes at sites both near and far from the HIS3 promoter. Time courses of digestion of uninduced and induced sucrose gradient-purified TA-HIS3 chromatin with exogenous plasmid DNA as internal control, analyzed by indirect end-labeling. (A) Accessibility of the MscI site in HIS3: the phosphorimage of a Southern blot probed with a fragment of the HIS3 gene is shown. (*, Spurious band derived from plasmid control.) (B) Quantification of the data in A: uninduced (Unind) (•) and induced (Ind) (▪) TA-HIS3 chromatin; control plasmid DNA mixed with uninduced (○) or induced (□) chromatin. (C–E) Time courses of digestion by XbaI, HincII, and PleI, respectively. Symbols are as in B. See Fig. 1A for the locations of restriction sites.
Induction-Dependent Loss of Negative Supercoils from HIS3 Chromatin Requires the Presence of the Gcn4p Activator and the SWI/SNF Remodeling Complex in Vivo.
The roles of the Gcn4p activator and the SWI/SNF complex in remodeling HIS3 plasmid chromatin were examined. Mutant strains (gcn4Δ or snf2Δ) were transformed with TA-HIS3. The topology of chromatin purified from induced cells was determined by using two-dimensional chloroquine gels (Fig. 4A). Chromatin purified from induced snf2Δ or gcn4Δ cells was essentially fully supercoiled, unlike chromatin purified from induced wild-type cells, indicating that loss of supercoils required the presence in vivo of Gcn4p and the SWI/SNF complex.
The effects of these mutations on HIS3 transcription were examined by Northern blot analysis, using ACT1 as a reference (Fig. 4B). HIS3 transcription was reduced ≈5-fold in gcn4Δ cells and ≈2.5-fold in snf2Δ cells, relative to wild-type cells. These results confirm the positive roles of Gcn4p and the SWI/SNF complex in regulation of HIS3. The effect of induction on HIS3 expression in wild-type cells was small (≈1.5-fold), which probably reflected a partial induction of HIS3 as the multiplying cells depleted the medium of histidine. Thus, the variation in the proportions of the weakly and fully supercoiled forms of TA-HIS3 chromatin discussed above might reflect varying levels of induction of HIS3 in culture, which is consistent with the observation that TA-HIS3 chromatin purified from gcn4Δ cells was always fully supercoiled.
To confirm the central role of Gcn4p in remodeling, the Gcn4p-binding site was mutated (Fig. 4C). Deletion of a single T residue in the Gcn4p-binding site blocks induction of HIS3 (the his3-142 mutation; ref. 32). TA-HIS3 chromatin carrying this mutation was fully supercoiled after purification from induced cells, indicating that an intact Gcn4p-binding site was required for remodeling of HIS3 plasmid chromatin structure, which is consistent with the requirement for Gcn4p (Fig. 4A).
To address the role of transcription in remodeling, the region from −83 to −11 in the HIS3 promoter, which contains the consensus and the nonconsensus TATA boxes, was deleted (as in ref. 24). The TATA boxes were not required for loss of supercoils (Fig. 4C). However, this result did not completely rule out a role for transcription in remodeling because Northern blot analysis (not shown) indicated that HIS3 transcripts were still being synthesized at low levels. Despite this finding, it is unlikely that chromatin remodeling was the direct result of transcription, because HIS3 plasmid chromatin from snf2Δ and gcn4Δ cells did not lose supercoils, even though some HIS3 transcription was occurring (Fig. 4 A and B).
In conclusion, the large-scale remodeling of HIS3 plasmid chromatin structure occurring on induction depended on the presence of the SWI/SNF remodeling complex and the Gcn4p activator in vivo but did not require the HIS3 TATA boxes.
Induced Chromatin Has a Labile Structure.
The topology of HIS3 plasmid chromatin inside cells was determined by direct extraction of DNA from induced and uninduced cells without prior purification of the chromatin (Fig. 5A). No difference in topology was observed between induced and uninduced chromatin inside cells; both contained an average of 10–11 negative supercoils (Fig. 5B). Therefore, the loss of supercoils from induced chromatin must have occurred during purification. This finding was verified by testing chromatin samples for loss of supercoils at each stage of the purification; it was found that the supercoils were lost at the last stage, when the chromatin is thoroughly washed in a Centricon filter with a molecular weight cutoff of 50,000 (not shown). It is emphasized that loss of supercoils depended on induction; purified uninduced chromatin retained its supercoils during purification (it contained 10–11 negative supercoils both in vivo and after purification; compare Figs. 1 and 5), which indicates that induced chromatin, unlike uninduced chromatin, has an unstable chromatin structure, from which the negative supercoils are easily removed during purification. This lability extended to most or all of the nucleosomes in induced HIS3 plasmid chromatin. It is worth noting that two possible structures exist for the fraction of induced chromatin that retained its supercoils (Fig. 1): it could represent stable chromatin essentially identical to that found in uninduced cells, as suggested above, or potentially labile chromatin that did not lose its supercoils during purification. It is concluded that induction resulted in a large-scale destabilization of HIS3 chromatin structure.
Discussion
Three lines of evidence have been presented indicating that induction of HIS3 results in remodeling of an entire chromatin domain to create a structure in vivo that is labile during purification: (i) a dramatic, induction-dependent loss of negative supercoils that can be accounted for quantitatively only if most or all of the nucleosomes were involved (Fig. 1), (ii) a large reduction in the sedimentation rate of induced chromatin (Fig. 2), and (iii) increased accessibility of induced chromatin to restriction enzymes at sites located both near and far from the HIS3 promoter (Fig. 3). This remodeling required the presence of the SWI/SNF complex and the Gcn4p activator in vivo but was not due to transcription (Fig. 4).
The simplest structural model for the labile chromatin domain is that induction results in remodeling in vivo to yield destabilized nucleosomes that are easily lost from DNA during purification. The negative supercoils released as a result of histone loss would be relaxed by copurifying topoisomerases. We verified that the chromatin preparations contain topoisomerase activity by demonstrating that protein-free supercoiled DNA was relaxed when it was mixed with purified plasmid chromatin (not shown). In this model, purified induced chromatin would be mostly protein-free DNA with two or three nucleosomes remaining. This model can account for the loss of supercoils and increased accessibility to restriction enzymes, but the sedimentation data are less consistent. Induced chromatin clearly sedimented faster than protein-free plasmid (Fig. 2) and also sedimented considerably faster than plasmid chromatin containing three nucleosomes reconstituted with purified core histones (not shown), which suggests that induced chromatin contains significantly more protein than the equivalent of just three nucleosomes.
An alternative model that takes the known properties of the purified SWI/SNF complex into account should also be considered. We have shown that formation of the labile chromatin structure requires the presence of the SWI/SNF complex in vivo: induced chromatin did not display loss of supercoils when purified from an snf2 mutant (Fig. 4A). It should also be noted that remodeling could not have occurred during purification, because the buffers lacked the ATP required for the remodeling reaction and contained EDTA. In the SWI/SNF model, it is argued that the SWI/SNF complex is recruited to the HIS3 promoter by Gcn4p, where it directs the remodeling of nucleosomes on HIS3. This finding should result in a loss of negative supercoils apparent in vivo, but this was not the case (Fig. 5). It is therefore necessary to propose that the nucleosomes are remodeled, but the negative supercoils released as a result of remodeling were not relaxed by topoisomerase in vivo. We speculate that the negative supercoils are protected from relaxation by an unknown factor. During purification, this factor might dissociate from the chromatin, releasing the supercoils for relaxation by copurifying topoisomerases, finally revealing the remodeled state. In this model, purified induced chromatin would contain remodeled nucleosomes similar to those created by the SWI/SNF complex in vitro. Such a structure would display reduced supercoiling per nucleosome, reduced sedimentation rate, and increased accessibility to restriction enzymes, as observed. Although we have not yet been able to distinguish clearly between the two types of model, we have shown that HIS3 chromatin is dramatically remodeled in vivo as a result of induction, in a process that involves most or all of the nucleosomes on the plasmid.
It is proposed that the SWI/SNF complex is recruited to the HIS3 promoter by Gcn4p and then remodels nucleosomes both near and far from the promoter to create a domain of remodeled, destabilized chromatin structure. In support of this model, it has been shown that a direct interaction occurs between Gcn4p and the SWI/SNF complex (23, 33) and that recruitment of the SWI/SNF complex to a promoter containing a Gcn4p site depends on Gcn4p in vivo (34). Long-range remodeling is also implied by the observation that nucleosome positioning far upstream of the FLO1 promoter is affected in an snf2 mutant, although in this case it was not determined whether the transcribed region was affected (20). The extent to which our observations concerning HIS3 might hold true for other genes is unclear; the effects of induction on HIS3 chromatin were revealed by a new approach to the problem. If this approach were applied to some other well studied genes currently thought to be targets of localized disruption at the promoter, such as PHO5 (35), gene-wide remodeling might turn out to be a quite general phenomenon.
The use of plasmid chromatin as a model enabled us to measure topological changes, but the question remains as to how our observations might translate to the normal location of HIS3, which is not in a small circle but in a linear segment of a relatively large chromosome. A possibility is that the SWI/SNF complex, once recruited to the promoter by Gcn4p, forms a topologically isolated loop in the chromosome, containing HIS3, and then remodels the nucleosomes in the loop (15). In a circular plasmid, the formation of a loop would actually create two loops, perhaps accounting for the fact that TRP1 chromatin was remodeled, as well as that of HIS3. This proposal is consistent with the observation that the SWI/SNF complex is capable of forming loops in reconstituted chromatin in vitro (14).
The remodeling of transcribed regions has implications for activated transcription, suggesting that RNA polymerase II would have to transcribe through remodeled, rather than canonical, nucleosomes. Remodeling might remove the substantial barrier that a nucleosome presents to elongation by RNA polymerase II (36). That is, remodeled nucleosomes might be much easier to transcribe through than canonical nucleosomes. Indeed, evidence exists that the SWI/SNF complex might play a role in transcript elongation (37, 38).
Acknowledgments
We thank Alan Hinnebusch, Brehon Laurent, and Chang-Hui Shen for strains. We thank Ann Dean, Jurrien Dean, Gary Felsenfeld, and Alan Hinnebusch for comments on the manuscript and Jerry Workman for helpful discussion.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Luger K., Mäder, A. W., Richmond, R. K., Sargent, D. F. & Richmond, T. J. (1997) Nature 389 251-260. [DOI] [PubMed] [Google Scholar]
- 2.Kadonaga J. T. (1998) Cell 92 307-313. [DOI] [PubMed] [Google Scholar]
- 3.Kingston R. E. & Narlikar, G. J. (1999) Genes Dev. 13 2339-2352. [DOI] [PubMed] [Google Scholar]
- 4.Jenuwein T. & Allis, C. D. (2001) Science 293 1074-1080. [DOI] [PubMed] [Google Scholar]
- 5.Vignali M., Hassan, A. H., Neely, K. E. & Workman, J. L. (2000) Mol. Cell. Biol. 20 1899-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson C. L. & Workman, J. L. (2000) Curr. Opin. Genet. Dev. 10 187-192. [DOI] [PubMed] [Google Scholar]
- 7.Sudarsanam P. & Winston, F. (2000) Trends Genet. 16 345-351. [DOI] [PubMed] [Google Scholar]
- 8.Neely K. E., Hassan, A. H., Wallberg, A. E., Steger, D. J., Cairns, B. R., Wright, A. P. H. & Workman, J. L. (1999) Mol. Cell 4 649-655. [DOI] [PubMed] [Google Scholar]
- 9.Yudkovsky N., Logie, C., Hahn, S. & Peterson, C. L. (1999) Genes Dev. 13 2369-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson R. T., Thoma, F. & Brubaker, J. M. (1985) Cell 42 799-808. [DOI] [PubMed] [Google Scholar]
- 11.Kwon H., Imbalzano, A. N., Khavari, P. A., Kingston, R. E. & Green, M. R. (1994) Nature 370 477-481. [DOI] [PubMed] [Google Scholar]
- 12.Lorch Y., Cairns, B. R., Zhang, M. & Kornberg, R. D. (1998) Cell 94 29-34. [DOI] [PubMed] [Google Scholar]
- 13.Schnitzler G., Sif, S. & Kingston, R. E. (1998) Cell 94 17-27. [DOI] [PubMed] [Google Scholar]
- 14.Bazett-Jones D. P., Côté, J., Landel, C. C., Peterson, C. L. & Workman, J. L. (1999) Mol. Cell. Biol. 19 1470-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havas K., Flaus, A., Phelan, M., Kingston, R., Wade, P. A., Lilley, D. M. J. & Owen-Hughes, T. (2000) Cell 103 1133-1142. [DOI] [PubMed] [Google Scholar]
- 16.Gavin I., Horn, P. J. & Peterson, C. L. (2001) Mol. Cell 7 97-104. [DOI] [PubMed] [Google Scholar]
- 17.Hirschhorn J. N., Brown, S. A., Clark, C. D. & Winston, F. (1992) Genes Dev. 6 2288-2298. [DOI] [PubMed] [Google Scholar]
- 18.Reinke H., Gregory, P. D. & Hörz, W. (2001) Mol. Cell 7 529-538. [DOI] [PubMed] [Google Scholar]
- 19.Lomvardas S. & Thanos, D. (2001) Cell 106 685-696. [DOI] [PubMed] [Google Scholar]
- 20.Fleming A. B. & Pennings, S. (2001) EMBO J. 20 5219-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen C.-H., Leblanc, B. P., Alfieri, J. A. & Clark, D. J. (2001) Mol. Cell. Biol. 21 534-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hope I. A. & Struhl, K. (1985) Cell 43 177-188. [DOI] [PubMed] [Google Scholar]
- 23.Natarajan K., Jackson, B. M., Zhou, H., Winston, F. & Hinnebusch, A. (1999) Mol. Cell 4 657-664. [DOI] [PubMed] [Google Scholar]
- 24.Kuo M., vom Baur, E., Struhl, K. & Allis, C. D. (2000) Mol. Cell 6 1309-1320. [DOI] [PubMed] [Google Scholar]
- 25.Reid J. L., Iyer, V. R., Brown, P. O. & Struhl, K. (2000) Mol. Cell 6 1297-1307. [DOI] [PubMed] [Google Scholar]
- 26.Moehle C. M. & Hinnebusch, A. G. (1991) Mol. Cell. Biol. 11 2723-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark D. J. (1998) in Chromatin: A Practical Approach, ed. Gould, H. (Oxford Univ. Press, Oxford), pp. 139–152.
- 28.Zakian V. A. & Scott, J. F. (1982) Mol. Cell. Biol. 2 221-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S., Mellor, J., Kingsman, A. J. & Kingsman, S. M. (1986) Mol. Cell. Biol. 6 4251-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Losa R., Omari, S. & Thoma, F. (1990) Nucleic Acids Res. 18 3495-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mai X., Chou, S. & Struhl, K. (2000) Mol. Cell. Biol. 20 6668-6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Struhl K. & Hill, D. E. (1987) Mol. Cell. Biol. 7 104-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neely K. E., Hassan, A. H., Brown, C. E., Howe, L. & Workman, J. L. (2002) Mol. Cell. Biol. 22 1615-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syntichaki P., Topalidou, I. & Thireos, G. (2000) Nature 404 414-417. [DOI] [PubMed] [Google Scholar]
- 35.Svaren J. & Hörz, W. (1997) Trends Biochem. Sci. 22 93-97. [DOI] [PubMed] [Google Scholar]
- 36.Kireeva M. L., Walter, W., Tchernajenko, V., Bondarenko, V., Kashlev, M. & Studitsky, V. M. (2002) Mol. Cell 9 541-552. [DOI] [PubMed] [Google Scholar]
- 37.Biggar S. R. & Crabtree, G. R. (1999) EMBO J. 18 2254-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sullivan E. K., Weirich, C. S., Guyon, J. R., Sif, S. & Kingston, R. E. (2001) Mol. Cell. Biol. 21 5826-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]