Abstract
Gaucher disease is a lysosomal storage disorder caused by deficient lysosomal β-glucosidase (β-Glu) activity. A marked decrease in enzyme activity results in progressive accumulation of the substrate (glucosylceramide) in macrophages, leading to hepatosplenomegaly, anemia, skeletal lesions, and sometimes CNS involvement. Enzyme replacement therapy for Gaucher disease is costly and relatively ineffective for CNS involvement. Chemical chaperones have been shown to stabilize various proteins against misfolding, increasing proper trafficking from the endoplasmic reticulum. We report herein that the addition of subinhibitory concentrations (10 μM) of N-(n-nonyl)deoxynojirimycin (NN-DNJ) to a fibroblast culture medium for 9 days leads to a 2-fold increase in the activity of N370S β-Glu, the most common mutation causing Gaucher disease. Moreover, the increased activity persists for at least 6 days after the withdrawal of the putative chaperone. The NN-DNJ chaperone also increases WT β-Glu activity, but not that of L444P, a less prevalent Gaucher disease variant. Incubation of isolated soluble WT enzyme with NN-DNJ reveals that β-Glu is stabilized against heat denaturation in a dose-dependent fashion. We propose that NN-DNJ chaperones β-Glu folding at neutral pH, thus allowing the stabilized enzyme to transit from the endoplasmic reticulum to the Golgi, enabling proper trafficking to the lysosome. Clinical data suggest that a modest increase in β-Glu activity may be sufficient to achieve a therapeutic effect.
The lysosome is an important organelle for the catabolism and recycling of macromolecules within a cell. Defects in lysosomal enzymes lead to accumulation of their substrates, resulting in a lysosomal storage disease. More than 40 lysosomal storage diseases have been characterized in humans (1). Lysosomal storage of nondegraded substrates leads to a range of phenotypes including enlargement of affected organs, neurologic abnormalities, skeletal lesions, and premature death (2). The missense mutations that cause lysosomal storage diseases manifest themselves through active-site impairment, pH-dependent protein instability, protein degradation mediated by the cellular quality-control machinery, improper trafficking, or disruption of protein–protein interactions required for enzyme activation (3). Current therapeutic strategies include inhibition of substrate production using small molecule enzyme inhibitors and replacement of the defective enzyme (4). Enzyme replacement can be accomplished by protein infusions. Enzyme replacement for the most prevalent lysosomal storage disease, Gaucher disease, costs between $100,000 and $750,000 per year and is not very effective for the treatment of CNS involvement. Although the enzyme has been modified to take advantage of mannose receptor-mediated endocytosis by macrophages, studies in rats suggest that less than 7% of the enzyme is taken up into liver macrophages (5), and the uptake in human bone marrow macrophages is so low that it is difficult to detect (6). Transplantation of hematopoietic stem cells can also reverse the disease, but thus far, attempts at gene transfer have been unsuccessful.
Some lysosomal storage diseases appear to be caused by lysosomal enzyme variants that retain catalytic activity but are predisposed to misfolding or mistrafficking in the cell (7). The use of chemical chaperones to template proper folding within the secretory pathway, prevent postsecretory misfolding, or stabilize proteins with a predilection to misfold is well documented (8). Antagonists have been shown to increase cell-surface expression of vasopressin V2 receptor variants (9). Ligands of δ opiod receptors also promote receptor maturation (10). Of particular interest, the inhibitor 1-deoxy-galactonojirimycin has been shown to chaperone the enzyme α-galactosidase in a rare variant of Fabry disease, another lysosomal storage disease (11, 12). Fabry lymphocytes cultured with inhibitor for 4 days exhibited a 7-fold increase in α-galactosidase activity that persisted 5 days after removal of drug. Transgenic mice to which 1-deoxy-galactonojirimycin was administered orally exhibited elevated α-galactosidase activity in major organs. These results suggest that small molecule binding may stabilize the mutant enzyme at neutral pH, allowing it to be transported to the lysosome, where it remains stable because of the high substrate concentrations and low pH environment.
Gaucher disease is the most prevalent lysosomal storage disorder with an estimated incidence of 1 in 40,000–60,000 in the general population (13) and 1:800 among the Ashkenazi Jewish population (14). Five mutant alleles of β-glucosidase (β-Glu, glucocerebrosidase) account for the majority of reported cases (15). Accumulation of the substrate (glucosylceramide) leads to hepatomegaly, splenomegaly, bone crisis, anemia, and CNS involvement. Clinically, Gaucher disease is subdivided into three types: type 1 is nonneuronopathic with adult onset, type 2 is infantile onset with severe CNS involvement, and type 3 is typically childhood or early adult onset with milder CNS involvement.
β-Glu is a 497-residue membrane-associated lysosomal glycoprotein whose activity is enhanced by negatively charged phospholipids and the activator protein saposin C (16). There is broad phenotypic diversity in Gaucher disease that cannot be explained by enzyme activity measurements in vitro (16, 17). In fact, there is significant disagreement in the literature regarding the activity associated with numerous β-Glu variants, many of which are known to be unstable (18, 19). We hypothesize that certain catalytically active variants of β-Glu may be identified by the quality-control machinery of the cell as deficient in folding during translocation into the endoplasmic reticulum (ER) and degraded by the ER-associated proteosome pathway, precluding sufficient trafficking to the lysosome. This class of β-Glu variants should be amenable to chemical chaperoning. The c.1226 A > G (N370S) Gaucher disease mutation is found in >98% of Jewish patients and about half of non-Jewish patients (20). We now demonstrate that the β-Glu activity of cells homozygous for the N370S mutation can be increased up to 2-fold in a cell line by using the chemical chaperone N-(n-nonyl)deoxynojirimycin (NN-DNJ) at a concentration of 10 μM, suggesting that this variant has folding deficiencies.
Materials and Methods
Fibroblast Culture.
Primary skin fibroblast cultures were established from a patient homozygous for the N370S (c.1226 A > G) mutation. Type 2 (infantile) Gaucher disease fibroblasts (GM0877) and normal fibroblast cultures (GM05659, GM00498) were obtained from the Coriell Cell Repositories, Camden, NJ. Fibroblasts were cultured in MEM with Earle's salts and nonessential amino acids (GIBCO) supplemented with 16.5% FBS (Irvine Scientific) and 1% glutamine pen-strep (Irvine Scientific) at 37°C in a 5% CO2 atmosphere in air. Monolayers were passaged on reaching confluency with trypsin-EDTA (Irvine Scientific). All cells used in this study were between the fourth and 18th passages. Total cell protein was determined by using Coomassie Plus (Pierce) or Micro BCA (Pierce) assay reagent.
Intact Cell Lysosomal β-Glu Assay.
The intact cell assay was modified from a procedure in ref. 21. Cells were plated into 24-well assay plates in media. The media were replaced with media containing potential chemical chaperones dissolved in DMSO after cell attachment. The total DMSO content in the media was <1% and had no effect on cell viability or β-Glu activity according to control experiments. Untreated and chaperone-treated media was replaced every 3–4 days when incubations longer than 4 days were used. Potential chaperones were evaluated at all concentrations in quadruplicate. The enzyme activity assay was performed after removing media supplemented with potential chaperones. The monolayers were washed with Dulbecco's PBS solution (Irvine Scientific, pH 7.2). Eighty microliters of PBS and 80 μl of 0.2 M acetate buffer (pH 4.0) were added to each well. The reaction was started by the addition of 5 mM 4-methylumbelliferyl β-d-glucoside (100 μl, Sigma) to each well, followed by incubation at 37°C for 1 h. The cells appeared intact when examined by light microscopy after treatment as described. The reaction was stopped by lysing the cells with 2 ml of 0.2 M glycine buffer (pH 10.8). Liberated 4-methylumbelliferone was measured (excitation 365 nm, emission 445 nm) with an Aviv Associates (Lakewood, NJ) fluorimeter. The fluorescence was also evaluated on a SpectraMax Gemini plate reader (Molecular Devices) in 24-well format by using identical excitation and emission wavelengths. The fluorescence of untreated cells was compared with those treated with potential chaperones. Conduritol B epoxide (Toronto Research Chemicals, Downsview, ON, Canada) was routinely added to control wells to evaluate the extent of nonspecific β-Glu activity (22).
Lysed Cell β-Glu Assay.
Intact cells were harvested and the pellet was lysed in PBS by sonication. Before assay, the cell lysate was diluted with an equal volume of pH 5.0 acetate buffer. All lysed cell β-Glu assays were performed in the presence of 0.1% Triton X-100 (Fisher) and 0.2% taurodeoxycholic acid (Calbiochem). Substrate (20 mM 4-methyllumbelliferyl β-d-glucoside) was added to initiate the reaction, and the samples were incubated at 37°C for 30 min. The reaction was terminated by the addition of glycine buffer (pH 10.8) and the fluorescence was measured as described above. IC50 values were determined by preincubating acidified lysate with small molecules for 15 min in the presence of taurodeoxycholic acid and Triton X-100 at 37°C. Small molecule concentrations ranged from 10.0 nM to 1.0 mM. Substrate (20 mM 4-methyllumbelliferyl β-d-glucoside) was added, and the samples were incubated for an additional 30 min. The reaction was quenched with glycine buffer and the fluorescence was recorded.
In Vitro Stabilization of β-Glu.
An assessment of the ability of chemical chaperones to stabilize β-Glu against denaturation was performed by using a commercially available highly purified human placental β-Glu preparation, Ceredase (alglucerase injection containing albumin, Genzyme). Ceredase remaining in injection vials after administration to patients was recovered, pooled, and used without purification. Enzyme aliquots (20 μl, pH 7.4) were incubated with 0, 50, or 100 μM chemical chaperone on ice for 1 h. The samples were heated at 48°C as a function of time in an attempt to heat-inactivate (denature) β-Glu, and then the samples were diluted into 3 vol of 0.1 M acetate-phosphate buffer (pH 5.0). The enzyme was immediately incubated with 20 μl of substrate (20 mM 4-methyllumbelliferyl β-d-glucoside) in the presence of 0.l % Triton X-100 and 0.2% taurodeoxycholic acid for 10 min at 37°C before quenching with glycine buffer. Liberated 4-methylumbelliferone was measured (excitation 365 nm, emission 445 nm) with an Aviv Associates fluorimeter. Enzyme activity was reported relative to unheated enzyme.
β-Glu Inhibitors and Related Molecules to Probe the Requirements for N370S β-Glu Chaperoning.
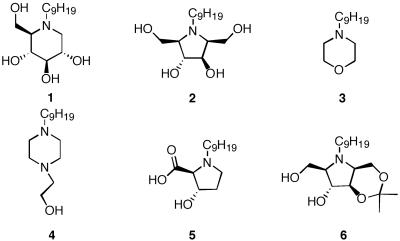
N-(n-butyl)deoxynojirimycin (NB-DNJ), N-(7-oxadecyl)deoxynojirimycin, NN-DNJ, and N-(n-dodecyl)deoxynojirimycin were purchased from Toronto Research Chemicals. Nonanoic acid, nonyl aldehyde, decyl alcohol, 1-nonanesulfonic acid, dodecylamine, morpholine, 1-(hydroxyethyl)piperazine, and diethylamine were purchased from Aldrich. Trans-4-hydroxy-l-proline was purchased from Fluka. Following published procedures, 1-deoxynojirimycin (DNJ) was prepared from 2,3,4,6-tetra-O-benzyl-α-glucopyranose (23). 2,5-Anhydro-imino-d-glucitol was prepared from 5-keto-d-fructose according to Baxter and Reitz's method (24, 25). N-nonyl compounds (2, 3, 4, 5, and diethyl nonylamine) were prepared via reductive amination of nonyl aldehyde with 2,5-anhydro-imino-d-glucitol, morpholine, 1-(hydroxyethyl)piperazine, trans-4-hydroxy-l-proline, and diethylamine, respectively. Likewise, reductive amination of octyl aldehyde and DNJ gave N-(n-octyl)deoxynojirimycin. Compound 6 was generated by the treatment of N-nonyl-2,5-anhydro-imino-d-glucitol 2 and 2-methoxypropene in the presence of p-toluene sulfonic acid (catalytic amount) (26, 27). Structural characterization was accomplished by 1H-NMR, 13C-NMR, and high-resolution MS (see Supporting Text, which is published as supporting information on the PNAS web site, www.pnas.org).
Results and Discussion
DNJ Analogs Inhibit Enzymes That Make and Break Glucosyl Bonds.
DNJs are known to inhibit several enzymes including the endoplasmic reticulum oligosaccharide-processing enzymes α-glucosidase I/II, ceramide glucosyl transferase, and both nonlysosomal and lysosomal β-Glu, the latter enzyme being deficient in Gaucher disease. Alkylation of DNJ is not required to inhibit α-glucosidases, as DNJ presumably acts as a transition-state mimetic (28). DNJ alkylation is required for glucosyl transferase inhibition, with increasing chain length modestly increasing potency (29). The proposed mechanism of inhibition is through ceramide mimicry. The nonpolar side chain may help target the inhibitor to the membrane-localized enzyme. NB-DNJ has been used in cell lines and tested in clinical trials to inhibit the formation of the substrate (glucosylceramide) that accumulates in Gaucher disease (30, 31). It is established that DNJ inhibition of lysosomal and nonlysosomal β-Glu also improves dramatically with chain length, and nanomolar inhibition of nonlysosmal β-Glu is observed when DNJ is alkylated with a hydrophobic group such as adamantyl. Because lysosomal and nonlysosomal β-Glu show distinct specificity toward artificial substrates, activators, and inhibitors, it should be possible to find selective active site-directed molecules (22). A variety of alkylated DNJs were therefore procured or synthesized and studied.
Alkylated DNJ Analogues Increase Lysosomal β-Glu Activity.
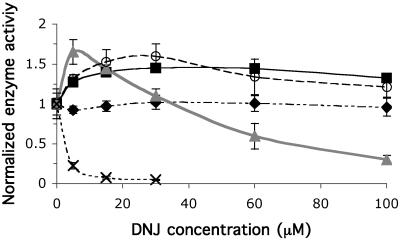
Because DNJ analogues are known to bind to the active site of WT lysosomal β-Glu, these compounds served as a starting point for chemical chaperone screening. DNJ analogues of varying alkyl chain length were cultured with fibroblasts for 5 days, with a homozygous N370S cell line derived from a Gaucher patient. An intact cell assay was performed at pH 4 (see Materials and Methods) by using conduritol B epoxide (CBE) as a control to specifically measure lysosomal β-Glu activity (22). CBE covalently inhibits lysosomal β-Glu, but not nonlysosomal β-Glu. As expected, CBE-insensitive activity was negligible (typically <5%) at pH 4. Several of the alkylated DNJ analogs increased intracellular N370S β-Glu activity at lower concentrations and inhibited the enzyme at high concentrations (Fig. 1). NN-DNJ displayed the activity profile expected of a chemical chaperone. At low concentrations (<30 μM), β-Glu activity was elevated when compared with untreated cells. Up to a 1.65-fold increase in N370S β-Glu activity was observed after adding 5 μM NN-DNJ to the culture media for 5 days. Dose-dependent inhibition occurred at concentrations >60 μM, with <20% activity remaining at a culture concentration of 100 μM. Although NN-DNJ did not cause cell death over a 4- to 5-day incubation period, notable cell death occurred at high concentrations (>60 μM) during longer incubation periods. N-(n-dodecyl)deoxynojirimycin (ND-DNJ) was highly inhibitory at all concentrations evaluated, even at nanomolar concentrations (data not shown). At ND-DNJ concentrations higher than 30 μM, complete cell death occurred within 24 h, potentially because of membrane disruption. The known β-Glu inhibitor NB-DNJ showed no activity, even at concentrations as high as 100 μM. This finding is consistent with Priestman et al.'s (32) demonstration that β-Glu was not inhibited by low doses of NB-DNJ in mice receiving β-Glu infusions. The β-Glu IC50 value of NB-DNJ as determined in WT fibroblast lysates was ≈300 μM, hence the small molecule concentration in tissue culture may simply have been too low to observe chaperone activity. Both N-(7-oxadecyl)deoxynojirimycin and N-(n-octyl)deoxynojirimycin increased β-Glu activity over a wide range of concentrations, resulting in no inhibition through 100 μM. Decreasing compound lipophilicity with oxygen-substituted alkyl chains [N-(7-oxadecyl)deoxynojirimycin] has been shown to decrease toxicity (29). N-(n-octyl)deoxynojirimycin was well tolerated by the cells after 9 days of exposure at concentrations as high as 100 μM.
Fig 1.
The influence of selected alkylated DNJs on β-Glu activity in N370S fibroblasts cultured for 5 days with butyl (⧫), octyl (▪), 7-oxadecyl (○), nonyl (▴), and dodecyl (×) DNJ. β-Glu activity was evaluated at pH 4.0 by using 4-methyllumbelliferyl β-d-glucoside as a substrate in intact cells. Enzyme activity is normalized to untreated cells, assigned a relative activity of 1. Mean values ± SD are shown for quadruplicate experiments.
To be confident that the β-Glu activity enhancement observed in intact cells was not caused by changes in fibroblast membrane permeability, experiments were also performed with membrane-lysed cell preparations (see Materials and Methods). N370S cells that had been cultured with NN-DNJ were sonicated, and an activity assay was performed on the lysates. These data were compared with those obtained in intact cells (Table 1). β-Glu activity assays have been typically performed on lysates at pH 5.0 in the presence of Triton X-100 and taurodeoxycholate or deoxycholate, and therefore we used these conditions (33). It is known that the activity of the N370S mutation is extremely sensitive to assay conditions; however, these were not optimized further for these studies. At longer incubation periods, NN-DNJ-induced activity increases were greater in detergent-stimulated lysates than in intact cells. However, these differences were small at shorter incubation periods (Table 1). These results demonstrate that substrate permeability is entirely adequate in intact cells and further demonstrate that the activity increase in the presence of NN-DNJ is not caused by changes in fibroblast membrane permeability. It is clear that the length of the alkyl chain has a significant effect on the ability of the putative chaperones to increase intracellular β-Glu activity. The amphiphilic character of alkylated DNJ mimics glucosylceramide, and increasing the alkyl chain length increases enzyme affinity by exploiting hydrophobic recognition elements in the active site. The β-Glu IC50 of NN-DNJ is 100-fold lower than NB-DNJ in lysates, which contributes to the observed difference in chaperone activity. The alkyl chain length also affects cell permeability and partitioning in cell culture experiments. Potential chemical chaperones with longer alkyl chains should be able to insert into the membrane and increase local concentration near membrane-associated β-Glu.
Table 1.
A comparison of β-Glu activity in intact and lysed cells
| Relative 4-day ICA increase | Relative 4-day LCA increase | Relative 9-day ICA increase | Relative 9-day LCA increase |
|---|---|---|---|
| 1.50 ± 0.10 | 1.50 ± 0.05 | 1.70 ± 0.10 | 2.00 ± 0.05 |
NN-DNJ (10 μM) was added to the culture medium of N370S fibroblasts for 4 or 9 days before the intact cells were assayed for activity at pH 4.0. Cell lysates pretreated in the same fashion were assayed at pH 5.0 with 0.1% Triton X-100 and 0.2% taurodeoxycholic acid. Enzyme activity is normalized to untreated cells, assigned a relative activity of 1. ICA, intact cell β-Glu activity; LCA, lysed cell β-Glu activity.
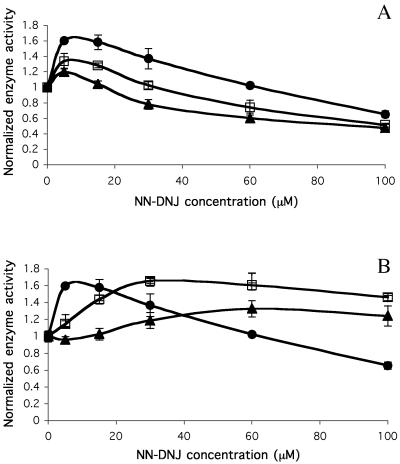
The NN-DNJ-Mediated Increase in N370S β-Glu Activity Increases with Culture Time and Persists on Removal of NN-DNJ from Fibroblast Cultures.
The increase of N370S β-Glu activity observed with low concentrations of NN-DNJ (up to ≈30 μM) increased with the duration of the putative chaperone incubation period (Fig. 2A). However, inhibition of enzyme activity persisted at concentrations >60 μM. Interestingly, cells that had been “pulsed” with NN-DNJ for 4 days and chased with untreated media also showed a significant activity enhancement for at least 6 days after removal of putative chemical chaperone from the fibroblast media (Fig. 2B). Although the shape of the activity curve shifted with time, all pulse concentrations resulted in elevation of enzyme activity during the chase periods, including those that initially were inhibitory. Importantly, no inhibition was observed even at high pulse concentrations, suggesting that we have not yet optimized the utilization of these compounds. Fan et al. (11) report similar elevation of mutant α-galactosidase A activity in Fabry lymphoblasts 5 days after removal of the pharmacological chaperone 1-deoxy-galactonojirimycin (DGJ). They believe that mutant α-galactosidase A synthesized in the presence of DGJ is stable for at least 5 days. Although this may also be the case for N370S β-Glu, an alternative possibility is that NN-DNJ and related alkylated iminocyclitols are incorporated into the cell membrane as discussed above, leading to sustained activity enhancement.
Fig 2.
The dependence of time of incubation with NN-DNJ on the activity of N370S β-Glu in intact fibroblasts. (A) N370S fibroblasts were cultured for 1 (▴), 2 (□), or 4 (•) days with NN-DNJ. (B) N370S fibroblasts were pulsed with NN-DNJ for 4 days and then chased with fresh media for 0 (•), 4 (□), or 6 (▴) days. The intact cells were assayed at pH 4.0 by using 4-methyllumbelliferyl β-d-glucoside as a substrate. Enzyme activity is normalized to untreated cells, assigned a relative activity of 1. Mean values ± SD are shown for quadruplicate experiments.
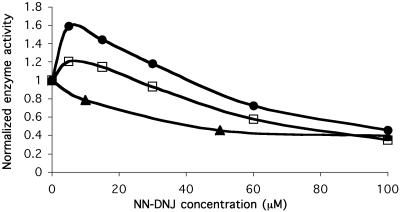
NN-DNJ Increases the Activity of WT and N370S β-Glu but Not L444P β-Glu.
Both WT and Gaucher disease type 2 cells homozygous for the c.1448 T > C (L444P) mutation were also incubated with NN-DNJ for 4 days (Fig. 3). The enzyme activity of the cells containing the N370S mutation exhibited the greatest enhancement, whereas the activity of the L444P mutant cells was only inhibited over the concentration range assayed. The WT cells consistently showed a 1.2-fold maximum increase in activity under these conditions. Interestingly, Asano et al. (12) also observed α-galactosidase A activity elevation in WT lymphoblasts on incubation with 1-deoxy-galactonojirimycin, the chaperone that corrects the activity deficit in one variant associated with Fabry disease. Both WT and N370S β-Glu activity can be increased by culturing with putative chaperones, whereas the L444P mutant is not amenable to this approach. Because not all Gaucher-associated β-Glu variants are defective in folding and trafficking (some mutations destroy the active site or prevent the activator protein, saposin C, from binding for example), NN-DNJ chaperoning should be ineffective in some cases. The inability of NN-DNJ to enhance the cellular activity of the L444P variant is reassuring in that it would be surprising if all mutant enzymes were amenable to this approach.
Fig 3.
The effect of NN-DNJ on β-Glu activity in three different cell lines. NN-DNJ was added to the culture medium of N370S (•), WT (□), or L444P (▴) fibroblasts for 4 days. The intact cells were assayed at pH 4.0 by using 4-methyllumbelliferyl β-d-glucoside as a substrate. Enzyme activity is normalized to untreated cells, assigned a relative activity of 1. The actual activity of L444P β-Glu ≪ N370S β-Glu ≪ WT β-Glu. Numbers were measured in triplicate, and SDs were <10%.
Probing the Requirements for N370S β-Glu Chaperoning with Simple Amphipathic Molecules and Alkylated Nitrogen Heterocycles.
β-Glu activity, especially in the case of the N370S mutant, is stimulated by detergents, bile salts, phosphatidylserine, and the activator protein Saposin C (19). The mechanisms by which these molecules enhance enzyme activity are not known, hence it was decided to test different types of amphipathic molecules. A series of charged and neutral amphipathic molecules were evaluated over a concentration range of 5–200 μM. We compared both WT and N370S cell lines because both proteins could potentially be stabilized by these molecules. Nonanoic acid, nonanal, 1-nonanesulfonic acid, decan-1-ol, dodecylamine, and diethyl-nonylamine were incubated with fibroblasts for 4 days. Dodecylamine was toxic to both WT and N370S fibroblasts at concentrations greater than 10 μM. A similar toxicity effect was observed when cells were treated with dodecyl-DNJ. All of the other compounds exhibit activity within 5% of untreated cells at low concentrations (<50 μM). At very high concentrations (200 μM), up to 20% inhibition was observed. In comparison, NN-DNJ-chaperoned WT cells show a 1.2-fold increase in activity and N370S cells show a 1.5-fold increase in activity under the same conditions. The activity of β-Glu is not increased by positively charged, negatively charged, or neutral simple amphipathic molecules.
A series of alkylated nitrogen heterocycles were prepared to study the core structure requirements for N370S activity enhancement (Table 2). Although all of the molecules contained the same nonyl alkyl chain and a nitrogen that would be protonated under the cellular conditions used, a range of activities was observed (see Supporting Text for activity profile for each compound). The iminocyclitol 2, a known transition-state mimetic, only slightly increased β-Glu activity, whereas related five-membered ring N-heterocycles 5 and 6 were inactive (34). Surprisingly, the morpholine- and piperazine-based molecules 3 and 4 showed some activity despite their inability to form numerous hydrogen bonds in the active site thought to be important for the binding of 1 to β-Glu. However, the latter compounds may be able to form an ion pair with the putative active-site carboxylate because of their structures. The piperazine and morpholine compounds had measurable IC50 values (high μM range) whereas 5 and 6 had IC50 values in the mM range. This finding may explain why the former compounds are active and the latter are not. NN-DNJ possessed the lowest IC50 in WT and N370S lysates and was the most promising compound at concentrations below 10 μM. When NN-DNJ was incubated in conjunction with the morpholine 3, the activity profile was identical to the expected profile for the tighter binding NN-DNJ (data not shown), suggesting that they competed for the same site in β-Glu. The concept that the best inhibitors are the best chaperones demonstrated by the Fabry disease study also seems to be recapitulated by β-Glu chaperones (12).
Table 2.
The effect of variable core structure with constant n-nonyl substructure on N370S and WT β-Glu activity
Various core structures N-alkylated with the n-nonyl group were added to the culture medium of fibroblasts for 5 days. β-Glu activity was assayed at pH 4.0 by using 4-methyllumbelliferyl β-d glucoside as a substrate. IC50 values were determined in WT and N370S lysates at pH 5.0 in the presence of 0.1% Triton X-100 and 0.2% taurodeoxycholic acid. Enzyme activity is normalized to untreated cells, assigned a relative activity of 1. ICA, intact cell β-Glu activity.
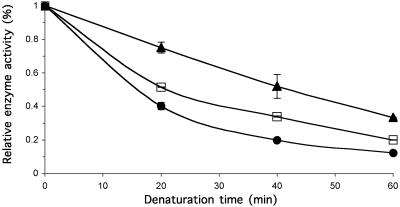
NN-DNJ Protects β-Glu from Thermal Denaturation.
Ceredase (alglucerase injection) was preincubated with 0, 50, or 100 μM NN-DNJ before being subjected to heat inactivation (48°C). Untreated β-Glu lost the most activity under these conditions, with only 20% activity remaining after 1 h of heating. β-Glu activity was retained by the inclusion of NN-DNJ, and the effect was improved with increasing NN-DNJ concentration (Fig. 4). Enzyme incubated with 100 μM NN-DNJ retained twice as much activity as untreated enzyme at all time points evaluated, suggesting that NN-DNJ binding stabilized WT β-Glu against thermal denaturation in vitro. These results imply that small molecule binding may also stabilize WT and N370S β-Glu within cells allowing proper folding and trafficking, leading to increased enzyme activity (Fig. 1). These data are also consistent with observations that NB-DNJ-treated mice receiving enzyme infusions exhibited an increased β-Glu serum half-life (32).
Fig 4.
Stabilization of isolated β-Glu by NN-DNJ evaluated in vitro by using heat inactivation. Ceredase (alglucerace injection) aliquots were incubated with 0 μM (•), 50 μM (□), or 100 μM (▴) NN-DNJ at pH 7.4. The samples were heated at 48°C for the indicated amount of time and then assayed for activity at pH 5.0 with 0.1% Triton X-100 and 0.2% taurodeoxycholic acid. Enzyme activity is reported relative to unheated enzyme. Mean values ± SD are shown for duplicate experiments.
In conclusion, we have shown that the incubation of fibroblasts with μM concentrations of active site-directed molecules can lead to increased N370S β-Glu intracellular activity. The N370S mutation is the most common variant causing Gaucher disease. The activity of this variant increased with putative chaperone incubation time and persisted for at least 6 days on removal of the small molecule from the culture medium. The nature of the core structure and the length of the N-alkyl chain strongly influenced the potency of the putative chaperone; however, it is clear that there is room for improvement. DNJ alkylated with short chains (four carbons) produced inactive compounds at low concentrations, whereas long alkyl chains (12 carbons) proved to be toxic. DNJs modified with alkyl side chains having 8–10 carbons were well tolerated and enhanced β-Glu activity significantly. These chaperones seem to function through both ceramide-mimicry and by targeting the small molecule to the membrane, increasing its local concentration. NN-DNJ has been shown to increase the activity of both N370S and WT β-Glu in fibroblasts, presumably through protein stabilization, as demonstrated in vitro. However, it does not enhance the intracellular activity of the L444P variant. Although further studies must be conducted to determine the detailed mechanism by which these chemical chaperones increase β-Glu activity, it appears that they enhance stability and enable trafficking to the lysosome. Clinical data indicate that a small increase in enzyme activity may be effective in treating disease. Although patients receiving Cerezyme infusions experience reduced hepatosplenomegaly, improved blood counts, and amelioration of bone crises, the increase of enzyme activity in bone marrow caused by treatment can be quite small. After infusion of either 1.15 units/kg or 60 units/kg of enzyme into five patients, a 1.7- to 9.6-fold increase of β-Glu activity was observed (6). This finding suggests that the 1.5- to 2-fold increase in activity caused by NN-DNJ chemical chaperoning may be clinically useful. Because the intracellular half-life of much of the infused Cerezyme is only hours and enzyme is often administered only every 2 weeks, the average increase in patients is miniscule compared with the increases we observed in tissue culture with chaperone treatment. Although it was not an effective chaperone in our in vitro system, it is possible that the therapeutic effectiveness of NB-DNJ (30, 31) might be related to an in vivo chaperoning effect that we cannot detect. Like NB-DNJ, the chaperone molecules we have studied are likely to be effective by the oral route. There is good reason to be optimistic that better compounds and optimized dosing regimes will provide even greater activity enhancements.
Supplementary Material
Acknowledgments
We thank Juliana Conkright, Terri Gelbart, Helen Plutner, Natalia Reixach, and Joleen White for excellent technical assistance, Sara Werner for chemistry advice, and Joel Buxbaum for helpful discussions on assay development. This work was supported by The Skaggs Institute for Chemical Biology, the Lita Annenberg Hazen Foundation, and a National Science Foundation Predoctoral Fellowship (to A.R.S.).
Abbreviations
β-Glu, β-glucosidase
DNJ, deoxynojirimycin
NN-DNJ, N-(n-nonyl)deoxynojirimycin
NB-DNJ, N-(n-butyl)deoxynojirimycin
References
- 1.Winchester B., Vellodi, A. & Young, E. (2000) Biochem. Soc. Trans. 28 150-154. [DOI] [PubMed] [Google Scholar]
- 2.Rapola J. (1994) Pathol. Res. Pract. 190 759-766. [DOI] [PubMed] [Google Scholar]
- 3.Durand P., Fabrega, S., Henrissat, B., Mornon, J.-P. & Lehn, P. (2000) Hum. Mol. Genet. 9 967-977. [DOI] [PubMed] [Google Scholar]
- 4.Schiffmann R. & Brady, R. O. (2002) Drugs 62 733-742. [DOI] [PubMed] [Google Scholar]
- 5.Bijsterbosch M. K., Donker, W., van de Bilt, H., van Weely, S., van Berkel, T. J. & Aerts, J. M. (1996) Eur. J. Biochem. 237 344-349. [DOI] [PubMed] [Google Scholar]
- 6.Beutler E., Kuhl, W. & Vaughan, L. M. (1995) Mol. Med. 1 320-324. [PMC free article] [PubMed] [Google Scholar]
- 7.Berg-Fussman A., Grace, M. E., Ioannou, Y. & Grabowski, G. A. (1993) J. Biol. Chem. 268 14861-14866. [PubMed] [Google Scholar]
- 8.Morello J. P., Bouvier, M., Petaja-Repo, U. E. & Bichet, D. G. (2000) Trends Pharmacol. Sci. 21 466-469. [DOI] [PubMed] [Google Scholar]
- 9.Morello J.-P., Salahpour, A., Laperriere, A., Bernier, V., Arthus, M.-F., Lonergan, M., Petaja-Repo, U., Angers, S., Morin, D., Bichet, D. G. & Bouvier, M. (2000) J. Clin. Invest. 105 887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petaja-Repo U. E., Hogue, M., Bhalla, S., Laperriere, A., Morello, J.-P. & Bouvier, M. (2002) EMBO J. 21 1628-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan J.-Q., Ishii, S., Asano, N. & Suzuki, Y. (1999) Nat. Med. 5 112-115. [DOI] [PubMed] [Google Scholar]
- 12.Asano N., Ishii, S., Kizu, H., Ikeda, K., Yasuda, K., Kato, A., Martin, O. R. & Fan, J.-Q. (2000) Eur. J. Biochem. 267 4179-4186. [DOI] [PubMed] [Google Scholar]
- 13.Grabowski G. A. (1993) Adv. Hum. Genet. 21 377-441. [PubMed] [Google Scholar]
- 14.Beutler E., Nguyen, N. J., Henneberger, M. W., Smolec, J. M., McPherson, R. A., West, C. & Gelbert, T. (1993) Am. J. Hum. Genet. 52 85-84. [PMC free article] [PubMed] [Google Scholar]
- 15.Beutler E., Gelbart, T., Kuhl, W., Zimran, A. & West, C. (1992) Blood 79 1662-1666. [PubMed] [Google Scholar]
- 16.Zhao H. & Grabowski, G. A. (2002) Cell. Mol. Life Sci. 59 694-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maire I. (2001) J. Inherited Metab. Dis. 24 57-61. [DOI] [PubMed] [Google Scholar]
- 18.Beutler E. & Kuhl, W. (1986) Proc. Natl. Acad. Sci. USA 83 7472-7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grace M. E., Newman, K. M., Scheinker, V., Berg-Fussman, A. & Grabowski, G. A. (1994) J. Biol. Chem. 269 2283-2291. [PubMed] [Google Scholar]
- 20.Beutler E. & Gelbart, T. (1993) Br. J. Haematol. 85 401-405. [DOI] [PubMed] [Google Scholar]
- 21.Beutler E., Kuhl, W., Trinidad, F., Teplitz, R. & Nadler, H. (1971) Am. J. Hum. Genet. 23 62-66. [PMC free article] [PubMed] [Google Scholar]
- 22.Overkleeft H. S., Renkema, G. H., Neele, J., Vianello, P., Hung, I. O., Strijland, A., Van Der Burg, A. M., Koomen, G.-J., Pandit, U. K. & Aerts, J. M. F. G. (1998) J. Biol. Chem. 273 26522-26527. [DOI] [PubMed] [Google Scholar]
- 23.Matos C. R. R., Lopes, R. S. C. & Lopes, C. C. (1999) Synthesis 4 571-573. [Google Scholar]
- 24.Baxter E. W. & Reitz, A. B. (1994) J. Org. Chem. 59 3175-3185. [Google Scholar]
- 25.Reitz A. B. & Baxter, E. W. (1990) Tetrahedron Lett. 31 6777-6780. [Google Scholar]
- 26.Wong C.-H., Provencher, L., Porco, J. A., Jr., Jung, S.-H., Wang, Y.-F., Chen, L., Wang, R. & Steensma, D. H. (1995) J. Org. Chem. 60 1492-1501. [Google Scholar]
- 27.Wang Y.-F., Takaoka, Y. & Wong, C.-H. (1994) Angew. Chem. Int. Ed. Engl. 33 1242-1244. [Google Scholar]
- 28.Fleet G. W. J. (1988) Spec. Publ. R. Soc. Chem. 65 149-162. [Google Scholar]
- 29.Butters T. D., Van den Broek, L. A. G. M., Fleet, G. W. J., Krulle, T. M., Wormald, M. R., Dwek, R. A. & Platt, F. M. (2000) Tetrahedron Asymmetry 11 113-124. [Google Scholar]
- 30.Cox T., Lachmann, R., Hollak, C., Aerts, J., van Weely, S., Hrebicek, M., Platt, F., Butters, T., Dwek, R., Moyses, C., et al. (2000) Lancet 355 1481-1485. [DOI] [PubMed] [Google Scholar]
- 31.Platt F. M., Jeyakumar, M., Andersson, U., Priestman, D. A., Dwek, R. A., Butters, T. D., Cox, T. M., Lachmann, R. H., Hollak, C., Aerts, J. M. F. G., et al. (2001) J. Inherited Metab. Dis. 24 275-290. [DOI] [PubMed] [Google Scholar]
- 32.Priestman D. A., Platt, F. M., Dwek, R. A. & Butters, T. D. (2000) Glycobiology 10 iv-vi. [PubMed] [Google Scholar]
- 33.Raghavan S. S., Topol, J. & Kolodny, E. H. (1980) Am. J. Hum. Genet. 32 158-173. [PMC free article] [PubMed] [Google Scholar]
- 34.Sears P. & Wong, C.-H. (1999) Angew. Chem. Int. Ed. 38 2301-2324. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.