Abstract
The thyroid-stimulating hormone/thyrotropin (TSH) is the most relevant hormone in the control of thyroid gland physiology in adulthood. TSH effects on the thyroid gland are mediated by the interaction with a specific TSH receptor (TSHR). We studied the role of TSH/TSHR signaling on gland morphogenesis and differentiation in the mouse embryo using mouse lines deprived either of TSH (pitdw/pitdw) or of a functional TSHR (tshrhyt/tshrhyt and TSHR-knockout lines). The results reported here show that in the absence of either TSH or a functional TSHR, the thyroid gland develops to a normal size, whereas the expression of thyroperoxidase and the sodium/iodide symporter are reduced greatly. Conversely, no relevant changes are detected in the amounts of thyroglobulin and the thyroid-enriched transcription factors TTF-1, TTF-2, and Pax8. These data suggest that the major role of the TSH/TSHR pathway is in controlling genes involved in iodide metabolism such as sodium/iodide symporter and thyroperoxidase. Furthermore, our data indicate that in embryonic life TSH does not play an equivalent role in controlling gland growth as in the adult thyroid.
The mouse thyroid gland begins to develop at embryonic day (E)8.5 as an endodermal thickening in the floor of the primitive pharynx. After loosing all connections with the pharynx, the thyroid bud migrates caudally, reaching its final position in front of the trachea ≈E13 (1). Only after completion of migration do thyroid follicular cells begin their differentiative program and express thyroid-specific genes such as thyroglobulin (Tg), thyroid-stimulating hormone/thyrotropin (TSH) receptor (TSHR), thyroperoxidase (TPO), and the sodium/iodide symporter (NIS) (2). Finally, primitive follicles appear, and the gland displays its final morphological organization. Since E8.5, thyroid precursor cells express a combination of transcription factors such as TTF-1 (encoded by the titf1/nkx2.1 gene) (3), TTF-2 (encoded by the titf2/foxe1 gene) (4), and Pax8 (5). Gene-targeting experiments demonstrated that all these factors are required for the early stages of thyroid development (6–8). However, it still is unclear what the mechanisms are that lead to the initiation of functional differentiation that only occurs at E14.
TSH is known as the main regulator of the adult thyroid gland. Indeed, after binding to its receptor, TSH stimulates the thyroid cells in almost every aspect of their metabolism including synthesis and secretion of thyroid hormones (9). Several groups have demonstrated clearly that TSH regulates mRNA levels of several thyroid-specific genes such as Tg (10–13), TPO (13–15), and NIS (16, 17).
TSH also stimulates the aggregation of porcine thyroid cells in follicles (18), and its presence is necessary to maintain the follicular architecture (19). In the rat, there is a temporal correlation between the increased expression of TSHR and the formation of follicles. Indeed, TSHR mRNA is expressed by E15 (3, 20), and its expression increases on E17–E18. At this stage, thyroid-specific genes are up-regulated, and the formation of colloid begins (20).
In this study we decided to investigate the role of TSH/TSHR signaling in differentiation and morphogenesis of the developing mouse thyroid. To this end we evaluated both the expression of thyroid-specific genes and the morphology of the developing gland in three mutant mouse lines deprived of either TSH or a functional TSHR. As a model of absence of TSH we used pitdw/pitdw (formerly Snell dwarf or dw/dw) mice (21–23). The pit1dw allele encodes a pit1 protein severely defective in DNA binding as a consequence of a Trp-to-Cys change in the POU homeodomain. Mice homozygous for this mutation are completely deprived of TSH, growth hormone, and prolactin (24). As model for the absence of TSHR we used both tshrhyt/tshrhyt (formerly hyt/hyt mice) mice (25) and a mouse model in which the tshr gene was inactivated by homologous recombination in embryonic stem cells (26). tshrhyt mice have a point mutation in the coding region of tshr gene that leads to the replacement of a highly conserved proline (Pro-556) in the transmembrane domain IV with a leucine. This mutation causes a defective binding with TSH (27).
Activation of the TSH/TSHR pathway occurs in mice at ≈E15. To determine whether some aspect of morphogenesis and differentiation depended exclusively on the activation of this pathway, we also generated a mouse in which a constitutively activated form of the human TSHR (28) is expressed from E8.5 onward, i.e., from the very beginning of thyroid development.
Here we show that the TSH/TSHR signaling is not a global regulator of thyroid function in the mouse embryo. We demonstrate that TSH and an intact TSHR are strictly required for NIS and TPO expression but not for Tg synthesis that occurs at nearly normal levels also in their absence. However, mice with an anticipated expression of a constitutively activated form of TSHR do not show any corresponding anticipation of NIS or TPO expression, indicating that additional mechanisms are involved in TSH-controlled gene expression. Finally, thyroid gland size and DNA synthesis in the fetal thyroid is unaffected by TSH/TSHR signaling, suggesting that different mechanisms control gland size in embryogenesis and adulthood.
Materials and Methods
Animals.
Heterozygous tshrhyt/+ (genetic background BALB/c) and pit1dw/+ (genetic background DW) mice were obtained from The Jackson Laboratory. We crossed both strains with C57BL/6 and backcrossed mutant offspring to C57BL/6 for at least four generations. Heterozygous mice were intercrossed to produce the homozygous mice used in the experiments shown here. We included as control both wild-type C57BL/6 and heterozygous animals from the same litter. Because for all parameters observed the two controls used behave equally, only the C57BL/6 controls are shown. TSHR-knockout (KO) mice are described in ref. 26.
Knock-In of a Constitutively Active Human TSHR cDNA into the titf1 Locus.
The mouse titf1 gene was isolated from a strain 129/Sv mouse genomic library (Stratagene) by using a probe corresponding to the 3′ UTR of rat TTF-1 (3). To prepare the targeting vector, a fragment extending from base pairs 4,656–10,443 of the reported mouse genomic sequence (GenBank accession no. U19755) containing the entire coding sequence for TTF-1 was cloned in pBlueScript SK(−). A fragment spanning from the translation start side of TTF-1 (base pair 7,957) to the end of homeobox (base pair 9,480) was removed and replaced by the sequence encoding a version of the human TSHR constitutively activated (TSHR*) by a mutation in the VI transmembrane segment (28). The simian virus 40 poly(A) sequence was inserted downstream of the TSHR* stop codon. The construct includes HSV-tk and PGK-neo cassettes for selection of transfected embryonic stem cells. R1 embryonic stem cells were electroporated and selected as described (8). Genomic DNA from the neomycin resistance clone was digested with BamHI and analyzed by Southern blotting using as a probe a 500-bp fragment spanning from nucleotides 10,512 to 11,042 of the mouse titf1 gene. Two embryonic stem cell clones in which the targeting vector had been integrated properly were injected into C57BL/6 blastocysts. Mating of chimeras was done on a C57BL/6 background. The heterozygous titf1+/KI-TSHR* mice were backcrossed for at least 10 generations to C57BL/6 mice.
Genotyping.
A piece from the tail (both in the case of adult mice and embryos) was incubated overnight at 60°C with lysis buffer (50 mM Tris⋅HCl/100 mM EDTA/100 mM NaCl/1% SDS/0.5 mg/ml proteinase K), and genomic DNA was extracted by adding 0.3 volumes of 6 M NaCl and precipitated with isopropanol.
Selected regions of the mouse tshr and pit1 genes were amplified and analyzed by the single-strand conformation polymorphism (SSCP) method. The primers used for the amplification were: HYT forward, 5′-GAT GGT ACG GCA TGA CCT TC-3′; HYT reverse, 5′-AGA CGA CAA CAA AGG CAA CAA-3′; PIT forward, 5′-TTC CTC GCA GGA GAT CAT GC-3′; and PIT reverse, 5′-CCT TGG AAA TAG AGA ACA GGC-3′.
PCR products of 270 bp (HYT primers) and 140 bp (PIT primers) were diluted two times in 98% deionized formamide/10 mM EDTA/0.025% xylene cyanol/0.025% bromophenol blue, denatured at 95°C for 5 min, and loaded on a 12.5% polyacrylamide nondenaturing gel (Amersham Pharmacia). The gel was run by using the Multiphore II electrophoresis unit (Amersham Pharmacia) at 15°C, 600 V, 5 mA, and 30 W for 150 min. Gels were stained by using PlusOne silver-staining kit (Amersham Pharmacia) according to the supplied instructions.
To genotype titf1+/KI-TSHR* mice, genomic DNA was digested with BamHI and analyzed by Southern blotting.
Preparation of Anti-Pax8 Antibody.
The sequence encoding full-length mouse Pax8 was cloned in the BamHI site of pET 22b(+) vector (Novagen), in frame with a sequence encoding for a six-His stretch at its COOH terminus (Pax8-6H). The protein, expressed in BL21(DE3)pLysS cells, was localized in inclusion bodies. For immunization, inclusion bodies were solubilized in 6 M urea, dialyzed against 4 M urea, and injected into rabbits. A total of six rabbits were injected s.c. with Pax8-6H every 2 weeks for 10 weeks. On the 12th week, all the rabbits were killed, and the immune sera were pooled, precipitated in ammonium sulfate to 50% saturation, and dissolved in 10 mM Tris, pH 7.5/100 mM NaCl to obtain a crude antibody fraction.
To purify anti-Pax8 antibodies, we immobilized highly purified Pax8-6H on Affigel 10 resin (Bio-Rad). In this case, Pax8-6H containing inclusion bodies was solubilized in 6 M guanidinium hydrochloride, and Pax8-6H was purified by nickel-chelate affinity chromatography as described in the manual provided with the resin (Chelating Sepharose FastFlow, Amersham Pharmacia). The purified protein was dialyzed against 1 M guanidinium hydrochloride/50 mM phosphate buffer, pH 7.0/0.2M NaCl/1 mM EDTA, pH 8.0 and finally coupled to Affigel 10 by using the aqueous coupling procedure.
Crude antibody fractions were loaded on the Affigel Pax8-6H resin. The anti-Pax8 antibody was eluted from the column in 0.1 M glycine, pH 2.5, neutralized with Tris, and dialyzed against PBS, pH 7.4.
The affinity-purified anti-Pax8 antibody was tested for specificity by Western blot analysis using thyroid and nonthyroid cell extracts. In our experimental conditions the antibody recognized a single band at the expected apparent molecular mass of Pax8 (46 kDa) exclusively in thyroid cell extracts (data not shown). Furthermore, in immunohistochemistry experiments, the antibody reveals exclusively thyroid and kidney, the two major sites of Pax8 expression.
Histology.
Animals were killed by cervical dislocation. Staged embryos were obtained by dissection of pregnant females. The day at which the vaginal plug was detected was designed as E0.5. Adult thyroid glands were obtained from 2-month-old mice. Thyroids and embryos were fixed overnight at 4°C in 4% paraformaldehyde in PBS at pH 7.2, dehydrated through ethanol series, cleared in xylene, and embedded in paraffin, and 7-μm sections were cut. For cryo-sectioning, paraformaldehyde-fixed thyroids were treated overnight at 4°C in 30% sucrose in PBS and embedded in OCT compound (BDH), and 10-μm sections were cut.
For histological examinations, slides were stained with Harry's hematoxylin/eosin (BDH) according to manufacturer instructions.
Immunohistochemistry.
Sections were dewaxed by standard techniques, and heat treatment to retrieve the antigen sites was performed. To quench endogenous peroxidases, the sections were treated with 1.5% hydrogen peroxide in methanol at room temperature. The sections were incubated for 1 h at room temperature with blocking solution (3% BSA/5% goat serum/20 mM MgCl2/0.3% Tween 20 in PBS) and then with primary antibodies overnight at 4°C. Staining procedures and chromogenic reactions were carried out according to the protocols of the Vectastain ABC kit protocol (Vector Laboratories). The primary antibodies used were: anti-rat TTF-1 (3), anti-rat TTF-2 (29), anti-mouse Pax8, anti-human Tg (Dako), and anti-rat NIS (17).
BrdUrd Labeling and Detection.
Pregnant mice at E15.5 and E16.5 were injected i.p. with BrdUrd solution (100 mg/kg body weight, Sigma) and killed after 4 h by cervical dislocation. Paraffin-embedded embryos were sectioned and adjacent sections were processed for BrdUrd detection. BrdUrd detection was performed as described (30). The secondary antibody, a biotinylated goat anti-mouse (Sigma), was diluted 1:200 (vol/vol) in 1.5% goat serum in Tris-buffered saline (TBS, 0.1 M Tris⋅HCl, pH 7.5/0.15 M NaCl).
In Situ Hybridization of TPO mRNA.
E15.5 mouse embryo total RNA was extracted by the guanidine-isothiocyanate method (31) and reverse-transcribed by using Superscript first-strand synthesis system (Invitrogen) according to manufacturer instructions. cDNA was amplified with the mouse TPO-specific primers 5′-AAA GAT GTT AAT GAG TGT GC-3′ and 5′-AGG ATA TCT GAG CCA GAC CAG C-3′. The resulting PCR product, corresponding to the fragment from nucleotide 2,347 to 3,170 of the reported TPO sequence (GenBank accession no. X60703), was cloned in pBlueScript SK(−) and used to prepare a digoxigenin-labeled probe using DIG-labeling RNA kit (Roche Molecular Biochemicals) according to manufacturer instructions. Hybridization was performed as described (29).
Northern Blot Analysis.
RNA from lung of adult animals was prepared by the guanidine-isothiocyanate method (31), and Northern blot analysis was carried out as described (3). To detect the expression of the exogenous human TSHR in the lung of titf1+/KI-TSHR* mice, the Northern blot was hybridized with a 32P-labeled fragment corresponding to the 3′ UTR of the human TSHR (28). A probe derived from the 3′ UTR of rat TTF-1 cDNA (3) was used to detect TTF-1 expression in the lung.
Results
Role of TSH/TSHR Signaling in Thyroid Gland Morphogenesis.
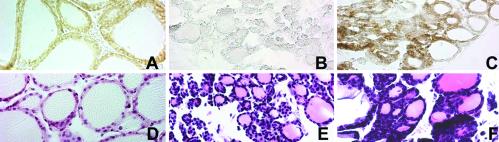
We performed a detailed analysis of thyroid gland of tshrhyt/tshrhyt and pitdw/pitdw mutant mice. In Fig. 1 A–C, sagittal sections of E17 embryos are shown. The size of the thyroid is the same in mutants and control mice. Consistent with this observation, the number of proliferating cells, as detected by BrdUrd incorporation, is comparable with tshrhyt/tshrhyt and wild-type embryos at E16.5 (Fig. 1 G–J).
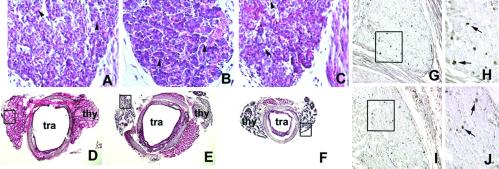
Fig 1.
Hematoxylin/eosin stain of wild-type and mutant mouse thyroid gland and BrdUrd incorporation in developing mouse thyroid gland. (A–C) Sagittal sections of E17 wild-type (A), tshrhyt/tshrhyt (B), and pit1dw/pit1dw (C) mouse embryos. The black arrowheads in A–C point to small follicles. These data were representative of at least two independent experiments performed on embryos coming from different littermates. As controls, wild-type C57BL/6 embryos were used. (D–F) Transversal sections of thyroid gland and trachea of wild-type (D), tshrhyt/tshrhyt (E), and pit1dw/pit1dw (F) of 2-month-old mice. thy, thyroid; tra, trachea. These data were representative of three independent experiments (two homozygous males and one homozygous female for both tshrhyt and pitdw strains). The mice were generated in different littermates. As controls, wild-type C57BL/6 mice were used. (G–J) Sagittal sections of E16.5 wild-type (G and H) and tshrhyt/tshrhyt (I and J) embryos. H and J show higher magnification of the boxed areas in G and H, respectively. These data are representative of three independent experiments. Embryos from untreated pregnant mice at the same days of gestation were used as a negative control. Cells that incorporated BrdUrd are visible as black dots (arrows).
Conversely, as reported before (21, 22, 25, 32), the thyroid of 2-month-old tshrhyt/tshrhyt or pit1dw/pit1dw animals is smaller than that of control mice (Fig. 1 D–F). Hence we can conclude that TSH does not have a relevant effect on proliferation of the thyroid cells during development but is necessary for the maintenance of both the size and functional structure of the thyroid gland in the postnatal life.
Role of TSH in Expression of Differentiation Markers in the Embryonic Thyroid Gland.
Experiments in thyroid cell lines have shown that TSH can regulate the expression of mRNA of several thyroid-specific genes such as Tg (10, 11), TPO (14, 15), NIS (17), TTF-2 (33), and Pax8 (34).
Animal models allow assessing the relevance of TSH/TSHR signaling in controlling the expression of thyroid-specific genes in vivo. As reported previously, Tg and TPO are expressed in thyroid gland since E14.5 (3) and E16 (20), respectively. Because the onset of NIS expression during embryonic development has not been described yet, we studied the presence of the NIS protein at different stages of thyroid development using a specific antibody (17). Interestingly, we found that NIS is detectable only starting at E16 (Fig. 2 C and D), 1 day later than the first appearance of Tg (Fig. 2 A and B).
Fig 2.
Onset of NIS expression in the developing mouse thyroid gland. Serial sagittal sections of E14.5 (A and C) and E16 (B and D) embryos were stained with anti-Tg (A and B) and anti-NIS (C and D) antibodies. These data are representative of three independent experiments.
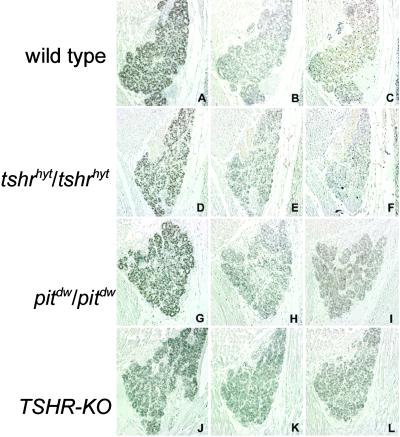
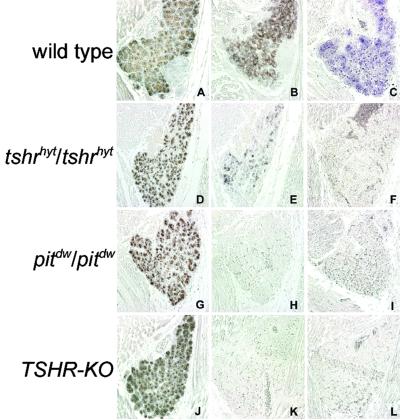
We next analyzed the expression of thyroid-specific genes in E17 embryos (Figs. 3 and 4) and 2-month-old adult thyroids (data not shown) from wild-type and mutated mice. Comparable amounts of TTF-1, TTF-2, and Pax8 (Fig. 3) are present in the thyroid gland of wild type and in all mutant embryos. A small but noticeable reduction of Tg expression (Fig. 4, A, D, G, and J) was observed in both tshrhyt/tshrhyt and pit1dw/pit1dw, whereas TSHR-KO mice show the same amount as the controls. NIS protein (Fig. 4B) is clearly detected in wild-type E17 thyroid but is reduced greatly in tshrhyt/tshrhyt thyroid (Fig. 4E), and it is almost absent in both pit1dw/pit1dw and TSHR-KO E17 thyroids (Fig. 4 H and K). Furthermore, TPO mRNA is absent in all the mutants examined (Fig. 4, F, I, and L).
Fig 3.
Expression of thyroid transcription factors in developing mouse thyroid gland. Serial sagittal sections of wild-type (A–C), tshrhyt/tshrhyt (D–F), pit1dw/pit1dw (G–I), and TSHR-KO (J–L) E17.5 embryos were stained with anti-TTF-1 (A, D, G, and J), anti-TTF-2 (B, E, H, and K), and anti-Pax8 (C, F, I, and L) antibodies. These data are representative of at least two independent experiments. As controls, wild-type C57BL/6 embryos were used.
Fig 4.
Expression of Tg, NIS, and TPO in developing mouse thyroid gland. Serial sagittal sections of wild-type (A–C), tshrhyt/tshrhyt (D–F), pit1dw/pit1dw (G–I), and TSHR-KO (J–L) E17.5 embryos were stained with anti-Tg (A, D, G, and J) and anti-NIS (B, E, H, and K) or hybridized with TPO antisense probe (C, F, I, and L). These data are representative of at least two independent experiments. As controls, wild-type C57BL/6 embryos were used.
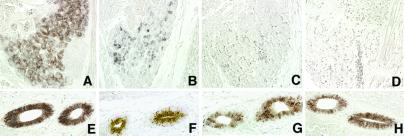
Because NIS is expressed also in the salivary gland (35), we asked whether the observed NIS down-regulation as a consequence of absent TSH/TSHR signaling is a thyroid-specific effect. As Fig. 5 shows, NIS is still present in the salivary gland ducts of both mutant and wild-type embryos, suggesting that the strict TSH/TSHR requirement operates through a thyroid-specific mechanism.
Fig 5.
Thyroid-specific down-regulation of NIS protein. Sagittal sections of wild-type (A and E), tshrhyt/tshrhyt (B and F), pit1dw/pit1dw (C and G), and TSHR-KO (D and H) E17.5 thyroids and salivary glands were stained with anti-NIS antibody. Salivary glands shown in E–H belong to the embryos shown in A–D, respectively. NIS expression is very scarce in the thyroid of mutant embryos. In contrast, a clear signal is present in the salivary glands. These data are representative of at least two independent experiments. As controls, wild-type C57BL/6 embryos were used.
Deregulated Expression of a Ligand-Independent TSHR Does Not Anticipate NIS Expression but Rescues It in tshrhyt/tshrhyt and pit1dw/pit1dw Mice.
Given the great relevance of the TSH/TSHR pathway in the expression of NIS and TPO, we asked whether the same pathway was responsible also for the temporal control of their expression during mouse thyroid development. The expression of TPO and NIS is observed in the developing thyroid only starting from E16 (Fig. 2; ref. 20). We generated, using a knock-in strategy (Fig. 6A), a mouse strain in which the sequence encoding a constitutively activated form of human TSHR (TSHR*; ref. 28) has been inserted into the titf1 gene. In such a line the signaling pathway downstream of the receptor should be activated in a TSH-independent manner, since E8.5, i.e., when TTF-1 expression begins.
Fig 6.
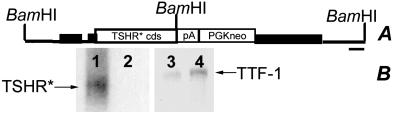
Structure of the titf1 locus modified by homologous recombination and expression of the targeted allele. (A) The black box indicates titf1 exons, and the black bar indicates the probe used to identify the targeted allele. (B) Human TSHR RNA expression in lung of adult targeted mice. Lanes 1 and 3, lung RNA from titf1+/KI-TSHR* animals; lanes 2 and 4, lung RNA from wild-type animals. (Left) Hybridization with human TSHR probe. (Right) Hybridization with rat TTF-1 probe.
To show that the allele titf1KI-TSHR* directs the expression of TSHR* in the TTF-1 expression domain, we demonstrated by Northern blot the presence of human TSHR mRNA in lungs of adult mice carrying it (Fig. 6B). We then introduced the titf1KI-TSHR* allele in both the tshrhyt/tshrhyt and pit1dw/pit1dw genetic backgrounds by appropriate crossings and analyzed the phenotype of the adult thyroid of these mice.
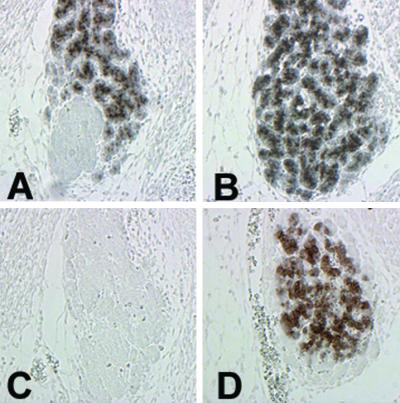
In Fig. 7 we show NIS expression in 2-month-old thyroid glands from wild-type (Fig. 7A), pit1dw/pit1dw (Fig. 7B), and double-mutant mice titf1+/KI-TSHR*;pit1dw/pit1dw (Fig. 7C). The presence of NIS in the double mutant demonstrates that the knock-in allele is able to rescue the expression of NIS in the thyroid gland of pit1dw/pit1dw mice. Thus the receptor encoded by the titf1KI-TSHR* allele is functioning. The same result was obtained in the case of the double-mutant mice titf1+/KI-TSHR*;tshrhyt/tshrhyt (data not shown). The rescue obtained shows that the TSHR* allele used by us encodes a receptor that is activated in a TSH-independent manner.
Fig 7.
Rescue of NIS expression. Serial sagittal sections of adult wild-type (A and D), pit1dw/pit1dw (B and E), and titf1+/KI-TSHR*;pit1dw/pit1dw (C and F) thyroids were stained with anti-NIS antibody (A–C) or hematoxylin/eosin (D–F). NIS expression is absent in the thyroid gland of pit1dw/pit1dw (B), whereas its expression is rescued in double mutants titf1+/KI−TSHR*;pit1dw/pit1dw (C). These data are representative of at least two independent experiments.
We then analyzed developing thyroid from titf1+/KI-TSHR* heterozygous mice. Neither at E12.5 (data not shown) or E13.5 were we able to detect NIS protein or TPO mRNA in the thyroid of these knock-in mice (Fig. 8). Hence we have to conclude that TSH/TSHR signaling is necessary for NIS and TPO expression, but it is not sufficient for expression at early developmental stages.
Fig 8.
Analysis of titf1+/KI-TSHR* E13.5 embryos. Serial sagittal sections were stained with anti-TTF-1 (A) or anti-NIS (B) or hybridized with TPO antisense probe (C). These data are representative of at least two independent experiments.
Discussion
From E8.5 up to ≈E13.5 the developing thyroid consists of a mass of endodermal cells that bud from the endoderm of the pharyngeal floor and migrate toward the trachea. These thyroid precursor cells can be identified unequivocally by the simultaneous presence of three transcription factors, TTF-1, TTF-2, and Pax8. At E14 molecular and morphological differentiation of the thyroid gland occurs, thus completing organogenesis. Only at this stage, thyroid-specific genes such as Tg, TPO, and TSHR start to be expressed (2), whereas NIS appears at E16, as shown in this study. By E16.5 thyroid follicles begin to be recognizable. The mechanisms that drive these late stages of organogenesis are still unknown.
Just before the final morphological differentiation of the gland, both TSHR and its ligand are expressed (3, 36). We decided to investigate the role of TSH/TSHR signaling on the thyroid development and differentiation using different mouse strains in which this pathway is severely impaired. We demonstrated that during the embryonic life, TSH/TSHR signaling is essential for the expression of some of the genes required for thyroid hormone biosynthesis such as TPO and NIS, whereas it is not required for the onset of Tg expression that occurs in all mutants examined. Furthermore, we show that the TSH/TSHR pathway is not involved in controlling DNA synthesis in the developing thyroid and, hence, in the growth of the embryonic gland. On the contrary, it is well established that in postnatal life the TSH/TSHR pathway is important in both maintaining the follicular organization of the thyroid and controlling gland size (9, 37).
The animal models we used, tshrhyt/tshrhyt and pit1dw/pit1dw mice, have been described already. These mice are all affected by a severe hypothyroidism associated with thyroid hypoplasia. Histological analysis showed that the follicles of the mutant thyroids were smaller than those of the wild type (22, 27).
Here we show that at E17 the size of the thyroid gland in tshrhyt/tshrhyt or pit1dw/pit1dw mice is rather similar to that of wild-type thyroid. We also observed a similar follicular structure between mutants and wild-type thyroid, with the presence of small follicles filled with colloid, intermingled with epithelial cords not yet organized in follicles, indicating that the folliculogenesis initiates also in the absence of TSH signals. The presence of follicles in the embryonic thyroid of tshrhyt/tshrhyt mice is in contrast with a previous study (38). Such a difference could be due to the different genetic background of the mice used. On the contrary, as observed before (22, 27), the architecture of the thyroid gland of tshrhyt/tshrhyt and pit1dw/pit1dw mice is severely impaired after birth. Our data suggest that TSH/TSHR signaling might be required to maintain rather than initiate folliculogenesis.
Data obtained from both cell cultures (39) and animal models (37) demonstrate that TSH has a mitogenic effect on thyroid follicular cells. In contrast, we did not observe any difference in size between mutant and wild-type embryonic thyroids. Furthermore, BrdUrd incorporation in the developing thyroid gland is the same in the tshrhyt/tshrhyt embryos as well as in the wild-type embryos. We conclude that TSH does not control the growth of thyroid precursor cells. We can hypothesize that other factors are responsible for proliferation of these cells. Indeed, other growth factors such as insulin-like growth factor 1 or epidermal growth factor can promote the proliferation of thyroid cells in culture (40, 41). These factors are also expressed during embryogenesis (42, 43) and could be the primary regulators of thyroid cell growth. However, we cannot exclude that TSH is capable of acting on growth of embryonic thyroid cells, but in the absence of TSH other factors functionally substitute for it.
The TSH was proposed as the main agent regulating the activity of the thyroid gland. Stimulation of thyroid follicular cells by TSH results in the rapid activation of almost every aspect of their metabolism including the regulation of synthesis of thyroid-specific proteins such as Tg, TPO, and NIS. Many observations in cultured thyroid cells (10, 11), in hypophysectomized rats (12), and in tissue slices (13) agree with the notion that TSH controls the levels of Tg, TPO, and NIS mRNAs. However, discrepancies exist on the extent of TSH control, mostly for Tg, that in some studies shows a 2-fold decrease (10) or disappearance in the absence of TSH (12).
It has been observed also that the mechanisms involved in the TSH regulation of Tg and TPO expression are different (13, 44). Furthermore, in transgenic mice expressing the A2 adenosine receptor, which activates the adenylyl cyclase pathway (45), the level of TPO mRNA is strongly up-regulated, whereas Tg mRNA is not affected. Our data show, in comparison to the wild type, only a slight reduction of Tg expression in both tshrhyt/tshrhyt and pit1dw/pit1dw mutant embryos. No reduction was observed in TSHR-KO embryos. Although the minor differences observed in the three models could be due to differences in genetic background, these observations all point to a limited role of TSH in controlling the onset of Tg expression in the developing thyroid. On the contrary, in all the animal models we used, TPO expression is strongly down-regulated in the absence of TSH/TSHR signaling, indicating that in vivo TPO expression is regulated largely by TSH.
TSH stimulates thyroid I− accumulation (46). Previous data suggested that TSH stimulation of iodide accumulation results, at least in part, from the cAMP-mediated increased biosynthesis of NIS (17). In addition, NIS mRNA also is increased by TSH signaling (16). Our experiments clearly show that in tshrhyt/tshrhyt mice the level of NIS protein is decreased strongly, and in pit1dw/pit1dw and TSHR-KO mice NIS protein is almost absent. Higher levels of NIS in tshrhyt/tshrhyt could be explained by postulating a residual activity of the hyt TSHR that could be induced by the high level of circulating TSH, although we cannot exclude that the small differences observed between the mutants is due to the diverse genetic background. However, taken together, the data show that, at variance from Tg, NIS and TPO expression are regulated tightly in vivo by TSH. The regulation of NIS expression is thyroid-specific as tested by the presence of similar levels of the protein in salivary glands (see Fig. 6).
It is worth noting that some cases of congenital hypothyroidism with a phenotype resembling that of tshrhyt/tshrhyt mice were reported (47–49). These patients had high levels of TSH in the blood, low serum levels of T4, and Tg levels in the normal range. Physical examination and 99mTc scintigraphy failed to demonstrate the presence of thyroid tissue, but careful ultrasonography revealed a very hypoplastic thyroid. A loss of expression of NIS consequent to the TSHR mutation in the thyroid tissue of the patients could cause the reported phenotype.
In conclusion, our data show that TSH, at least in mice, does not coordinately control the thyroid-differentiated phenotype, because we can distinguish genes tightly controlled (TPO and NIS) and others with expression that is largely TSH-independent. Furthermore, we have presented evidence demonstrating that the increase in number of thyroid cells during embryogenesis does not depend on a functional TSH/TSHR pathway. This observation suggests that in thyroid other factors must be responsible for controlled growth of embryonal cells, which is at variance from the case of the pituitary somatotroph cells, where growth hormone-releasing hormone controls both physiology and development (50) via the cAMP pathway. However, it is still conceivable that elevated TSH levels, such as in hypothyroid patients with hormonogenesis defects, could induce growth, cooperating with other, yet-unidentified mechanisms that control growth of fetal thyroid. This mechanism could explain the presence of goiter at birth in some severely hypothyroid children.
Acknowledgments
We thank Dr. N. Carrasco (Department of Molecular Pharmacology, Albert Einstein College of Medicine, Bronx, NY) and Dr. B. Rousset (Faculte de Medecine Lyon RTH-Laennec, France) for the gift of anti-NIS antibody. This work was supported in part by Telethon Grant GP0208Y01, an Associazione Italiana per la Ricerca sul Cancro grant (to R.D.L.), and Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica Grant “I geni cell'uomo” cluster 01. A.R. and P.M. are supported by a Biogem s.c.a.r.l. salary.
Abbreviations
E, embryonic day
Tg, thyroglobulin
TSH, thyroid-stimulating hormone/thyrotropin
TSHR, TSH receptor
TPO, thyroperoxidase
NIS, sodium/iodide symporter
KO, knockout
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kaufman M. H. & Bard, J., (1999) The Anatomic Basis of Mouse Development (Academic, San Diego), pp. 165–166.
- 2.Di Lauro R. & De Felice, M. (2001) in Endocrinology, eds. DeGroot, L. J. & Jameson, J. L. (Saunders, Philadelphia), Vol. 2, pp. 1268–1278. [Google Scholar]
- 3.Lazzaro D., Price, M., De Felice, M. & Di Lauro, R. (1991) Development (Cambridge, U.K.) 113 1093-1104. [DOI] [PubMed] [Google Scholar]
- 4.Zannini M., Avantaggiato, V., Biffali, E., Arnone, M. I., Sato, K., Pischetola, M., Taylor, B. A., Phillips, S. J., Simeone, A. & Di Lauro, R. (1997) EMBO J. 16 3185-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plachov D., Chowdhury, K., Walther, C., Simon, D., Guenet, J. L. & Gruss, P. (1990) Development (Cambridge, U.K.) 110 643-651. [DOI] [PubMed] [Google Scholar]
- 6.Kimura S., Hara, Y., Pineau, T., Fernandez-Salguero, P., Fox, C. H., Ward, J. M. & Gonzalez, F. J. (1996) Genes Dev. 10 60-69. [DOI] [PubMed] [Google Scholar]
- 7.Mansouri A., Chowdhury, K. & Gruss, P. (1998) Nat. Genet. 19 87-90. [DOI] [PubMed] [Google Scholar]
- 8.De Felice M., Ovitt, C., Biffali, E., Rodriguez-Mallon, A., Arra, C., Anastassiadis, K., Macchia, P. E., Mattei, M. G., Mariano, A., Scholer, H., Macchia, V. & Di Lauro, R. (1998) Nat. Genet. 19 395-398. [DOI] [PubMed] [Google Scholar]
- 9.Dumont J. E. & Vassart, G. (2001) in Endocrinology, eds. DeGroot, L. J. & Jameson, J. L. (Saunders, Philadelphia), Vol. 2, pp. 1301–1313. [Google Scholar]
- 10.Avvedimento V. E., Tramontano, D., Ursini, M. V., Monticelli, A. & Di Lauro, R. (1984) Biochem. Biophys. Res. Commun. 122 472-477. [DOI] [PubMed] [Google Scholar]
- 11.Santisteban P., Kohn, L. D. & Di Lauro, R. (1987) J. Biol. Chem. 262 4048-4052. [PubMed] [Google Scholar]
- 12.Van Heuverswyn B., Streydio, C., Brocas, H., Refetoff, S., Dumont, J. & Vassart, G. (1984) Proc. Natl. Acad. Sci. USA 81 5941-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerard C. M., Lefort, A., Christophe, D., Libert, F., Van Sande, J., Dumont, J. E. & Vassart, G. (1989) Mol. Endocrinol. 3 2110-2118. [DOI] [PubMed] [Google Scholar]
- 14.Chazenbalk G., Magnusson, R. P. & Rapoport, B. (1987) Mol. Endocrinol. 1 13-17. [DOI] [PubMed] [Google Scholar]
- 15.Zarrilli R., Formisano, S. & Di Jeso, B. (1990) Mol. Endocrinol. 4 39-45. [DOI] [PubMed] [Google Scholar]
- 16.Kogai T., Endo, T., Saito, T., Miyazaki, A., Kawaguchi, A. & Onaya, T. (1997) Endocrinology 138 2227-2232. [DOI] [PubMed] [Google Scholar]
- 17.Levy O., Dai, G., Riedel, C., Ginter, C. S., Paul, E. M., Lebowitz, A. N. & Carrasco, N. (1997) Proc. Natl. Acad. Sci. USA 94 5568-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lissitzky S., Fayet, G., Giraud, A., Verrier, B. & Torresani, J. (1971) Eur. J. Biochem. 24 88-99. [DOI] [PubMed] [Google Scholar]
- 19.Yap A. S., Stevenson, B. R., Keast, J. R. & Manley, S. (1995) Endocrinology 136 4672-4680. [DOI] [PubMed] [Google Scholar]
- 20.Brown R. S., Shalhoub, V., Coulter, S., Alex, S., Joris, I., De Vito, W., Lian, J. & Stein, G. S. (2000) Endocrinology 141 340-345. [DOI] [PubMed] [Google Scholar]
- 21.Bartke A. (1964) Anat. Rec. 149 225-236. [DOI] [PubMed] [Google Scholar]
- 22.Cordier A. C., Denef, J. F. & Haumont, S. M. (1976) Cell Tissue Res. 171 449-475. [DOI] [PubMed] [Google Scholar]
- 23.Snell G. D. (1929) Proc. Natl. Acad. Sci. USA 15 733-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S., Crenshaw, E. B., III, Rawson, E. J., Simmons, D. M., Swanson, L. W. & Rosenfeld, M. G. (1990) Nature 347 528-533. [DOI] [PubMed] [Google Scholar]
- 25.Beamer W. J., Eicher, E. M., Maltais, L. J. & Southard, J. L. (1981) Science 212 61-63. [DOI] [PubMed] [Google Scholar]
- 26.Marians R. C., Ng, L., Blair, H. C., Unger, P., Graves, P. N. & Davies, T. F. (2002) Proc. Natl. Acad. Sci. USA 99 15776-15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein S. A., Oates, E. L., Hall, C. R., Grumbles, R. M., Fernandez, L. M., Taylor, N. A., Puett, D. & Jin, S. (1994) Mol. Endocrinol. 8 129-138. [DOI] [PubMed] [Google Scholar]
- 28.Porcellini A., Ciullo, I., Pannain, S., Fenzi, G. & Avvedimento, E. (1995) Oncogene 11 1089-1093. [PubMed] [Google Scholar]
- 29.Dathan N., Parlato, R., Rosica, A., De Felice, M. & Di Lauro, R. (2002) Dev. Dyn. 224 450-456. [DOI] [PubMed] [Google Scholar]
- 30.Xuan S., Baptista, C. A., Balas, G., Tao, W., Soares, V. C. & Lai, E. (1995) Neuron 14 1141-1152. [DOI] [PubMed] [Google Scholar]
- 31.Chomczynski P. & Sacchi, N. (1987) Anal. Biochem. 162 156-159. [DOI] [PubMed] [Google Scholar]
- 32.Stein S. A., Shanklin, D. R., Krulich, L., Roth, M. G., Chubb, C. M. & Adams, P. M. (1989) Neuroendocrinology 49 509-519. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz L., Zannini, M., Di Lauro, R. & Santisteban, P. (1997) J. Biol. Chem. 272 23334-23339. [DOI] [PubMed] [Google Scholar]
- 34.Van Renterghem P., Vassart, G. & Christophe, D. (1996) Biochim. Biophys. Acta 1307 97-103. [DOI] [PubMed] [Google Scholar]
- 35.Ajjan R. A., Kamaruddin, N. A., Crisp, M., Watson, P. F., Ludgate, M. & Weetman, A. P. (1998) Clin. Endocrinol. (Oxford) 49 517-523. [DOI] [PubMed] [Google Scholar]
- 36.Japon M. A., Rubinstein, M. & Low, M. J. (1994) J. Histochem. Cytochem. 42 1117-1125. [DOI] [PubMed] [Google Scholar]
- 37.Dumont J. E., Lamy, F., Roger, P. & Maenhaut, C. (1992) Physiol. Rev. 72 667-697. [DOI] [PubMed] [Google Scholar]
- 38.Beamer W. G. & Cresswell, L. A. (1982) Anat. Rec. 202 387-393. [DOI] [PubMed] [Google Scholar]
- 39.Medina D. L. & Santisteban, P. (2000) Eur. J. Endocrinol. 143 161-178. [DOI] [PubMed] [Google Scholar]
- 40.Kimura T., Dumont, J. E., Fusco, A. & Golstein, J. (1999) Eur. J. Endocrinol. 140 94-103. [DOI] [PubMed] [Google Scholar]
- 41.Errick J. E., Ing, K. W., Eggo, M. C. & Burrow, G. N. (1986) In Vitro Cell Dev. Biol. 22 28-36. [DOI] [PubMed] [Google Scholar]
- 42.Partanen A. M. (1990) Curr. Top. Dev. Biol. 24 31-55. [PubMed] [Google Scholar]
- 43.Bondy C. A., Werner, H., Roberts, C. T. J. & LeRoith, D. (1990) Mol. Endocrinol. 4 1386-1398. [DOI] [PubMed] [Google Scholar]
- 44.Isozaki O., Kohn, L. D., Kozak, C. A. & Kimura, S. (1989) Mol. Endocrinol. 3 1681-1692. [DOI] [PubMed] [Google Scholar]
- 45.Ledent C., Dumont, J. E., Vassart, G. & Parmentier, M. (1992) EMBO J. 11 537-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De La Vieja A., Dohan, O., Levy, O. & Carrasco, N. (2000) Physiol. Rev. 80 1083-1105. [DOI] [PubMed] [Google Scholar]
- 47.Abramowicz M. J., Duprez, L., Parma, J., Vassart, G. & Heinrichs, C. (1997) J. Clin. Invest. 99 3018-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bretones P., Duprez, L., Parma, J., David, M., Vassart, G. & Rodien, P. (2001) Thyroid 11 977-980. [DOI] [PubMed] [Google Scholar]
- 49.Gagne N., Parma, J., Deal, C., Vassart, G. & Van Vliet, G. (1998) J. Clin. Endocrinol. Metab. 83 1771-1775. [DOI] [PubMed] [Google Scholar]
- 50.Lin S. C., Lin, C. R., Gukovsky, I., Lusis, A. J., Sawchenko, P. E. & Rosenfeld, M. G. (1993) Nature 364 190-191. [DOI] [PubMed] [Google Scholar]