Abstract
We describe a previously uncharacterized function for changes in plant chemistry induced by phytophagous insects: to provide cues for mate location. Larvae of the gall wasp Antistrophus rufus Gillette (Hymenoptera: Cynipidae) feed within inconspicuous galls inside the flowering stems of the prairie perennials Silphium laciniatum L. and Silphium terebinthinaceum Jacquin (Asteraceae). Adult male A. rufus emerge before females and are challenged with locating mates that are sequestered within dead plant stems that occur in a matrix of dead vegetation. Allozyme studies revealed complete reproductive isolation between wasp subpopulations in the two plant species. In laboratory bioassays, males responded only to their natal plant species, antennating the stem surface. Males from S. laciniatum also responded to hexane extracts of S. laciniatum stems, and extracts contained much higher concentrations of monoterpenes (α-pinene, β-pinene, and camphene) than did S. terebinthinaceum. Ratios of “+” and “−” enantiomers of α- and β-pinene approximated 50:50 for nongalled S. laciniatum stems but strongly differed from 50:50 in galled stems, with “+” and “−” enantiomers strongly dominant in different plants. In bioassays, male wasps from S. laciniatum responded to a synthetic blend of the monoterpenes in enantiomeric ratios characteristic of galled stems. Male A. rufus rely entirely on olfaction to locate females within stems in a complex prairie habitat, and gall wasps themselves apparently influence the plant to modify ratios of monoterpene enantiomers. These plant volatiles serve as a signal for males, acting as a sex pheromone proxy for females concealed within plant tissues.
Keywords: Hymenoptera, Cynipidae, insect, mate location, volatiles
Phytophagous insects may induce release of plant volatile chemicals that relay information across trophic levels. For example, induced plant volatiles can attract natural enemies of herbivores (e.g., refs. 1 and 2), discourage oviposition by conspecific herbivores to prevent intraspecific competition between larvae (3), or serve both functions (4). Volatile compounds associated with insect-feeding damage also induce physiological resistance in conspecific plants (5) or across plant species (6). In this paper, we describe a previously uncharacterized function for changes in plant chemistry induced by phytophagous insects: to provide cues for mate location. Although earlier studies have revealed that plant volatiles play an important role in insect mate location (e.g., refs. 7 and 8), ours is to our knowledge the first to suggest that insects alter the chemical composition of plant volatile bouquets for purposes of mate location.
Antistrophus rufus larvae feed within galls inside the flowering stems of four species of Silphium that occur in prairies of the Midwestern United States (9, 10). These galls form entirely within the stem and are not discernable externally. Silphium laciniatum L. and Silphium terebinthinaceum Jacquin cooccur throughout the Midwest, including at our prairie study sites in central Illinois (J.F.T. and L.M.H., unpublished results); they are closely related (11) and occasionally hybridize (12). Stems of S. laciniatum bolt in mid-May, whereas those of S. terebinthinaceum appear about 1 mo later. Consequently, the period during which adult female A. rufus are ovipositing into stems shows a bimodal pattern across the two hosts, with ≈1 mo between peaks (J.F.T. and L.M.H., unpublished results). The gall wasp larvae develop during summer. In fall, Silphium stems senesce, detach from their taproot, and fall to the ground, where they lie through the winter in a matrix of dead plants of various species, the remnants of the plant community.
Male A. rufus are protandrous, emerging in spring from the dead and desiccated plant stems, and show an allochronic emergence pattern corresponding to plant phenology: males emerge from S. laciniatum ≈1 mo earlier than from S. terebinthinaceum but are present on both species for a period of ≈30 d (J.F.T. and L.M.H., unpublished results). Males walk along the dead stems, rapidly drumming the tips of their antennae on the surface, and position themselves over sites where females will emerge. Males guard these sites, driving off rival males by charging and head-butting (J.F.T. and L.M.H., unpublished results). After mating, females walk to nearby bolting stems (11). In fact, both sexes of adult A. rufus show a pronounced disinclination to take flight, usually walking on host stems or along the ground (J.F.T. and L.M.H., unpublished results). In hundreds of field observations, we have seen only occasional short hopping flights (<1 m distance) to adjacent plants. A disinclination to fly is adaptive for insects such as A. rufus that are small-bodied and live in windy habitats where they are subject to involuntary aerial dispersal (13) or occupy perennial late-successional habitats such as prairies, obviating the need to disperse (14).
Male A. rufus are challenged with locating mates that are sequestered within dead plant stems, searching through a three-dimensional matrix of dead vegetation for stems that contain females. The difficulty in locating a mate is compounded by the brief life span of A. rufus males, averaging <9 d for starved individuals and only 16 d when provided water and a sugar source in the laboratory (J.F.T. and L.M.H., unpublished data). Males are probably relatively short-lived in the field because they are not known to visit flowers of other plant species to feed and are active well before Silphium plants bloom. Location of mates is further complicated by interactions with spiders and other predaceous arthropods, resulting in male A. rufus dropping to the ground and recommencing their meandering search for host plants.
The purpose of our study was to determine the chemical basis for host plant species recognition and mate location in male A. rufus. To evaluate the degree to which males move between the two plant species in seeking mates, we conducted an allozyme analysis to evaluate the level of gene flow between A. rufus subpopulations on S. laciniatum and S. terebinthinaceum. That study revealed that the subpopulations were reproductively isolated, suggesting that males mate assortatively across plant species and are under selection to search for mates only on stems of their natal host species. We next demonstrated that males could discriminate between stems of the two Silphium species and overwhelmingly preferred their natal host plant species. Analysis of plant volatile compounds revealed that stems of S. laciniatum produced three monoterpenes (α-pinene, β-pinene, and camphene) that were in very low abundance in stems of S. terebinthinaceum. Ratios of monoterpene enantiomers may provide a cue for males to discriminate between stems that contain galls and those that do not: ratios of both α- and β-pinene differed significantly between galled and nongalled S. laciniatum stems. In bioassays using pure standards, we elicited searching behavior of male gall wasps of the S. laciniatum subpopulation by adjusting the abundance of terpenoids and stereochemistry to simulate galled stems of S. laciniatum. Our findings suggest that galls of A. rufus induce changes in enantiomeric ratios of monoterpenes, providing a cue for locating mates that persists long after the host plant dies.
Materials and Methods
Source of Specimens.
To provide adult A. rufus for our experiments, we collected dead stems of S. laciniatum and S. terebinthinaceum during the winter of 2000–2001 from four tallgrass prairie sites: Meadowbrook Prairie (Champaign County, N 40° 04.72, W 88° 12.41); Buckley Prairie (Iroquois County, ≈40° 33, W 88° 03); Loda Cemetery Prairie Nature Preserve (LCP; Iroquois County, N 40° 31.61, W 88° 04.57); and Prospect Cemetery Prairie Nature Preserve (Ford County, N 40° 26.71, W 88° 05.87). Stems were cut into ≈30-cm sections and placed in outdoor rearing cages. Wasps began emerging in late May and were kept in covered paper cups, separated by host plant species, in an incubator at 13°C (15 h light/9 h dark) with honey for food and moistened cotton wicks for water or were stored at 4°C for later use.
We collected dead Silphium stems of both species from the same sites, as well as three other prairie sites, including Red Bison Prairie Corridor in central Illinois (Champaign County, N 40° 04.81, W 88° 14.83); Nardi Prairie in Indiana (Parke County, ≈N 39° 38, W 87° 22); and Clinton Park Prairie in Kansas (Douglas County, N 38° 55.47, W 95° 22.01). We also used plants in a common garden of 4- to 5-yr-old S. laciniatum and S. terebinthinaceum plants (Missouri Wildflower Nursery, Jefferson City, MO) that we established in spring 2000 at the Landscape Horticulture Research Center of the University of Illinois (40° 05.43, W 88° 13.04). In spring 2001 and 2002, we manipulated the degree to which these common garden plants were galled by A. rufus by enclosing developing plant stems in organdy cages and releasing mated female A. rufus of the appropriate subpopulations in cages, randomly assigning “galled” treatments (50 wasps) and “nongalled” treatments (0 wasps) to at least 15 plants of each plant species. Gall wasps had been reared from stems of S. laciniatum and S. terebinthinaceum stems from field sites LCP and Prospect Cemetery Prairie Nature Preserve.
Gene Flow Between Subpopulations on Different Host Plants.
To evaluate the amount of mating between individuals of the two subpopulations, we screened 14 enzyme loci in larvae and adults from field sites LCP, Buckley Prairie, and Meadowbrook Prairie by using both starch and cellulose acetate electrophoresis [abbreviations and EC numbers: aconitase (ACON, 4.2.1.3); adenylate kinase (AK, 2.7.4.3); aldolase (ALD, 4.1.2.13); fumarase (FUM, 4.2.1.2); glucose-phosphate isomerase (GPI, 5.3.1.9); glutamate-oxaloacetate transaminase (GOT, 2.6.1.1); glyceraldehyde-3-phosphate dehydrogenase (G3PDH, 1.2.1.12); hexokinase (HEX, 2.7.1.1); isocitrate dehydrogenase (IDH, 1.1.1.42); malate dehydrogenase (MDH, 1.1.1.37); malic enzyme (ME, 1.1.1.40); NADH-dependent diaphorase (DIA-1, 1.6.2.2); peptidase A (PEP, 3.4.11); and phosphoglucomutase (PGM, 2.7.5.1)]. We homogenized A. rufus larvae or adults in 20 μl of grinding buffer and analyzed homogenates by electrophoresis on either 12% starch gels or Titan III cellulose acetate plates (Helena Laboratories, Beaumont, TX). Our method for starch gel electrophoresis was that of Berlocher and Smith (15). Cellulose acetate electrophoresis was performed for 30 min (GPI, G3PDH, MDH, ME) or 1 h (PGM, IDH) at 150 V, using Tris-citrate buffer (pH 8.6) with the gel apparatus chilled with ice. Enzyme staining for both electrophoretic techniques followed standard procedures (e.g., ref. 16).
Recognition of Host Plant Species.
To determine whether male A. rufus were able to recognize stems of their natal host, we designed a bioassay in which walking individuals from both plant species were presented a choice between dead stems of S. laciniatum and S. terebinthinaceum collected at field site LCP from which all gall wasps recently had emerged. A dead stem (20 cm long) of the nonhost plant Solidago altissima L. served as the base of a “Y” configuration with the arms being stems of equal length of the two Silphium species (separated by an ≈80° angle). The “Y” was positioned in an enamel pan at an incline of ≈20° with arms resting on the lip and directed toward a north-facing window and the intersection of stems supported by a Petri dish. Wasps released at the base of the “Y” invariably walked uphill, where they encountered the Silphium stems. We allowed wasps to “respond” to a Silphium stem by remaining on it for at least 2 min; individuals that did not respond to either Silphium stem within 5 min were recorded as “no response.” We repeated this bioassay until we reached 20 responding individuals from both Silphium species, replacing stems and switching plant species between the arms after every five wasps to control for location effects. We used the χ2 goodness of fit test (17) to test for differences among wasps in host plant choice.
Isolation of Volatile Chemical Cues.
Response of male A. rufus of the S. laciniatum subpopulation to S. laciniatum confirmed that they could discriminate between Silphium species, and their antennating behavior suggested they used volatile cues (see Results and Discussion). To test the short-range response of wasps to plant volatiles, we serially doused individual ≈30-cm lengths of S. laciniatum stems from field site LCP with 5 ml of ethanol (polar) and hexane (nonpolar), collected the extracts, reduced their volume under nitrogen to 1 ml, and applied the entire fraction to filter paper (Qualitative No. 1, Whatman). We allowed the solvent to evaporate and cut 2.5-cm squares that contained one stem-section equivalent of extracted compounds. We tested the activity of hexane and ethanol extracts separately and in combination by arranging four paper squares, two with the extracts and two solvent controls, in a circular pattern in a 10-cm-diameter Petri dish. Individual gall wasps were released at the dish center; we covered the dish and recorded how long they remained on each paper square during a 5-min period, noting their behavior. Wasps that remained stationary for a 2-min period were relocated to the dish center. The bioassay was repeated 20 times each for ethanol and hexane extracts individually and in combination. We tested differences between treatments in the amount of time wasps spent on paper squares with Student's t test (17).
Identification of Volatile Chemical Cues.
We prepared hexane extracts (see Results and Discussion) of each of at least 20 Silphium stems of both plant species from field site LCP by the method already described. Because these hexane extracts did not yield sufficient concentrations of chemicals for resolving ratios of enantiomers in some cases (see Results and Discussion), we also extracted monoterpenes from stems by hydrodistillation: we collected dead stems of S. laciniatum and S. terebinthinaceum from our common garden (n = 5 per species). Stems of 20-cm sections were cut into small pieces (≈3 mm) and boiled for 3.5 h in 500 ml of distilled water under reflux in an all-glass hydrodistillation apparatus with water-cooled condenser (18). We used 1 ml of hexane as the collection solvent.
To determine the source of variation in monoterpene content and enantiomeric ratios between Silphium species and galled and nongalled stems, we also studied volatile compounds released by bolting stems in our common garden. We trapped plant volatiles from three galled and nongalled plants by enclosing stems in a Reynolds oven cooking bag (made of inert materials) supported by a cylindrical wire cage (≈30 cm height, 20 cm diameter). Air entering the bag was purified with activated charcoal, and air was drawn out of the bag (≈0.8 liters/min) through a column of the adsorbent SuperQ (Alltech Industries, Deerfield, IL) with a 1-hp vacuum cleaner on a variable power supply. We eluted the SuperQ with 2 ml of methylene chloride.
We identified volatile components of extracts in the lab of L.M.H. with a Hewlett–Packard 5973 mass spectrometer interfaced to a HP 6890 gas chromatograph, using a HP-5MS (cross linked 5% phenylmethyl siloxane) capillary column (30 m × 0.25 mm × 0.25 μm film thickness) in splitless mode with helium as the carrier gas (42.1 kPa head pressure, linear velocity ≈36 cm/s). Oven temperature was held at 38°C for 4 min and then ramped to 90°C at 2°C/min. Injector temperature was 250°C, and the transfer line temperature was 280°C. Identifications were verified by comparing retention times and spectra with pure compounds (Aldrich). We measured relative abundances of compounds in hexane extracts by comparison to an internal standard (hexadecane) and tested differences between plant species with the nonparametric Kruskall–Wallis tests because zero values in some classes violated assumptions of ANOVA (17, 19).
We studied enantiomeric compositions of galled and nongalled stems of field-collected S. laciniatum plants [number of wasps/plant = 282 ± 103 (mean ± SD) for “galled” stems and 2.0 ± 2.7 for “nongalled” stems] as well as volatiles sampled from bolting stems of S. laciniatum in the common garden. We do not know the levels of galling in these green bolting plants, because the study was conducted before wasps had completed development and adults emerged; however, we observed female gall wasps ovipositing in stems of plants in the gall treatment during summer 2002, and this method has consistently resulted in high densities of galls in previous years. Enantiomeric compositions of α- and β-pinene were resolved by W.A.K. by using a 25-m fused silica column with the chiral stationary phase octakis(6-O-methyl-2,3-di-O-pentyl)-γ-cyclodextrin (30% in polysiloxane OV 1701) at 40°C column temperature and 50 kPa hydrogen as a carrier gas (21). Enantiomeric composition of camphene was determined with a 50-m fused silica capillary column with heptakis(6-O-methyl-2,3-di-O-pentyl)-β-cyclodextrin (30% in polysiloxane OV 1701) at 40°C column temperature and 80 kPa hydrogen as a carrier gas (20). We compared the ratios of enantiomers of α- and β-pinene of galled and nongalled stems by using the nonparametric two-tailed Kolmogorov–Smirnov test (17, 19).
Verification of Response to Volatile Chemical Cues.
To verify the response of male A. rufus to the three monoterpenes, we diluted pure synthetic compounds in hexane, individually and in combination, to approximate one equivalent of a 30-cm section of plant stem with ratios of enantiomers characteristic of stems containing galls (≈70% “+” for both α- and β-pinene; see Results and Discussion). We also tested the response of males to standards of the monoterpenes in which ratios were maximally skewed (100% “+” α-pinene and 100% “−” β-pinene). The response of gall wasps was tested by using a bioassay similar to the previous study but improved to exploit the preference of the wasps to walk upside down on the Petri dish lid: We applied 3 ml of standards or pure solvent to two paper squares each (four total), stapled the squares to a disk of organdy (≈14 cm diameter) in a circular arrangement, secured the organdy over a plastic 9-cm-diameter Petri dish with a rubber band, and covered the organdy with a second Petri dish to create a chamber. The apparatus was inverted on a ring stand; we released individual wasps into the chamber and observed with a mirror their behavior through the bottom dish.
Results and Discussion
Gene Flow Between Subpopulations on Different Host Plants.
For both starch and cellulose acetate electrophoresis, eight of the loci we screened were invariant, but we found scoreable differences at six loci: GPI, PGM, G3PDH, MDH, ME, and IDH. For each of these six loci, there were consistent and fixed differences between gall wasp subpopulations on the two host plant species (Table 1): no alleles were shared between subpopulations, providing very strong evidence of complete reproductive isolation. Nei's (21) genetic distance was 0.560, and this degree of genetic divergence suggests that the two subpopulations probably represent separate species (22). In our subsequent discussions, we label individuals from S. laciniatum as “ArSl ” and those from S. terebinthinaceum as “ArSt” to reflect the probable species-level distinction between gall wasps associated with the two plant species.
Table 1.
Allelic frequencies of A. rufus reared from stems of S. laciniatum (S. lac.) and S. terebinthinaceum (S. ter.) from three populations in central Illinois
| Locus/mobility
|
Gall wasp population | |||||
|---|---|---|---|---|---|---|
| LCP | BP | MP | ||||
| S. lac. (30) | S. ter. (30) | S. lac. (15) | S. ter. (15) | S. lac. (10) | S. ter. (10) | |
| GPI | ||||||
| 100 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 | 0.000 |
| 63 | 0.000 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 |
| PGM | ||||||
| 100 | 0.000 | 0.483 | 0.000 | 0.679 | 0.000 | 0.550 |
| 86 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 | 0.000 |
| 59 | 0.000 | 0.517 | 0.000 | 0.321 | 0.000 | 0.450 |
| G3PDH | ||||||
| 100 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 | 0.000 |
| 90 | 0.000 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 |
| MDH | ||||||
| 100 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 | 0.000 |
| 84 | 0.000 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 |
| ME | ||||||
| 100 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 | 0.000 |
| 62 | 0.000 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 |
| IDH | ||||||
| 100 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 | 0.000 |
| 80 | 0.000 | 1.000 | 0.000 | 1.000 | 0.000 | 1.000 |
Sample sizes are in parentheses. The most anodal band was assigned the value of 100, with electrophoretic mobilities of other bands calculated relative to this value based on measurements of cellulose acetate gels (for starch mobilities, contact authors). Populations are named after their prairies (see Materials and Methods). BP, Buckley Prairie; MP, Meadowbrook Prairie.
The results of the allozyme study were consistent with mating trials we have conducted that confirmed that both ArSl and ArSt males attempted to mate only with females from their natal host species but showed no response whatsoever to females from the other host species (unpublished data). We therefore conclude that male wasps should be under strong selection to search for mates only on the Silphium species that was their natal host, further reducing the portion of the habitat where potential mates would be available.
Recognition of Host Plant Species.
In the “Y” bioassay with plant stems, males from both plant species discriminated between Silphium species and showed a marked preference for their natal host species: 20 of 22 male ArSl responded to stems, with 18 choosing stems of S. laciniatum (χ2 = 12.8, P < 0.001); 20 of 24 male ArSt responded, with 15 choosing stems of S. terebinthinaceum (χ2 = 5.0, P < 0.025). When encountering stems of their natal host, all males actively antennated the stem surface in a manner identical to their behavior in the field but did not show this behavior on the other plant species.
Isolation of Volatile Chemical Cues.
In Petri dish bioassays, male ArSl spent significantly more time on paper treated with the combined hexane and ethanol extracts of S. laciniatum stems (36.8 ± 10.7 sec) than on solvent-treated paper (10.3 ± 3.5 sec; t = 2.36, P = 0.028). All males responded to extract-treated paper as they did plant stems, antennating the surface, but did not display this behavior on solvent-treated paper. Males also responded to hexane extracts alone, spending 38.7 ± 8.6 sec on extract-treated paper compared with 12.2 ± 3.8 sec on solvent controls (t = 2.84, P = 0.009), with the typical antennating behavior. There was no significant response to the ethanol extract alone (21.9 ± 6.7 sec on extract-treated paper versus 16.6 ± 3.1 sec on control; t = 0.72, P = 0.48). These findings suggest that chemical cues by which male ArSl recognized host stems were contained in the hexane extract.
Identification of Volatile Chemical Cues.
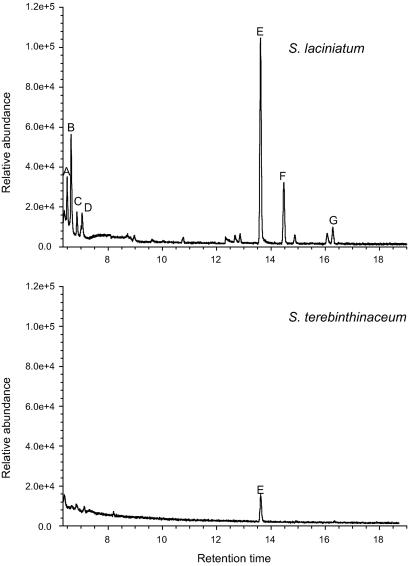
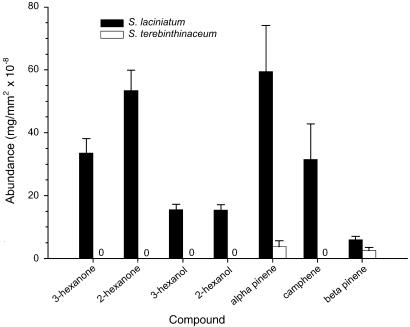
Hexane extracts of galled S. laciniatum contained α-pinene, β-pinene, camphene, 3-hexanone, 2-hexanone, 3-hexanol, and 2-hexanol, but extracts of S. terebinthinaceum revealed only very minute quantities of α- and β-pinene (Figs. 1 and 2; differences between plant species for all seven compounds were significant, with Kruskall–Wallis P < 0.0001, except β-pinene with P < 0.03). An independent study revealed similar differences between the plant species in the production of volatiles from developing stems in the common garden: 15-min aerations of S. laciniatum stems yielded significant amounts of monoterpenes, but S. terebinthinaceum stems produced such minute amounts that they were marginally detectable even when aeration periods were increased ≈15-fold (unpublished data).
Fig. 1.
Representative total ion chromatograms of hexane extracts of S. laciniatum and S. terebinthinaceum stems. A, 3-hexanone; B, 2-hexanone; C, 3-hexanol; D, 2-hexanol; E, α-pinene; F, camphene; G, β-pinene.
Fig. 2.
Abundance of compounds (in mg/mm2 × 10−8 of stem surface) present in hexane extracts of dead stems of S. laciniatum and S. terebinthinaceum.
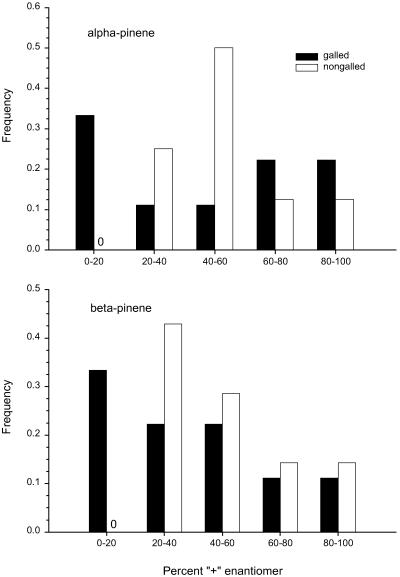
In addition, enantiomeric ratios of monoterpenes differed between S. laciniatum stems that contained galls and those that did not (Fig. 3): for both α- and β-pinene, ratios of “+” and “−” enantiomers varied around 50:50 in nongalled stems, but these ratios were significantly skewed in stems that contained galls (α-pinene: Kolmogorov–Smirnov statistic = 0.14, P = 0.003; β-pinene: statistic = 0.24, P < 0.0001). A preliminary study of volatiles of S. terebinthinaceum revealed that enantiomeric ratios of α-pinene show a similar shift with galling, but this effect was absent in β-pinene (unpublished data).
Fig. 3.
Frequency distribution of the percentage of “+” enantiomers of α- and β-pinene in galled and nongalled stems of S. laciniatum. Data presented include ratios of enantiomers from both hydrodistillation of dead stems (n = 5 plants) and volatile aerations of green stems from plants (n = 3) in our common garden plot. Proportions of enantiomers for both α- and β-pinene did not differ significantly between hydrodistilled dead stems and aerated green plants (ANOVA F2,15 = 1.10, P = 0.36).
The variation in our data on enantiomeric ratios of monoterpenes of α- and β-pinene (Fig. 3) in galled stems may reflect differences between sections of plant stems in density of galls. The effect of gall wasps on enantiomeric ratios may be very localized within plants, inducing changes in adjacent tissues rather than a systemic effect on the whole plant, as is the case with the salivary secretions of gall wasps that induce gall formation (23). In that case, ratios of enantiomers (summed across plant sections) would be more highly skewed for stems with high densities of evenly distributed galls but would approach 50:50 for stems that lack galls in some sections. Future studies will evaluate the spatial aspects of induced changes in enantiomers with galling of stems.
Verification of Response to Volatile Chemical Cues.
In Petri dish bioassays, male ArSl showed no response to α-pinene, β-pinene, and camphene tested separately in enantiomeric ratios representative of galled stems (mean time spent on paper squares: 21.3 ± 16.5, 51.0 ± 18.7, 50.3 ± 20.5, and 21.3 ± 16.5 sec, for α-pinene, β-pinene, camphene, and control, respectively; ANOVA F3,30 = 0.83, P = 0.49). When the three compounds were combined with the same ratios of enantiomers as found in galled host material, however, male ArSl spent nearly twice as much time on paper treated with the monoterpenes (81.9 ± 11.8 sec) than on controls (41.7 ± 11.2 sec; t = 2.48, P < 0.020) but, more importantly, responded to the synthetic blend by antennating the paper just as they did in searching the stems of host plants. Male wasps also significantly responded to the blend of 100% “+” α-pinene and 100% “−” β-pinene, remaining on monoterpene-treated paper more than four times as long as on solvent controls (23.1 ± 6.5 sec and 5.2 ± 1.0, respectively; t = 2.7, P = 0.013). The considerably shorter times for the monoterpene treatment in this study (23.1 min) than in the previous study (81.9) suggest that males were more responsive to ratios simulating those of plants; however, the two bioassays were conducted independently, and their results therefore are not directly comparable. Our findings support the notion that male ArSl recognize stems of host plants that contained gall wasps by skewed ratios of monoterpene enantiomers.
We conclude from these findings that male ArSl are reproductively isolated from ArSt and rely on olfaction to locate females within the stems of their natal host plant species in a structurally and taxonomically complex prairie habitat. Chemical differences between plants in our common garden plot, where galling treatments were assigned randomly to plants, confirm that S. laciniatum and S. terebinthinaceum stems differ in the composition of their monoterpene profiles, and that gall wasps alter ratios of monoterpene enantiomers in both plant species. Thus, volatile bouquets of host plants serve as a signal for males that are searching for mates, and plant volatiles take on the functional role of sex pheromones for females that are sequestered within plant tissues. Our findings attest to the intimate and elaborate interactions characteristic of gall-forming insects and their host plants (24). Gall formers are known to manipulate host plant chemistry for their own benefit (25), but our results expand the inventory of host plant manipulation and show that gall formers influence plants in ways more extensive and subtle than previously reported.
Acknowledgments
We thank May Berenbaum for suggesting PNAS as a venue for our research findings and for editorial suggestions that greatly improved the manuscript. For the allozyme work, we appreciate the advice of Stewart Berlocher and the technical assistance of Shelia Lyons-Sobaski and Jeff Heilveil. We are grateful for the advice and research assistance provided by Arthur Zangerl, James Nardi, Ellie Kron, Amy Crumrin, John Westberg, and Megan Weaver Tooker. Thanks to Hank Wilkinson and Daryl Huffstutler of the Landscape Horticulture Research Center of the University of Illinois at Urbana–Champaign (UIUC) for providing land for our common garden and maintaining the plantings. Thanks also to Todd Ashenbach (University of Kansas) for providing stems of S. laciniatum and Zhiwei Liu (Field Museum, Chicago) for identifying the cynipid species. We also appreciate the access to prairie sites provided by the Illinois Nature Preserves Commission, the Nature Conservancy, the Canadian National Railroad, the Urbana Park District, and Red Bison (UIUC Registered Student Organization). This work was in partial fulfillment of a Ph.D. degree for J.F.T. from UIUC and was supported in part by a Summer Research Grant from the Program in Ecology and Evolutionary Biology in the School of Integrative Biology, UIUC.
Abbreviations
ArSl, Antistrophus rufus associated with Silphium laciniatum
ArSt, A. rufus associated with Silphium terebinthinaceum
LCP, Loda Cemetery Prairie Nature Preserve
References
- 1.Turlings T. C. J., Loughrin, J. H., McCall, P. J., Rose, U. S. R., Lewis, W. J. & Tumlinson, J. H. (1995) Proc. Natl. Acad. Sci. USA 92 4169-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thaler J. S. (1999) Nature 399 686-688. [Google Scholar]
- 3.De Moraes C. M., Mescher, M. C. & Tumlinson, J. H. (2001) Nature 410 577-580. [DOI] [PubMed] [Google Scholar]
- 4.Kessler A. & Baldwin, I. (2001) Science 291 2141-2144. [DOI] [PubMed] [Google Scholar]
- 5.Dolch R. & Tscharntke, T. (1999) Oecologia 125 504-511. [DOI] [PubMed] [Google Scholar]
- 6.Karban R. & Maron, J. L. (2002) Ecology 83 1209-1213. [Google Scholar]
- 7.Hanks L. M. (1999) Annu. Rev. Entomol. 44 485-505. [DOI] [PubMed] [Google Scholar]
- 8.Ruther J., Reinecke, A. & Hilker, M. (2002) Ecol. Entomol. 27 76-83. [Google Scholar]
- 9.Gillette C. P. (1891) Bull. Ill. State Lab. Nat. Hist. 3 191-197. [Google Scholar]
- 10.Beutenmüller W. (1910) Bull. Am. Mus. Nat. Hist. 28 137-144. [Google Scholar]
- 11.Clevinger J. A. & Panero, J. L. (2000) Am. J. Bot. 87 565-572. [PubMed] [Google Scholar]
- 12.Fisher T. R. (1959) Brittonia 11 250-254. [Google Scholar]
- 13.Wagner D. & Liebherr, J. K. (1992) Trends Ecol. Evol. 7 216-220. [DOI] [PubMed] [Google Scholar]
- 14.Barbosa P., Krischik, V. & Lance, D. (1989) Am. Midl. Nat. 34 262-274. [Google Scholar]
- 15.Berlocher S. H. & Smith, D. C. (1983) J. Hered. 74 337-340. [DOI] [PubMed] [Google Scholar]
- 16.Murphy R. W., Sites, J. W., Jr., Buth, D. G. & Haufler, C. N. (1996) in Molecular Systematics, eds. Hillis, D. M., Moritz, C. & Mable, B. K. (Sinaur, Sunderland, MA), pp. 51–120.
- 17.Sokal R. R. & Rohlf, F. J., (1995) Biometry (Freeman, New York).
- 18.Sprecher E. (1963) Deutsch Apoth. Ztg. 103 213-214. [Google Scholar]
- 19.Analytical Software, (2000) STATISTIX User Manual (Analytical Software, Tallahassee, FL), Rel. 7.0.
- 20.Koenig W. A., Icheln, D., Runge, T., Pforr, I. & Krebs, A. (1990) J. High Res. Chromatogr. 13 702-707. [Google Scholar]
- 21.Nei M. (1978) Genetics 89 583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorpe J. P. (1982) Annu. Rev. Ecol. Syst. 13 139-168. [Google Scholar]
- 23.Askew R. R. (1984) in Biology of Gall Insect, ed. Ananthakrishnan, T. N. (Oxford, New Delhi), pp. 223–271.
- 24.Bronner R. (1992) in Biology of Insect-Induced Galls, eds. Shorthouse, J. D. & Rohfritsch, O. (Oxford, New York), pp. 118–140.
- 25.Abrahamson W. G. & Weis, A. E. (1987) in Nutritional Ecology of Insects, Mites, Spiders and Related Invertebrates, eds. Slansky, F., Jr. & Rodriquez, J. G. (Wiley, New York), pp. 235–258.