Abstract
The spread of exotic species and climate change are among the most serious global environmental threats. Each independently causes considerable ecological damage, yet few data are available to assess whether changing climate might facilitate invasions by favoring introduced over native species. Here, we compare our long-term record of weekly sessile marine invertebrate recruitment with interannual variation in water temperature to assess the likely effect of climate change on the success and spread of introduced species. For the three most abundant introduced species of ascidian (sea squirt), the timing of the initiation of recruitment was strongly negatively correlated with winter water temperature, indicating that invaders arrived earlier in the season in years with warmer winters. Total recruitment of introduced species during the following summer also was positively correlated with winter water temperature. In contrast, the magnitude of native ascidian recruitment was negatively correlated with winter temperature (more recruitment in colder years) and the timing of native recruitment was unaffected. In manipulative laboratory experiments, two introduced compound ascidians grew faster than a native species, but only at temperatures near the maximum observed in summer. These data suggest that the greatest effects of climate change on biotic communities may be due to changing maximum and minimum temperatures rather than annual means. By giving introduced species an earlier start, and increasing the magnitude of their growth and recruitment relative to natives, global warming may facilitate a shift to dominance by nonnative species, accelerating the homogenization of the global biota.
Predicted increases in global temperature (1) likely will have dramatic effects on the structure and function of ecosystems worldwide (2–4). One approach to predicting the biological effects of climate change has been to examine the direct physiological consequences to resident species of changes in temperature or CO2 concentration (5–8). Other studies have focused on the indirect consequences of altered temperature on interactions among resident species and the cascading effects on local community composition (9–12). Climatic warming on the time scale of decades also may alter the composition of the resident biota by facilitating the poleward spread of species characteristic of warmer temperature regimes (9, 13–15). However, it is also possible that climate change could facilitate quantum leaps in the range of species across ocean basins or continents. Humans already inadvertently transport countless species around the globe (16), and, although many of these inoculations presumably fail because of inhospitable climate in the recipient region (17, 18), global warming may relax this constraint. Despite considerable interest in predicting the spread and success of “invasive” species because of their significant effect on native communities (19), there are few data to evaluate the effects of climate change on this type of invasion (4).
Understanding the effect of shorter-term (annual) fluctuations in environmental conditions is one approach to developing better predictions regarding the ecological effects of future climate change. Since 1991, we have been monitoring the settlement and recruitment of sessile marine invertebrates at weekly intervals during the primary recruitment season in eastern Long Island Sound near Avery Point, CT. Briefly, we exposed four replicate, 100-cm2 PVC panels for consecutive, 1-week periods during the recruitment season (May–October) from 1991 to 1997 and continuously since 1997 at Avery Point, CT. The panels were suspended from a floating dock face down 1 m below the water's surface and ≈2 m above the bottom. Each week, all individuals on each panel were identified to species under a dissecting microscope and counted. Previous work demonstrated no differences in settlement of the organisms on different types of natural and artificial substrate (R.W.O. and R.B.W., unpublished data). General seasonal patterns of recruitment at this site are qualitatively similar to those at additional sites in southern New England (refs. 20 and 21 and unpublished data), suggesting that the long-term study site is representative of the region. Concurrent data for near-surface water temperature were collected ≈8 km from the sampling station at Millstone Point (22). A 3-year temperature time series from the site at which recruitment was measured showed that temperature patterns at Millstone reflect those at the study site for the period in which the two data sets overlap (R2 = 0.999, P < 0.0001).
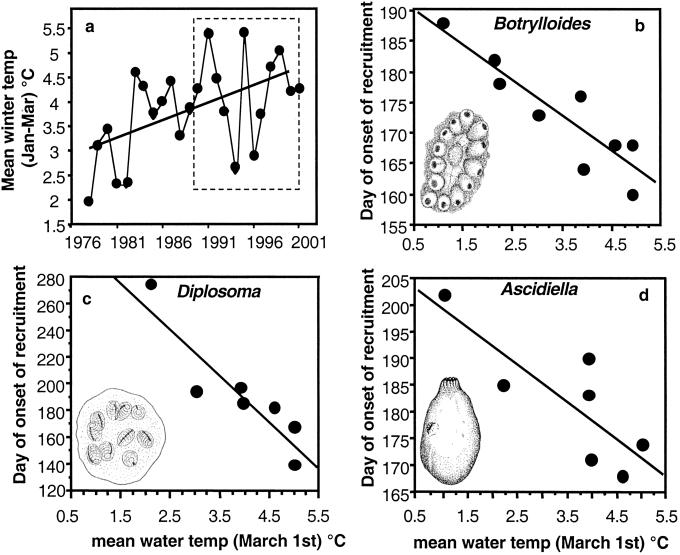
Over the last 25 years, mean annual ocean surface temperature has increased in this region, with the most pronounced increase in the mean winter water temperature (Fig. 1a). Similar patterns have been found throughout the New England region of eastern North America over the past 60 years (National Oceanic and Atmospheric Administration data; www.cpc.noaa.gov/charts.htm). This winter warming trend is expected to continue in the mid- and high latitudes of the Northern Hemisphere throughout the next century (1). Water temperatures in coastal southern New England cover a significant range, from as low as −1°C in the winter to >23°C in the summer, presenting a formidable thermal regime for many invertebrates. Moderation of winter temperatures, in particular, could facilitate the establishment and persistence of invaders from warmer temperate and even subtropical climates.
Fig. 1.
(a) Annual mean winter (January 1–March 31) water temperature (°C) for 1976–2001 at Millstone, CT. The dashed box indicates the time period for which recruitment data have been collected at Avery Point. Least-squares regression was used for curve fitting. Slope = 0.07, P < 0.01, R2 = 0.24. (b–d) March 1 water temperature is strongly negatively correlated with the timing of the onset of recruitment for three nonnative ascidians, B. violaceous, D. listerianum, and A. aspersa. The vertical axis is the number of days since the beginning of the calendar year (i.e., day 181 = July 1). For each of these nonnative species, recruitment begins earlier in years with warmer winters. Native ascidians showed no correlations between timing of onset of recruitment and interannual variation in water temperature. For analysis, see Table 1.
Interestingly, we found strong differences in the responses of native and introduced ascidians (sea squirts) to interannual variation in temperature. Over the past 12 years, the date of the initiation of recruitment of the three most abundant introduced species (the solitary Ascidiella aspersa and the colonial Botrylloides violaceous and Diplosoma listerianum) was strongly negatively correlated with early March water temperatures (Fig. 1 b– d and Table 1), meaning they recruit earlier in warmer years. Similar, but slightly weaker, correlations were found between water temperature in April and May and the timing of recruitment. In contrast, there were never significant (P ≥ 0.10), negative relationships between water temperature and the date of onset of recruitment for any of the native ascidians (Table 1). The outcome of competition among sessile invertebrates and, thus, community composition often is controlled by the order in which a species colonizes a habitat (20, 26). By giving introduced species an earlier start, rising winter temperatures have the potential to shift the community dominance to nonnatives.
Table 1.
Correlation of onset of recruitment with March 1 water temperature, 1991–2002
| Species | Mean date of onset | R2 | P | Origin | Date of first collection (ref. 23) |
|---|---|---|---|---|---|
| D. listerianum | July 10 | 0.79 | 0.002 | Bermuda | Late 1980s |
| S. Carolina (23) | |||||
| B. violaceous | June 23 | 0.72 | 0.001 | Japan (24) | Mid-1970s |
| A. aspersa | July 1 | 0.64 | 0.01 | Europe(23) | Mid-1980s |
| Ciona intestinalis | July 15 | 0.01 | 0.87 | Native (25) | — |
| Molgula manhattensis | July 4 | 0.15 | 0.29 | Native (25) | — |
| Botryllus schlosseri | June 13 | 0.27 | 0.10 | Native (25) | — |
Similar results are obtained for correlations between recruitment onset and temperature on April 1 and May 1, suggesting that springtime temperatures determine recruitment onset of invaders. Nonnative species are in bold type.
D. listerianum also has been referred to in previously published work as Diplosoma macdonaldi; however, D. listerianum is the appropriate name (G. Lambert, personal communication).
B. violaceous in New England also has been referred to as B. diegensis, but recent morphological evidence has confirmed the New England Botrylloides as B. violaceous, most likely native to Japan (see also ref. 24; G. Lambert, personal communication).
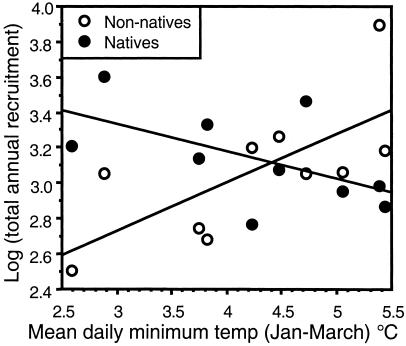
Of course, community composition also can be influenced by the relative magnitude of the recruitment of potential competitors. Total annual recruitment of introduced species was positively correlated with mean winter (January 1–March 31) temperatures (Fig. 2; slope = 0.280, P = 0.02, R2 = 0.46), whereas temperature and annual recruitment were negatively correlated for native species (Fig. 2; slope = −0.151, P = 0.08, R2 = 0.30). This suggests that rising temperature also confers a numerical advantage to introduced species. Averaged over the length of the record, native and introduced species recruitment did not differ [native: 1,700 ± 316; nonnative: 1,904 ± 651 recruits 100 cm−2⋅year−1 (mean ± 1 SE; P = 0.74, paired t test on log-transformed data)]. However, in the year after the warmest winter (1991), introduced species recruitment was two times greater than that of native species (1,537 vs. 745 recruits 100 cm−2⋅year−1). By contrast, during the recruitment season after the coldest winter (1994), native recruitment was five times greater than that of introduced species (1,634 vs. 322 recruits 100 cm−2 ⋅year−1). The difference in mean temperature between the warmest and coldest winters was only 3°C, highlighting the large biological effects of even subtle changes in seasonal mean temperature. In contrast, annual mean temperatures were never correlated with the timing or magnitude of recruitment (P > 0.15). This suggests that ecological effects of climate change may be difficult to predict because effects may be concentrated during the portions of the year when temperatures are most extreme or when species pass through critical life-history stages.
Fig. 2.
Total annual recruitment of introduced (nonnative) species is positively correlated with mean water temperature during the preceding winter (Jan 1–March 31), whereas native species recruitment is negatively correlated with winter temperatures. Total recruitment data were log-transformed to meet assumptions of ANCOVA. ANCOVA results: temperature F = 0.94, P = 0.348; native status F = 11.99, P = 0.003; temperature⋅status F = 11.57, P = 0.004. Parameter estimates of linear curve fits: Nonnatives: slope = 0.28, P = 0.007, R2 = 0.49, slope P = 0.007; natives: slope = −0.15, P = 0.108, R2 = 0.32.
Although interannual variation in summer temperatures did not affect recruitment timing or magnitude, because environmental temperature and metabolic processes often are closely correlated in invertebrates, we also examined the effects of experimentally enhanced water temperature on the growth of one native and two introduced ascidians in the laboratory. We restricted this experiment to three ecologically similar colonial ascidians to facilitate interspecific comparisons. Colonies of the native B. schlosseri and the introduced Botrylloides and Diplosoma were collected as new recruits (<10 zooids) on artificial substrates in the field and maintained in a 1-liter container of unfiltered seawater within a temperature-controlled water bath. Each day, colonies were transferred into new containers containing fresh, unfiltered seawater of the same temperature. Growth was measured as the percentage change in the number of zooids after 1 week. Not surprisingly, growth increased with temperature for all species (P < 0.001). However, the slope of the temperature vs. growth curve was steeper for the two introduced species [Botrylloides (slope ± 1 SE = 0.769 ± 0.20, R2 = 0.26) and Diplosoma (1.49 ± 0.15, R2 = 0.46)] than for the native Botryllus (slope = 0.45 ± 0.08, R2 = 0.25), resulting in a significant interaction between temperature and species in the analysis of covariance [temperature (df = 1), F = 81.7, P < 0.0001; species (df = 2), F = 11.9, P < 0.0001; temperature⋅species (df = 2), F = 16.8, P < 0.0001 (error df = 257)].
When the continuous temperature data are placed into discrete categories, it becomes clear that growth did not differ among species at moderate temperatures (<19°C), but the two introduced species had much higher growth rates than the native at the highest temperatures (Fig. 3). Specifically, Botrylloides and Diplosoma both grew faster than Botryllus at temperatures between 19 and 21°C. However, Diplosoma grew much faster than both species at 23°C (Fig. 3). Its enhanced performance at high temperature is not surprising given that Diplosoma's previous range limit on the east coast of the U.S. was South Carolina, where temperatures well in excess of 23°C are common. In most years, temperatures higher than 21°C occur for only a relatively brief period in the summer at our study sites (mean = 14 days per year, range = 0–50; data from ref. 22). However, the duration and magnitude of these warm water periods are likely to increase as a result of climatic warming (1), which could further favor introduced species. Interestingly, extreme warm temperatures producing the greatest differences in growth and recruitment between natives and nonnatives are likely to occur most frequently in shallow bays and estuaries, areas that often are also the most heavily invaded (27).
Fig. 3.
Native (Botryllus) and introduced (Botrylloides, Diplosoma) ascidians show similar growth rates at moderate temperature, but at higher temperatures the nonnative species have greater growth rates than an ecologically similar native. ANOVA results: 16.1–19.0°C: F = 0.96, P = 0.39; 19.1–21.0°C: F = 7.9, P = 0.0006; 21.1–23.3°C: F = 42.7, P < 0.0001. ANOVA was not done for 14.5–16.0°C because of poor replication for Botrylloides; unpaired t test indicated that growth of Botryllus and Diplosoma did not differ at this temperature (df = 29; P = 0.14). Different letters above each bar indicate significant differences in growth among species within that temperature range by using Tukey–Kramer post hoc tests at P < 0.05.
The interspersion of warm and cold winters throughout the years of our recruitment record (Fig. 1a) strengthens our conclusions regarding the influence of temperature variability on invasion success. If we had observed a concurrent increase in both introduced species abundance and temperature over time, causation would be more difficult to infer as confounding variables such as time since initial establishment or increases in transoceanic shipping also could be invoked as explanations (4). However, the correlation we observed between introduced species recruitment and interannual temperature variation suggests that, over longer time periods (e.g., decades), ocean warming will facilitate the establishment and spread of introduced species, particularly those from warmer (or at least less extreme) climates.
Indeed, the establishment of these introduced ascidians in New England was concurrent with increases in winter water temperatures from the 1970s to 1990s (Fig. 1 and Table 1). Propagules from these species probably have been arriving via boat traffic and other vectors for much of the 20th century, yet none established until the 1970s and 1980s (23, 27), when the current warming trend began in earnest (1). With the moderation of winter water temperature in the past few decades (Fig. 1a), invaders native to warmer or more temperate waters are increasingly likely to establish and persist in coastal New England. Given that winter warming is expected to continue in the mid- and high latitudes of the Northern Hemisphere throughout the next century (1), increased dominance by nonindigenous species and new invasions are likely. In fact, during the last 3 years, a period with particularly mild winter and warm summer water temperatures (22), a didemnid ascidian, previously unknown in the western Atlantic (G. Lambert, personal communication), has become established at our study locations. The past few decades have seen a rapid acceleration in the rate of establishment of introduced species in coastal waters (27). Coupled with enhanced global transport of species, increasing coastal ocean temperatures may provide a fuller explanation for these increasing rates of invasion by nonindigenous species.
Acknowledgments
Temperature records were supplied by James Foertch at the Millstone Environmental Laboratory, Waterford, CT. This work was supported by grants from National Science Foundation Biological Oceanography and Long Term Research in Environmental Biology (LTREB) programs and the National and Connecticut Sea Grant programs.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.IPCC (2001) in Climate Change 2001: The Scientific Basis. Technical Summary of the Working Group I Report, eds. Albritton D. L. & Meira Filho, L. G.www.ipcc.ch/pub/spm22-01.
- 2.Walther G.-M., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T. J. C., Fromentin, J.-M., Hoegh-Guldberg, O. & Bairlein, F. (2002) Nature 416 389-395. [DOI] [PubMed] [Google Scholar]
- 3.Dukes J. S. & Mooney, H. A. (1999) Trends Ecol. Evol. 14 135-139. [DOI] [PubMed] [Google Scholar]
- 4.Carlton J. T. (2001) in Invasive Species in a Changing World, eds. Mooney, H. A. & Hobbs, R. J. (Island Press, Washington, DC), pp. 31–53.
- 5.Dawson W. R. (1992) in Global Warming and Biological Diversity, eds. Peters, R. L. & Lovejoy, R. E. (Yale Univ. Press, New Haven, CT), pp. 158–170.
- 6.Gates R. D. & Edmunds, P. J. (1999) Am. Zool. 39 30-43. [Google Scholar]
- 7.Gattuso J.-P., Allemand, D. & Frankignoulle, M. (1999) Am. Zool. 39 160-183. [Google Scholar]
- 8.DeLucia E. H., Hamilton, J. G., Naidu, S. L., Thomas, R. B., Andrews, J. A., Finzi, A., Lavine, M., Matamala, R., Mohan, J. E., Hendrey, G. R. & Schlesinger, W. H. (1999) Science 284 1177-1179. [DOI] [PubMed] [Google Scholar]
- 9.Southward A. J., Hawkins, S. J. & Burrows, M. T. (1995) J. Therm. Biol. 20 127-155. [Google Scholar]
- 10.Sanford E. (1999) Science 283 2095-2097. [DOI] [PubMed] [Google Scholar]
- 11.Bertness M. D., Leonard, G. H., Levine, J. M. & Bruno, J. F. (1999) Oecologia 120 446-450. [DOI] [PubMed] [Google Scholar]
- 12.Davis A. J., Jenkinson, L. S., Lawton, J. H., Shorrocks, B. & Wood, S. (1998) Nature 391 783-786. [DOI] [PubMed] [Google Scholar]
- 13.Sagarin R. D., Barry, J. P., Gilman, S. E. & Baxter, C. H. (1999) Ecol. Monogr. 69 465-490. [Google Scholar]
- 14.McGowan J. A., Chelton, D. B. & Conversi, A. (1996) Cal. Coop. Ocean. Fish. Invest. Rep. 37 45-68. [Google Scholar]
- 15.Holbrook S. J., Schmitt, R. J. & Stephens, J. S. (1997) Ecol. Appl. 7 1299-1310. [Google Scholar]
- 16.Carlton J. T. & Geller, J. B. (1993) Science 261 78-82. [DOI] [PubMed] [Google Scholar]
- 17.Williamson M., (1996) Biological Invasions (Chapman & Hall, London).
- 18.Chapman, J. W. (2000) Marine Bioinvasions: Proc. First Natl. Conf., 66–80.
- 19.Grosholz E. D. (2002) Trends Ecol. Evol. 17 22-27. [Google Scholar]
- 20.Osman R. W. (1977) Ecol. Monogr. 47 37-63. [Google Scholar]
- 21.Osman R. W., Whitlatch, R. B. & Malatesta, R. J. (1992) Mar. Ecol. Prog. Ser. 83 35-43. [Google Scholar]
- 22.Northeast Utilities Environmental Laboratory, (2001) Monitoring the Marine Environment of Long Island Sound at Millstone Nuclear Power Station (Northeast Utilities System, Waterford, CT).
- 23.Steneck R. S. & Carlton, J. T. (2001) in Marine Community Ecology, eds. Bertness, M. D., Hay, M. E. & Gaines, S. D. (Sinauer, Sunderland, MA), pp. 445–468.
- 24.Saito Y., Mukai, H. & Watanabe, H. (1981) Oka. Publ. Seto Mar. Biol. Lab. 26 357-368. [Google Scholar]
- 25.Van Name W. G. (1945) Bull. Am. Mus. Nat. Hist. 84 1-476. [Google Scholar]
- 26.Sutherland J. P. & Karlson, R. H. (1977) Ecol. Monogr. 47 425-446. [Google Scholar]
- 27.Ruiz G. M., Fofonoff, P. W., Carlton, J. T., Wonham, M. J. & Hines, A. H. (2000) Annu. Rev. Ecol. Syst. 31 481-531. [Google Scholar]