Abstract
Using selectable genes as proof of principle, a new high-throughput genotype-based mutation screen in mouse embryonic stem (ES) cells was developed [Chen et al. (2002) Nat. Genet. 24, 314–317]. If expanded to nonselectable genes, this approach would allow one to proceed quickly from sequence to whole-animal phenotypes. Here data are presented showing that a screen of a cryopreserved library of clonal, germ line competent, N-ethyl-N-nitrosurea (ENU) mutagenized ES cells can identify a large series of allelic mutations in Smad2 and Smad4, two nonselectable genes of the transforming growth factor β superfamily of signaling molecules. Whole animal phenotypic analyses of some of these alleles provided evidence for novel developmental processes mediated by these components of transforming growth factor β signaling, demonstrating the utility of non-null alleles created by chemical mutagens. The accurately assessed mutation load of the ES cell library indicates that it is a valuable resource for developing mouse lines for genetic and functional studies. This methodology can conceptually be applied for the generation of an allelic series of subtle mutations at any locus of interest in the mouse.
Allelic series of mutations in the mouse produced by chemical mutagens such as N-ethyl-N-nitrosourea (ENU) are valuable reagents to reveal a full spectrum of gene functions in vivo. Non-null alleles are useful in particular, as they allow for fine-tuned analysis of later functions of genes whose null allele results in lethality and for investigation of protein domains through isolated disruption of specific amino acid residues. The efficiency of ENU as a mutagen has made it the mutagen of choice in whole animal phenotype-based screens (1). Recently, experiments have shown that mutations in specific genes of interest can be obtained by screening DNA derived from mutagenized mice and reconstituting the mouse line from cryopreserved sperm (2). However, the large number of mice required to identify multiple mutations in any given gene with whole-animal mutagenesis may be prohibitive, thus more efficient gene-based methods will be needed to produce large numbers of subtle mutations in the mouse. We and others have shown that mouse embryonic stem (ES) cells can be efficiently mutagenized with chemical mutagens at several selectable loci, while retaining their ability to populate the germ line (3, 4). If this methodology could be extended to identify ES cells with mutations in specific genes of interest, one could then quickly proceed from gene sequence to developing a large allelic series of mutations in the mouse.
Smad2 and Smad4 are vertebrate members of the Smad family of intracellular transducers of transforming growth factor β (TGF-β) superfamily signaling (5). Current models posit Smad2 to be a receptor-regulated-Smad activated via phosphorylation in response to ACTIVIN, TGF-β, and NODAL signaling. Smad4 is the only known mammalian common-mediator Smad that interacts with receptor-regulated Smads after their activation. This Smad complex then translocates to the nucleus to effect transcription of target genes through direct interaction with a variety of other transcriptional regulators. The modulation of TGF-β signaling pathway by other signaling pathways, such as the mitogen-activated protein kinase pathways, is in part mediated by phosphorylation sites within the linker domain of receptor-regulated Smads (6, 7). Targeted disruption of either the murine Smad2 or Smad4 genes results in a peri-gastrulation lethality (8). These mutants have uncovered functions of these factors in a variety of developmental processes, including epiblast profileration, mesoderm formation, and extraembryonic tissue-mediated early embryonic patterning events (8). However, the requirements for these factors in later embryonic development and adult life remain largely unknown. Hypomorphic alleles that bypass the early embryonic requirement for Smad2 and Smad4 would be useful tools to analyze the functions of these factors in later life. Additionally, the importance of the various protein interactions and posttranslational modification sites in SMAD2 and SMAD4 can be analyzed in vivo with point mutations that specifically disrupt these protein domains.
We describe here the isolation of a large allelic series of mutations in Smad2 and Smad4 by combining ENU mutagenesis of mouse ES cells with high throughput mutation detection technology. Phenotypic analyses of some of these mutations in the mouse, including a hypomorphic allele of Smad2 and a splice mutant allele of Smad4, revealed previously undescribed functions of these factors. These allelic series of mutations in Smad2 and Smad4 will serve as useful genetic tools for studying biological processes mediated by Smad2 and Smad4. The methodology can be applied to most genes to develop an allelic series of mutations in the mouse, facilitating the functional annotation of the mammalian genome.
Materials and Methods
ES Cell Culture and Mutagenesis.
CT129 ES cells (9) were cultured as described. The cells were grown for two passages without feeders before ENU treatment. After the second passage onto gelatin-coated plates, the cells were incubated with 0.2 mg/ml ENU (Sigma) in culture medium for 2 h. The cells were then washed, trypsinised, and plated at the appropriate density to allow formation of individual colonies. Colonies were picked into gelatin-coated 96-well plates, grown, and split in duplicate. A total of 2,060 ES cells were picked from three separate mutagenesis experiments. One plate was cryopreserved as a mutagenized cell bank; the other plate was used to prepare total RNA and genomic DNA (Qiagen, Valencia, CA) for mutation detection.
Mutation Detection.
Total RNA from ENU-mutagenized ES cell clones was isolated for use in an RT-PCR-based amplification of the coding regions of mouse Smad2 and Smad4. Oligonucleotides specific to Smad2 or Smad4 were used to amplify overlapping amplicons to cover the entire coding regions of these genes (Table 4, which is published as supporting information on the PNAS web site, www.pnas.org). PCR products were denatured and annealed immediately after PCR in the thermocycler. Denaturing high performance liquid chromatography (DHPLC)-based heteroduplex analysis of the PCR products was performed by the WAVE fragment analysis system (Transgenomic, Omaha, NE) using UV absorbance for detection. Optimal DHPLC conditions for each amplicon were identified by analysis of point mutations created by in vitro mutagenesis. ES cell clones harboring candidate mutations were thawed and expanded, and RNA was isolated for RT-PCR. Presence of the mutation was confirmed by heteroduplex analysis as above. The base change of each mutation was identified by direct sequencing of the PCR product.
Generation of Mice.
ES cell lines harboring identified point mutations were thawed from their multiwell array and expanded. To assess whether each line was clonal, a subcloning procedure was performed. In brief, each ES cell line was grown and trypsinized, and single ES cells were picked by mouth pipette using a microscope and seeded onto feeders. After 7 days, 40 single cell colonies on these plates were picked into a multiwell plate and subsequently split for cryopreservation and RNA isolation. RNA was used for RT-PCR amplification and WAVE analysis as described. If all ES cell subclones of a given line were identified as carrying the point mutation, then the nonsubcloned parental line was used for injection; otherwise pools of three single-cell subclones carrying the point mutation were used for injection into blastocysts.
Blastocyst injections for production of chimeric mice were performed by standard procedures. Chimeras were mated to wild-type C57BL/6 or Black Swiss mice for testing germ-line transmission. Germ-line transmission was assessed by coat color and by genotyping with genomic DNA from tail biopsy. Genotyping was performed by heteroduplex analysis of PCR products amplifying the genomic region spanning the point mutation. Mouse stocks were maintained in the heterozygous state by crossing heterozygous mice to wild-type mice of the desired background for multiple generations.
Mouse Breeding.
To obtain mice carrying an ENU-induced point mutation within Smad2 or Smad4 over a targeted allele, mice heterozygous for a point mutation were crossed to heterozygous mice for targeted alleles of Smad2 (10) or Smad4 (11). Pups or embryos were genotyped by using tail- or yolk sac-derived DNA, using PCR-based methods to detect the targeted alleles as described, and heteroduplex formation to detect the presence of the point mutations (Table 5, which is published as supporting information on the PNAS web site). Intercrosses of mice heterozygous for a point mutation were performed to obtain mice homozygous for that mutation. Genotyping was accomplished by heteroduplex analysis. Homozygous wild-type and homozygous mutant PCR samples were distinguished by mixing an equimolar amount of wild-type control PCR product, annealed and subjected to heteroduplex detection.
Histology and Immunochemistry.
Embryos were fixed overnight in 4% paraformaldehyde, then washed in PBS. Platelet-endothelial cell adhesion molecule (PECAM) was detected in whole embryos with an anti-PECAM antibody (PharMingen) as described (12). Sectioning was performed by paraffin embedding and staining with nuclear fast red or hematoxylin and eosin.
Western Blotting.
The ES cells were lysed on ice for 30 min in buffer containing 50 mM Tris (pH 7.4), 0.5% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, and protease inhibitors (Roche). The lysate was cleared of debris by centrifugation. The total protein concentration was measured by using the Coomassie Plus Protein Assay Reagent (Pierce). Equal amounts of protein were separated on a SDS/10% PAGE gel and transferred to poly(vinylidene difluoride) membranes (Millipore). The SMAD4 protein was detected by using the B-8 monoclonal antibody (Santa Cruz Biotechnology). A polyclonal anti-ACTIN antibody (I-19, Santa Cruz Biotechnology) was used as a control for equal loading.
Results
Identification of ENU-Induced Smad2 and Smad4 Mutations.
To screen for ES cells with mutations in specific genes of interest, a cryopreserved library of mutagenized ES colonies was developed by using a slightly modified method of ENU treatment from what we described (3). A total of 2,060 mutagenized ES cell clones were cryopreserved from three separate experiments in a 96-well format to facilitate subsequent processing procedures. Total RNA and genomic DNA were isolated from individual mutagenized lines. Because both Smad2 and Smad4 are expressed in ES cells, RT-PCR was performed to amplify overlapping segments covering the entire coding regions of both genes. When a denaturing high performance liquid chromatography-based heteroduplex detection scheme was used (13), a total of 29 ES cell clones were identified with mutations in Smad2 or Smad4 (Table 1). All mutations were single nucleotide substitutions, which is consistent with the known mutagenic properties of ENU (14). Eighteen mutations (62%) resulted in missense alterations of the protein. Two mutations affected splicing through alterations in intronic sequences at the splice junctions (15). The remaining mutations (31%) were silent. The mutation frequencies of Smad2 and Smad4 were remarkably similar with an overall mutation rate of 1 in 464 kb and an average nonsilent mutation rate of 1 in 673 kb (Table 2).
Table 1.
Summary of ENU-induced mutations in Smad2 and Smad4
| Gene | Allele | ES cell clone | Nucleotide change | Codon change | Exon | Amino acid change | Region of protein |
|---|---|---|---|---|---|---|---|
| Smad2 | LM1-2G2 | T523A | CTG to CAG | 4 | L155Q | MH1 turn 5 | |
| LM2-2A6 | A1411G | AAA to AGA | 11 | K451R | MH2 domain, H5 | ||
| LM2-2E8 | G1205T | CTG to CTT | 10 | Silent L382 | |||
| LM2-3B1 | T781C | ATG to ACG | 6 | M241T | Linker, CaM kinase II phosphorylation motif (30) | ||
| m1Mag | LM2-3F6 | C886T | TCA to TTA | 8 | S276L | MH2, B1 sheet | |
| LM2-4A4 | A719G | ACA to ACG | 6 | Silent T220 | |||
| LM2-4H1 | C183G | CAA to GAA | 2 | Q41E | MH1 helix 2, calmodulin binding domain (31) | ||
| LM2-7D9 | T792G | TCT to GCT | 7 | S245A | Linker, putative proline-directed kinase site (7) | ||
| LM2-8C9 | A1059G | AGG to GGG | 8 | R334G | MH2, B5 sheet | ||
| LM3-1D6 | T818C | ACT to ACC | 7 | Silent T253 | |||
| LM3-2H10 | T1129A | ATC to AAC | 9 | I355N | MH2, between B6 and B7 sheets | ||
| LM3-5A6 | SA −7 T→G | NA | Int 2 | RN insertion after P78 | MH1, adjacent to SMAD2 unique region | ||
| LM3-5E7 | C718G | ACA to AGA | 6 | T220K | Linker, putative ERK phosphorylation site (7) | ||
| Smad4 | |||||||
| m3Mag | LM1-1H11 | G1467A | GGC to AGC | 9 | G383S | MH2, B5 sheet | |
| LM1-1H2 | T1228C | CAT to CAC | 7 | Silent H316 | |||
| LM1-2H8 | C812T | GAC to GAT | 4 | Silent D164 | |||
| LM2-5B6 | T1945A | ATG to AAG | 11 | M542K | MH2, H5 | ||
| LM2-9F5 | C1529T | GAC to GAT | 9 | Silent D403 | |||
| LM3-10D7 | C340T | ACA to ATA | 1 | T7I | Just N′ to MH1 | ||
| LM3-10E2 | A1588G | GAC to GGC | 9 | D423G | MH2, between H2 and B8 | ||
| m4Mag | LM3-1A5 | SD +1 G→A | Exon 10 deletion | Int 10 | aa436+19 out of frame aa | Deletion of QA insert and the rest of MH2 | |
| m1Mag | LM3-1A6 | T362A | GAT to GAA | 1 | D14E | Just N′ to MH1 | |
| LM3-1G1 | C1607T | TAC to TAT | 9 | Silent Y429 | |||
| LM3-2E3 | C1920T | CTC to TTC | 11 | L534F | MH2, H5 | ||
| LM3-3D1 | C1222T | TAC to TAT | 6 | Silent Y300 | |||
| LM3-3F5 | C346A | ACA to AAA | 1 | T9K | Just N′ to MH1 | ||
| m2Mag | LM3-4C12 | A858G | ACC to GCC | 4 | T180A | Linker, high homology with Medea (32) | |
| LM3-7C5 | C803T | TAC to TAT | 4 | Silent Y161 | |||
| LM3-7D6 | C1215T | CAT to TAT | 6 | H299Y | Linker, p300 binding, transactivation domain (33) |
Table 2.
Mutation frequencies at Smad2 and Smad4 loci
| Total base pairs screened, Mb | No. of mutations identified | ES cell clone mutation frequency | Nonsilent ES cell mutation frequency | Per-base mutation frequency, kb | Nonsilent per-base mutation frequency, kb | |
|---|---|---|---|---|---|---|
| Smad2 | 6.06 | 13 | 1 in 158 | 1 in 206 | 1 in 466 | 1 in 606 |
| Smad4 | 7.40 | 16 | 1 in 129 | 1 in 206 | 1 in 463 | 1 in 740 |
| Overall | 13.46 | 29 | 1 in 143 | 1 in 206 | 1 in 464 | 1 in 673 |
Thirteen karyotypically normal cell lines carrying nonsilent mutations were expanded for injection into blastocysts to create chimeric mice for germ-line transmission of the ENU-generated mutations. Consistent with our earlier work (3), mutagenized ES cells readily made germ line competent chimeras. Five mutations have been passed through the germ line. Complementation tests were performed by crossing each mutation against a corresponding targeted mutation in addition to crossing each mutation to homozygosity. A range of phenotypes was observed in mice derived from these crosses.
Smad2m1Mag Is a Hypomorphic Allele.
One ENU-induced allele, Smad2m1Mag, changed a serine to leucine in amino acid 276 within the edge of the Mad homology 2 domain. This serine residue is conserved amongst all vertebrate and most invertebrate receptor-regulated Smads, suggesting an important structural function of this precise region of the protein. However, no protein interactions have been localized to this region of SMAD2, nor has there been any report of serine-276 to be phosphorylated. Early embryonic defects were observed when the Smad2m1Mag allele was placed against a targeted allele of Smad2, Smad2Robm1 (10). Although Smad2+/Smad2m1Mag littermates were normal at 11.5 days of gestation (E11.5, Fig. 1A), a highly developed yolk sac devoid of any embryonic portion was observed in Smad2m1Mag/Smad2Robm1 heterozygotes (Table 3, Fig. 1B). Yolk sac blood islands were apparent, consistent with extraembryonic mesoderm formation. These phenotypes closely resemble those observed in homozygous Smad2Robm1 embryos and indicate a substantial loss of function associated with the Smad2m1Mag allele.
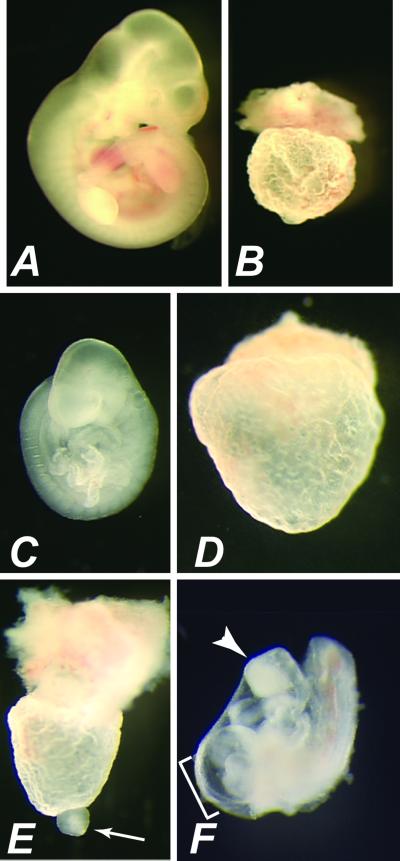
Fig 1.
Severe embryonic defects associated with Smad2m1Mag mutation. (A and B) Gross morphology of wild-type (A) and Smad2m1Mag/Smad2Robm1 (B) concepti at E11.5. Smad2m1Mag/Smad2Robm1 concepti consist of an empty yolk sac devoid of an embryo proper. (C) Wild-type embryo at E9.5. (D–F) Three classes of Smad2m1Mag/Smad2m1Mag embryos at E9.5. (D) The empty yolk sac closely resembles the phenotype observed in Smad2m1Mag/Smad2Robm1 (B). (E) A mass of tissue at the distal tip (arrow) is observed in another class of Smad2m1Mag/Smad2m1Mag embryo. This mass of tissue is contractile, indicating the presence of some cardiac muscle differentiation. (F) The most advanced class of Smad2m1Mag/Smad2m1Mag embryo exhibits many embryonic structures. Note the expanded pericardial cavity (bracket) and truncated anterior neurectoderm (arrowhead).
Table 3.
Complementation tests with targeted alleles (KO)
| ENU allele | +/+ | ENU/+ | +/KO | ENU/KO | Total |
|---|---|---|---|---|---|
| Smad2m1Mag | 14 | 6 | 8 | 0 (4) | 32 |
| Smad4m1Mag | 14 | 15 | 6 | 6 | 41 |
| Smad4m2Mag | 22 | 20 | 19 | 21 | 82 |
| Smad4m3Mag | 14 | 18 | 19 | 18 | 69 |
| Smad4m4Mag | 14 | 16 | 17 | 0 | 47 |
Smad2 analysis was performed at E9.5 to E11.5. Smad4 analysis was performed at postnatal stages.
All Smad2m1Mag/Smad2Robm1 concepti consisted of an empty yolk sac.
At E9.5 (Fig. 1 C–F), some homozygous Smad2m1Mag embryos consisted of an empty yolk sac, resembling Smad2m1Mag/Smad2Robm1 and Smad2Robm1/Smad2Robm1 embryos (54%, n = 34, Fig. 1D), though occasionally a malformed embryonic structure was present at the distal portion of the yolk sac (Fig. 1E). However, many Smad2m1Mag homozygous embryos (46%) exhibited considerably more advanced development. These embryos displayed obvious epiblast-derived structures, including a heart, tail bud, allantois, and somites (Fig. 1F). The allantois of these embryos failed to fuse to the chorion, resulting in allantoic tissue protruding from the posterior of the embryo (not shown). Severe anterior patterning defects were also observed in these Smad2m1Mag homozygous embryos. Anterior structures consisted of a truncated mass often with an unfolded neural plate, and the optic placode was not apparent. Although anterior midline tissues were morphologically apparent in wild-type embryos at E8.5 (Fig. 2 A and C), the absence of an anterior notochord and a small or absent foregut were observed in Smad2m1Mag homozygotes (Fig. 2 B and D). The advanced embryonic development of Smad2m1Mag/Smad2m1Mag embryos compared with that of the targeted alleles of Smad2, including the ability to form many embryonic tissues, strongly suggested that Smad2m1Mag is a hypomorph.
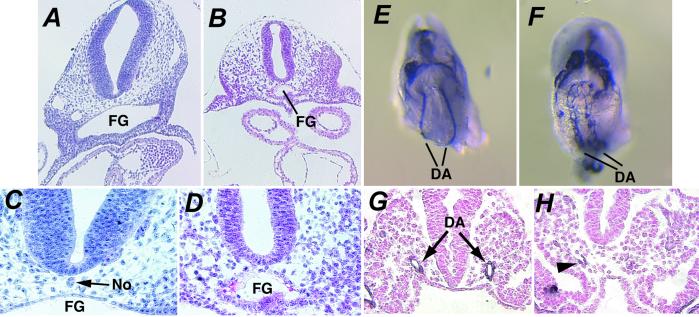
Fig 2.
Defects in the formation of the dorsal aorta, foregut, and notochord in Smad2m1Mag/Smad2m1Mag embryos. (A–D) Hematoxylin and eosin staining of transverse histological sections of wild-type (A and C) and Smad2m1Mag/Smad2m1Mag (B and D) embryos at E9.5 about the level of the heart at ×10 (A and B) and ×20 (C and D) objective magnification. Smad2m1Mag/Smad2m1Mag embryos display a small foregut (FG) compared with wild type and lack a morphologically obvious notochord (No). (E and F) Ventral view of the dorsal aortae (DA) at the region of the opening of the foregut of intact wild-type (E) and Smad2m1Mag/Smad2m1Mag (F) embryos at E8.5 visualized with an anti-PECAM antibody. Significant endothelial differentiation is observed in mutant embryos but the aorta fails to condense fully. (G and H) Transverse sections of PECAM-stained embryos at the level of the foregut entrance of wild-type (G) and Smad2m1Mag/Smad2m1Mag (H) embryos at E8.5. The cells of the dorsal aortae display strong PECAM expression about the aortic lumen in wild-type embryos. Although PECAM-positive endothelial cells are present in mutant embryos (H, arrowhead), cells fail to form an aortic lumen in this region of the embryo.
Vascular Defects in Smad2m1Mag Mutant Embryos.
Smad2m1Mag homozygous embryos with fused heart tubes displayed an expanded pericardial cavity (Fig. 1F) and erratic cardiac contractility, suggesting that cardiovascular defects may cause the embryonic lethality observed in these embryos after E9.5. Because vascular defects have been observed in several mutants of other TGF-β signaling components (16), the early embryonic vasculature was visualized with an anti-PECAM antibody. Although endothelial cells were present in both wild-type and mutant embryos (Fig. 2 E and F), the dorsal aorta in these Smad2m1Mag homozygotes was either highly constricted or lacked an aortic lumen present at this time in wild-type embryos (Fig. 2 G and H) and was generally more severe in the anterior of the embryo. The defects observed in Smad2m1Mag homozygous embryos confirmed the requirement for Smad2 in the proper formation of the anterior neurectoderm and foregut and demonstrate a function for Smad2 in chorioallantoic fusion and vascular development.
Viability of Several ENU-Induced Smad4 Alleles.
Three of the four ENU-induced Smad4 alleles (Smad4m1Mag, Smad4m2Mag, and Smad4m3Mag) resulted in missense substitutions of conserved amino acid residues (Table 1). Surprisingly, animals carrying each of these mutations survived to adulthood either in combination with the targeted Smad4tm1Cxd allele (17) or in the homozygous state (Table 3), indicating that these missense substitutions do not significantly perturb the functions of the SMAD4 protein during embryogenesis. To date, these animals have been aged for at least 5 months, and no overt phenotypes have been observed either in the compound heterozygotes or homozygotes.
Smad4m4Mag Is a Splice Mutant Allele.
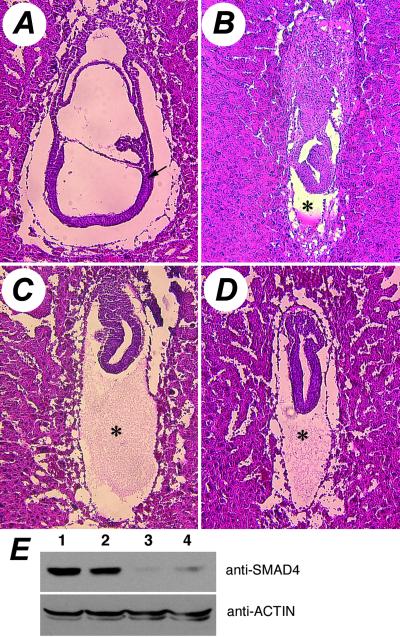
The fourth Smad4 allele, Smad4m4Mag, altered a conserved nucleotide in the splice donor of the intron 10, causing the deletion of exon 10. This aberrant transcript is predicted to produce a truncated protein with an additional 19 out-of-frame amino acids after amino acid 436 in the highly conserved Mad homology 2 domain. Consistent with the drastic alteration in the gene product, mice that are compound heterozygous for the Smad4m4Mag allele and the targeted allele Smad4tm1Cxd did not survive embryogenesis (Table 3). Compared with the wild-type E7.5 embryo (Fig. 3A), these animals were severely growth-retarded, failed to initiate gastrulation and consequently did not form mesoderm (Fig. 3C). Animals homozygous for Smad4m4Mag displayed similar phenotypes (Fig. 3D) and closely resembled those of the Smad4tm1Cxd homozygotes (Fig. 3B), indicating a severe loss of function associated with the Smad4m4Mag allele. However, examination of the steady-state SMAD4 protein levels in Smad4m4Mag heterozygous ES cells revealed that Smad4m4Mag is not a conventional null allele. Wild-type SMAD4 protein levels in the Smad4m4Mag heterozygous ES cell lines were dramatically lower than the expected level of 50% (Fig. 3E). Additionally, no truncated mutant SMAD4 protein, predicted to be 51 kDa, was detected. Similar reduction of the wild-type SMAD4 protein level as well as the absence of the truncated protein was also seen in primary embryonic fibroblasts of Smad4m4Mag heterozygotes (data not shown). The reduction of wild-type protein indicates that not only is the truncated mutant protein unstable, it possibly confers instability to the wild-type protein as well in heterozygous cells, suggesting a dominant negative property of the Smad4m4Mag allele.
Fig 3.
Defects associated with the Smad4m4Mag allele. Hematoxylin and eosin-stained sagittal sections of E7.5 embryos are shown. (A) Wild-type gastrulating embryo showing newly formed mesoderm (arrow) and other well patterned structures. (B) Homozygous Smad4tm1Cxd embryo. (C) Smad4m4Mag/Smad4tm1Cxd embryo. (D) Homozygous Smad4m4Mag embryo. (B–D) Compared with the wild-type E7.5 embryo (A), these mutant embryos are much smaller, with no sign of the initiation of gastrulation or the formation of mesoderm. Also note the disproportionally large cavity between the visceral and parietal endoderm (asterisks). (E) Steady-state level of SMAD4 protein in unmutagenized wild-type ES cells (lane 1) and three mutagenized sibling subclones (lane 2, wild-type for Smad4; lanes 3 and 4, heterozygous for Smad4m4Mag). The same blot was stripped and reprobed with an anti-ACTIN antibody to show equal loading of total cellular proteins.
Discussion
Our results demonstrate that combining chemical mutagenesis in mouse embryonic stem cells with high throughput mutation detection allows for the rapid identification of subtle mutations in nonselectible genes of interest. A variety of alleles can be quickly obtained in a modest sized genotype-based screen. Thus far we have obtained three viable alleles (Smad4m1Mag, Smad4m2Mag, Smad4m3Mag), a hypomorphic allele (Smad2m1Mag), and an allele (Smad4m4Mag) that resembles the null allele in the homozygous state but acts molecularly as a dominant negative in the heterozygous state.
Assessment of Mutation Load in Genotype-Based Screens.
Although predictions of mutation load have been attempted from whole animal phenotype-based screens (18), only with the recent introduction of genotype-based screens in the mouse has a per-base assessment of mutation load been possible (2, 18). However, highly differing numbers have been reported because of differences in sample size, the mutagen dose and the sensitivity of the mutation identification methods. The large sample size of our screen and consistent mutation frequencies between two independent loci indicate an accurate assessment of mutation load in our cryopreserved bank. With a mutation frequency of 1 in 464 kb, the average distribution of nonsilent coding sequence variations in this ES cell library is ≈1 per 13 centimorgans (cM), assuming a 1,600-cM, 2.7 Gb haploid mouse genome of which 3% is coding. Thus, the ES cell library is a useful reagent for developing mouse lines carrying mutations at specific loci. It is worth noting that a similar mutation frequency of 1 sequence variation per 482 kb was observed in a genotype-based screen in zebrafish exposed to a dose of ENU typically used in phenotype-driven screens (19). If desired, mutagenesis procedures can be readily altered in cell culture to lower mutation load, taking advantage of the ability to monitor the mutation load of a cryopreserved library in culture with selectable loci. Phenotypic analysis of mutations obtained in any chemical mutagenesis would be best analyzed by using compound heterozygotes, possibly by using alleles with similar phenotypic severity, which requires multiple mutant alleles of a gene. The high throughput nature of the ES-cell-based genotypic screen allows for the rapid development of such allelic series of mutations.
Smad2m1Mag Is a Tool to Analyze Smad2 Function in Embryonic Development.
The Smad2m1Mag allele demonstrates the utility of subtle mutations for uncovering previously uncharacterized gene function. Owing to the advanced development of some of these mutants, the ENU induced Smad2m1Mag allele suggests requirements for Smad2 not revealed in any Smad2 targeted mutation, including functions in chorioallantoic fusion and cardiovascular development. Additionally, this allele confirms functions for Smad2 in anterior development and endoderm formation (20, 21). The less severe class of Smad2m1Mag homozygous embryos is likely due to a partial or full rescue of the requirement of Smad2 in extraembryonic tissues to pattern the epiblast (10), suggesting that the mouse embryo must be highly sensitive to threshold levels of TGF-β-related signaling in extraembryonic tissues to mediate early embryo patterning. The distinct phenotypic classes of defects observed in Smad2m1Mag homozygotes resemble those observed in a hypomorphic allele of nodal, confirming the requirement for Smad2 in mediating NODAL signaling in a variety of embryonic stages (20).
Mutations in several other components of TGF-β signaling result in defects in vascular development, including Alk1, Smad5, and TGF-β1, which have similar phenotypes, including an enlarged dorsal aorta (16). The failure of normal dorsal aorta formation in Smad2m1Mag homozygotes appears phenotypically different from these mutations, suggesting that the Smad2m1Mag allele has uncovered potentially novel aspects of TGF-β-related signaling in vascular development. One intriguing possibility is that the notochord or foregut defects observed in Smad2m1Mag homozygotes underlie the vascular defects in these embryos. The failure of endoderm formation in Smad2m1Mag homozygotes is consistent with previous data showing that Smad2-deficient cells do not efficiently contribute to the definitive endoderm lineage (21). The Smad2m1Mag allele will thus provide a useful tool to analyze Smad2-mediated endoderm development, as well as the functional consequences of loss of endoderm-derived tissues in the early embryo.
SMAD4 Truncation Mutations and Dominant Negative Activities.
Several studies have shown that C-terminal truncations of the SMAD4 protein display dominant negative properties (22, 23) or are highly unstable and rapidly degraded via the ubiquitin-proteasome pathway (24). Although the Smad4m4Mag splice mutation deletes the C-terminal helix bundle thought to be important for homo-oligomerization (25), its intact Mad homology 1 domain, the linker region, and the remaining Mad homology 2 domain may be sufficient for oligomerization with the wild-type SMAD4 protein. Similar C-terminal truncations of SMAD4 protein have been shown to retain their ability to interact with the full-length wild-type protein (26). As cellular SMAD4 is found mostly in a homo-oligomer state (27), the decreased wild-type SMAD4 protein in the Smad4m4Mag heterozygous cells could be due to the association of mutant protein with the wild-type protein, which is then targeted for degradation. It is remarkable that the heterozygous ES cells and animals are phenotypically normal despite the low levels of SMAD4 protein, suggesting a low threshold level of SMAD4 protein required for normal development and function in the cell and tissue types examined. The nonallelic, noncomplementation of some of the receptor-regulated Smads (28) underscores the dosage sensitive aspect of the TGF-β-related signaling. The apparent dominant negative property of the Smad4m4Mag allele and hypomorphic nature of the Smad2m1Mag allele will provide unique sensitized backgrounds both in vitro and in vivo for studying the importance of these factors in various biological processes.
Supplementary Material
Acknowledgments
We thank E. Robertson and X. Deng for providing Smad2 and Smad4 targeted mice, respectively. This work was supported by National Institutes of Health postdoctoral fellowships (to J.L.V. and Y.C.), an American Heart Association postdoctoral fellowship (to J.L.V.), and a National Institutes of Health grant (to T.M.).
Abbreviations
ENU, N-ethyl-N-nitrosourea
TGF-β, transforming growth factor β
ES, embryonic stem
PECAM, platelet-endothelial cell adhesion molecule
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Russell W. L., Kelly, E. M., Hunsicker, P. R., Bangham, J. W., Maddux, S. C. & Phipps, E. L. (1979) Proc. Natl. Acad. Sci. USA 76 5818-5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coghill E. L., Hugill, A., Parkinson, N., Davison, C., Glenister, P., Clements, S., Hunter, J., Cox, R. D. & Brown, S. D. (2002) Nat. Genet. 30 255-256. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y., Yee, D., Dains, K., Chatterjee, A., Cavalcoli, J., Schneider, E., Om, J., Woychik, R. P. & Magnuson, T. (2000) Nat. Genet. 24 314-317. [DOI] [PubMed] [Google Scholar]
- 4.Munroe R. J., Bergstrom, R. A., Zheng, Q. Y., Smith, R., John, S. W., Schimenti, K. J., Libby, B. J., Browning, V. L. & Schimenti, J. C. (2000) Nat. Genet. 24 318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attisano L. & Tuen Lee-Hoeflich, S. (2001) Genome Biol. 2 3010.1-3010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kretzschmar M., Liu, F., Hata, A., Doody, J. & Massagué, J. (1997) Genes Dev. 11 984-995. [DOI] [PubMed] [Google Scholar]
- 7.Kretzschmar M., Doody, J., Timokhina, I. & Massague, J. (1999) Genes Dev. 13 804-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinstein M., Yang, X. & Deng, C. (2000) Cytokine Growth Factor Rev. 11 49-58. [DOI] [PubMed] [Google Scholar]
- 9.Threadgill D. W., Yee, D., Matin, A., Nadeau, J. H. & Magnuson, T. (1997) Mamm. Genome 8 390-393. [DOI] [PubMed] [Google Scholar]
- 10.Waldrip W. R., Bikoff, E. K., Hoodless, P. A., Wrana, J. L. & Robertson, E. J. (1998) Cell 92 797-808. [DOI] [PubMed] [Google Scholar]
- 11.Sirard C., de la Pompa, J. L., Elia, A., Itie, A., Mirtsos, C., Cheung, A., Hahn, S., Wakeham, A., Schwartz, L., Kern, S. E., et al. (1998) Genes Dev. 12 107-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlaeger T. M., Qin, Y., Fujiwara, Y., Magram, J. & Sato, T. N. (1995) Development (Cambridge, U.K.) 121 1089-1098. [DOI] [PubMed] [Google Scholar]
- 13.Xiao W. & Oefner, P. J. (2001) Hum. Mutat. 17 439-474. [DOI] [PubMed] [Google Scholar]
- 14.Skopek T. R., Walker, V. E., Cochrane, J. E., Craft, T. R. & Cariello, N. F. (1992) Proc. Natl. Acad. Sci. USA 89 7866-7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burset M., Seledtsov, I. A. & Solovyev, V. V. (2001) Nucleic Acids Res. 29 255-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zwijsen A., van Grunsven, L. A., Bosman, E. A., Collart, C., Nelles, L., Umans, L., Van de Putte, T., Wuytens, G., Huylebroeck, D. & Verschueren, K. (2001) Mol. Cell. Endocrinol. 180 13-24. [DOI] [PubMed] [Google Scholar]
- 17.Yang X., Li, C., Xu, X. & Deng, C. (1998) Proc. Natl. Acad. Sci. USA 95 3667-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beier D. R. (2000) Mamm. Genome 11 594-597. [DOI] [PubMed] [Google Scholar]
- 19.Wienholds E., Schulte-Merker, S., Walderich, B. & Plasterk, R. H. (2002) Science 297 99-102. [DOI] [PubMed] [Google Scholar]
- 20.Nomura M. & Li, E. (1998) Nature 393 786-790. [DOI] [PubMed] [Google Scholar]
- 21.Tremblay K. D., Hoodless, P. A., Bikoff, E. K. & Robertson, E. J. (2000) Development (Cambridge, U.K.) 127 3079-3090. [DOI] [PubMed] [Google Scholar]
- 22.Lagna G., Hata, A., Hemmati-Brivanlou, A. & Massague, J. (1996) Nature 383 832-836. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., Musci, T. & Derynck, R. (1997) Curr. Biol. 7 270-276. [DOI] [PubMed] [Google Scholar]
- 24.Maurice D., Pierreux, C. E., Howell, M., Wilentz, R. E., Owen, M. J. & Hill, C. S. (2001) J. Biol. Chem. 276 43175-43181. [DOI] [PubMed] [Google Scholar]
- 25.Shi Y., Hata, A., Lo, R. S., Massagué, J. & Pavletich, N. P. (1997) Nature 388 87-93. [DOI] [PubMed] [Google Scholar]
- 26.Hata A., Lo, R. S., Wotton, D., Lagna, G. & Massague, J. (1997) Nature 388 82-87. [DOI] [PubMed] [Google Scholar]
- 27.Jayaraman L. & Massague, J. (2000) J. Biol. Chem. 275 40710-40717. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein M., Monga, S. P., Liu, Y., Brodie, S. G., Tang, Y., Li, C., Mishra, L. & Deng, C. X. (2001) Mol. Cell. Biol. 21 5122-5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Y., Wang, Y. F., Jayaraman, L., Yang, H., Massague, J. & Pavletich, N. P. (1998) Cell 94 585-594. [DOI] [PubMed] [Google Scholar]
- 30.Wicks S. J., Lui, S., Abdel-Wahab, N., Mason, R. M. & Chantry, A. (2000) Mol. Cell. Biol. 20 8103-8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scherer A. & Graff, J. M. (2000) J. Biol. Chem. 275 41430-41438. [DOI] [PubMed] [Google Scholar]
- 32.Wisotzkey R. G., Mehra, A., Sutherland, D. J., Dobens, L. L., Liu, X., Dohrmann, C., Attisano, L. & Raftery, L. A. (1998) Development (Cambridge, U.K.) 125 1433-1445. [DOI] [PubMed] [Google Scholar]
- 33.de Caestecker M. P., Yahata, T., Wang, D., Parks, W. T., Huang, S., Hill, C. S., Shioda, T., Roberts, A. B. & Lechleider, R. J. (2000) J. Biol. Chem. 275 2115-2122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.