Abstract
Rearrangement of T cell receptor (TCR) genes is driven by transient expression of V(D)J recombination-activating genes (RAGs) during lymphocyte development. Immunological dogma holds that T cells irreversibly terminate RAG expression before exiting the thymus, and that all of the progeny arising from mature T cells express the parental TCRs. When single pancreatic islet-derived, NRP-A7 peptide-reactive CD8+ T cells from nonobese diabetic (NOD) mice were repeatedly stimulated with peptide-pulsed dendritic cells, daughter T cells reexpressed RAGs, lost their ability to bind to NRP-A7/Kd tetramers, ceased to transcribe tetramer-specific TCR genes, and, instead, expressed a vast array of other TCR rearrangements. Pancreatic lymph node (PLN) CD8+ T cells from animals expressing a transgenic NRP-A7-reactive TCR transcribed and translated RAGs in vivo and displayed endogenous TCRs on their surface. RAG reexpression also occurred in the PLN CD8+ T cells of wild-type NOD mice and could be induced in the peripheral CD8+ T cells of nondiabetes-prone TCR-transgenic B10.H2g7 mice by stimulation with peptide-pulsed dendritic cells. In contrast, reexpression of RAGs could not be induced in the CD8+ T cells of B6 mice expressing an ovalbumin-specific, Kb-restricted TCR, or in the CD8+ T cells of NOD mice expressing a lymphocytic choriomeningitis virus-specific, Db-restricted TCR. Extra-thymic reexpression of the V(D)J recombination machinery in certain CD8+ T cell subpopulations, therefore, enables further diversification of the peripheral T cell repertoire.
The recombination-activating gene (RAG)-1 and RAG2 proteins catalyze the rearrangement of the variable (V), diversity (D), and joining (J) DNA elements of antigen receptor genes in lymphocytes (1, 2). In the T lymphocyte lineage, RAG mRNAs and proteins are first detected in CD44+CD25+CD4−CD8− thymocytes (pre-T cells), where they initiate VβDβJβ recombination. Expression of functional T cell antigen receptor (TCR)β chains on the surface of preT cells activates their proliferation and down-regulates RAG transcription. RAGs then are reexpressed in quiescent CD4+CD8+ thymocytes where they induce VαJα rearrangements. Crosslinking of functional TCRαβ heterodimers on CD4+CD8+ thymocytes by self peptide/major histocompatibility complex (MHC) ligands on thymic epithelial cells induces their differentiation into CD4+CD8− or CD4−CD8+ thymocytes (positive selection) and irreversibly terminates RAG expression (3, 4). Thus, it is generally thought that mature T lymphocytes exit the thymus without expressing RAGs (5, 6), and that all of the progeny arising from mature T cells in response to antigenic stimulation express the parental TCRs.
Nonobese diabetic (NOD) mice develop overt diabetes after prolonged periods of pancreatic islet inflammation involving both CD4+ and CD8+ T cells. The initiation and progression of autoimmune diabetes requires the recruitment of β cell-reactive CD8+ T cells to the pancreatic lymph nodes, their activation by antigen, and their subsequent migration into pancreatic islets (7–9). We and others have shown that a significant fraction of NOD islet-associated CD8+ T cells express highly homologous TCRα chains (Vα17 and Jα42 joined by the same N-region sequence; refs. 10–12), and that they recognize the peptide NRP-A7 in the context of the MHC class I molecule H-2Kd (13).
The islet-associated, NRP-A7-reactive CD8+ T cell subpopulation engages antigen/MHC with increasing avidity as the mice age (9). To characterize TCRαβ heterodimers binding NRP-A7/Kd complexes with a range of affinities, we set out to generate clones from islet-derived, NRP-A7/Kd-reactive CD8+ T cell lines that bound tetramer with either low or high avidity. Unexpectedly, we found that these clones, which were derived from single, originally tetramer-positive cells, progressively lost their ability to bind tetramer. Subsequently, we found that NRP-A7-reactive CD8+ T cells reexpress RAGs and transcribe new TCR rearrangements in response to antigenic stimulation, both in vitro and in vivo. These results indicate that certain CD8+ T cell subpopulations can reexpress the V(D)J recombination machinery in the periphery.
Materials and Methods
Mice.
8.3-NOD and rag2−/− 8.3-NOD mice have been described (14). NOD/Lt (Thy1.2; H-2g7), NOD.Thy1.1, and C57BL/10 (B10; H-2b) mice were obtained from The Jackson Laboratory. B10.H-2g7 mice were obtained from L. Wicker and L. Peterson (Merck Research Labs, Rahway, NJ). 8.3-NOD.Thy1.1 and 8.3-B10.H-2g7 mice were produced by backcrossing the 8.3-TCR transgenes onto the NOD.Thy1.1 or B10.H-2g7 backgrounds for two and six generations, respectively. OT-1 TCR-transgenic B6 (15) and lymphocytic choriomeningitis virus (LCMV)-TCR-transgenic NOD mice (16) were provided by O. Bathe (University of Calgary, Alberta, Canada) and D. Serreze (The Jackson Laboratory), respectively.
Peptides and Tetramers.
T Cell Isolation.
Pancreatic islets were cultured for 7 days in RPMI medium 1640/10% (vol/vol) FBS and Takeda rIL-2. T cells were isolated from spleens, mesenteric lymph node (MLN), and pancreatic lymph nodes (PLN) using anti-CD8 (for 8.3-NOD mice) or anti-CD4/anti-CD8-coated magnetic beads (for NOD or B10 mice; Miltenyi Biotec, Auburn, CA). Purity was >95% T cells with <1% B220+ cells.
Adoptive T Cell Transfer.
Splenic CD8+ T cells from 7–12 week-old 8.3-NOD mice were labeled with carboxyfluorescein diacetate-succinimidyl ester (CFSE; Molecular Probes) and injected (107 cells per mouse) i.v. into NOD or B10.H-2g7 hosts. The MLNs and PLNs of the hosts were collected 6 days later for flow cytometry.
Tetramer Staining, Sorting, and T Cell Cloning.
T cells propagated from islets (NOD and 8.3-NOD mice) or freshly isolated from spleen (8.3-NOD, 8.3-B10.H-2g7, and OT-1 mice) were stained with tetramers and/or anti-CD8α mAb (9). NRP-A7 tetramer-positive and tetramer-negative CD8+ T cells were sorted at one cell per well into 96-well plates by flow cytometry and stimulated with NRP-A7-pulsed dendritic cells (DCs; 5 × 103 per well). DCs were prepared by culturing bone marrow cells in the presence of rmGM-CSF and rmIL4 (5 ng/ml) for 7–11 days (18). OT-1 clones were generated by stimulation with ova peptide (SIINFEKL)-pulsed B6 DCs. rIL-2 was added to all of the wells 4 days later. All cultures were restimulated biweekly. Clones were stained with phycoerythrin (PE)-labeled tetramers, anti-CD8-FITC, and 7-amino-actinomycin D (7-AAD), or with anti-CD8-PE, anti-TCRβ-FITC, and anti-B220-perCP. The anti-TCRβ mAb was titrated against a hamster IgG mixture as negative control. Dead cells (in the side/forward scatter plot, or if 7-AAD+) were gated out. In other experiments, clones were stained with anti-Thy1.1- or anti-Thy1.2-FITC, anti-CD8-PE, and 7-AAD. All stains were done at 4°C with sodium azide.
Anti-Vβ Staining.
Cells were stained with anti-CD4-biotin, streptavidin-perCP and anti-CD8-PE, anti-Vβ8.1/8.2-PE or NRP-A7 tetramer-PE, and a mixture of FITC-labeled mAbs against Vβ2, Vβ3, Vβ4, Vβ5.1/5.2 (excluded when staining OT-1 CD8+ cells), Vβ6, Vβ7, Vβ8.1/8.2 (excluded when staining 8.3- or LCMV-specific CD8+ cells), Vβ8.3, Vβ9, Vβ10, Vβ11, Vβ12, Vβ13, Vβ14, and Vβ17 (5 μl of prediluted stocks/mAb; PharMingen). Analyses were done on cells contained outside the PerCP+ gate (to exclude CD4+ cells in LNs, or background staining in clones). In other experiments, PLN cells were stained with tetramer-PE, Vβ panel-FITC, anti-CD69, or CD44-biotin and streptavidin-perCP.
RAG Reexpression in Vitro.
Purified MLN or splenic CD8+ T cells (5 × 104 per well, 24–36 wells per condition) or clones (≈5 × 104 cell per well; four to eight wells per condition) were cultured alone or with TUM-, NRP-A7-, ova-, or LCMV gp33 (KAVYNFATC)-pulsed, syngeneic DCs (1 × 104 per well) for 3, 24, and 48 h. Total RNA was examined for the presence of RAG transcripts by RT-PCR.
RT-PCR.
DNase I-treated RNA (0.4–1 μg) was reverse-transcribed with oligo-dT. TCR cDNAs were generated by anchor-PCR, cloned, and sequenced (11). PCR amplification of Vα17-Jα42 or Vα17-Jα−Cα cDNAs was done by amplifying 1% cDNAs with GCATGGCCCAGAAGGTAACACAG (Jα42) or GTCAAAGTCGGTGAACAGGCAGAG (Cα). Amplification of VβDβJβCβ cDNAs was done by amplifying 0.02% cDNAs with TGCTTTTGAGAGCTCAAACAAGGAGACCTTGGG (Cβ) and Vβ family-specific primers to yield products of similar size regardless of Vβ family usage (i.e., <20-bp differences). PCR products were cloned into the TOPO vector (Invitrogen) and sequenced. β-actin, RAG1, and RAG2 cDNAs were amplified (35 cycles, 1′ at 92°C, 1′ at 55°C and 2′ at 72°C) with the following intron-spanning primers (19): β-actin: CCTAAGGCCAACCGTGAAAAG and TCTTCATGGTGCTAGGAGCCA; RAG1: TGCAGACATTCTAGCACTCTG and ACATCTGCCTTCACGTCGATC; RAG2: CACATCCACAAGCAGGAAGTACAC or CTGCTACCTCCCACCTCTTC and TCCCTCGACTATACACCACGTCAA or CCAGTCAGGAGTCTCCATCTC, respectively. β-actin genomic DNA was amplified with: GTTACCAACTGGGACGACA and TGGCCATCTCCTGCTCGAA. Of each reaction, 5–20% was analyzed by Southern blotting by using alkaline-phosphatase (AP)-labeled RAG1 or RAG2 cDNA probes and a chemiluminescence detection kit (Amersham Pharmacia). Exposure times ranged from 5 min to 2 h. Linker-ligation PCR was done as described (20, 21). Briefly, genomic DNA was ligated to the BW-1/BW-2 linker (20) and amplified by nested-PCR using BW-1H and Dβ21- (first PCR) or Dβ22-specific primers (second PCR), or with BW-1H and Jα50.1 primers (21). The PCR products were probed with biotinylated Dβ25 or Jα50.2 primers, respectively (21), and the blots were developed with streptavidin-AP and chemiluminescence.
Western Blotting.
CD8+ T cell or whole thymus lysates were immunoprecipitated with 2 μg of goat anti-RAG2 antibodies (C19, Santa Cruz Biotechnology), electrophoresed in SDS/12% PAGE, and probed with mouse anti-RAG2 mAb (G110–461; PharMingen) and peroxidase-labeled goat anti-mouse IgG (Santa Cruz Biotechnology). The blots were developed with a chemiluminescence kit (Pierce).
Statistical Analyses.
Data were compared by Mann–Whitney U, χ2, or Fisher's t tests.
Results
Loss of Tetramer Reactivity by NRP-A7-Specific T Cell Clones in Vitro.
NRP-A7 tetramer-positive CD8+ T cells propagated from islets of 9- and 20-week-old NOD mice (binding tetramer with low and high avidity, respectively) were sorted by flow cytometry as bulk subpopulations and as single cells (Fig. 1A). Sequence analyses of cDNAs from TCR-specific cDNA libraries confirmed that the tetramer-reactive CD8+ T cells from 9- and 20-week-old mice expressed diverse TCRβ genes but nearly identical TCRα rearrangements (Vα17-MRD-Jα42). As expected, the tetramer-negative CD8+ T cell populations from both age groups expressed highly heterogenous TCRα repertoires (information available in Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org).
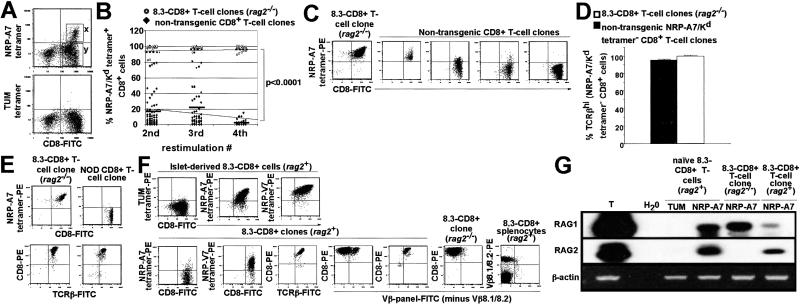
Fig 1.
Loss of tetramer reactivity by NRP-A7-specific CD8+ clones. (A) Pancreatic islets from nondiabetic NOD mice (n = 16–20 mice per experiment; two experiments) were cultured in the presence of rIL-2 for 7 days, and the islet-derived NRP-A7 tetramer-positive (x) and tetramer-negative (y) CD8+ cells were sorted by FACS at one cell per well. (B) Summary of NRP-A7 tetramer reactivity of T cell clones derived from single, originally tetramer-positive cells. Clones were derived from NOD (n = 77, 46, and 21 clones at each restimulation) or rag2−/− 8.3-NOD islets (n = 15–18 clones per restimulation) upon stimulation with NRP-A7-pulsed DCs. (C) Representative tetramer-staining patterns for clones derived from tetramer-positive cells. (D) TCR levels on clones from NOD or rag2−/− 8.3-NOD mice (n = 10 and 8, respectively). (E) Representative tetramer/TCRβ-staining patterns. (F) rag2+ 8.3-CD8+ clones express endogenous TCRs and decrease their avidity for NRP-A7 upon stimulation with NRP-A7-pulsed DCs. (Upper) Images correspond to an islet-derived 8.3-CD8+ T cell line before stimulation in vitro. (Lower) Images correspond to 8.3-CD8+ clones grown on NRP-A7-pulsed DCs or to splenic 8.3-CD8+ T cells. Dead cells were gated out. (G) Expression of RAG mRNAs by 8.3-CD8+ MLN cells and rag2+ and rag2−/− 8.3-CD8+ clones (harvested 1–2 weeks after the first or second restimulations, respectively) within 3 h of stimulation with NRP-A7-pulsed DCs. T, thymus RNA control.
To generate T cell clones, we sorted tetramer-reactive CD8+ cells at one cell per well into 96-well plates. The sorted cells were stimulated once every 2 weeks with NRP-A7 peptide-pulsed DCs and rIL-2. Surprisingly, tetramer-binding studies of clones arising from wells after the second and third restimulations revealed that many of the clones could no longer bind NRP-A7 tetramer (Fig. 1 B and C). Other clones displayed heterogeneous tetramer-binding profiles, implying that they comprised cells that bound tetramer with a range of intensities (Fig. 1 B and C). Repeated stimulations caused significant reductions in the number of viable clones (Fig. 1B), in the number of viable cells within individual clones (not shown), and in the percentage of live CD8+ cells that could bind tetramer (Fig. 1 B and C). This decline in cell viability with time was likely caused by the inability of tetramer-negative (and hence non-NRP-A7-reactive) cells to survive indefinitely in the absence of antigen, even in the presence of rIL-2. Importantly, this loss in the ability of NRP-A7-reactive T cells to recognize NRP-A7 upon antigenic stimulation could not be accounted for by down-regulation of CD3 or TCR, as all of the tetramer-negative CD8+ clones that were tested stained brightly with anti-CD3 (not shown) or anti-TCRβ mAbs (Fig. 1 D and E).
RAG-Dependent TCR Revision in NRP-A7-Reactive T Cell Clones in Vitro.
To ascertain whether the above phenomenon might be the result of TCR revision, we cloned NRP-A7 tetramer-binding CD8+ T cells from rag2−/− NOD or rag2+ NOD mice expressing a transgenic NRP-A7-reactive TCR (referred to as rag2−/− or rag2+ 8.3-CD8+ clones). As expected, none of the 18 rag2−/− 8.3-CD8+ clones that were followed lost their ability to bind NRP-A7 tetramer for at least four restimulations (Fig. 1B; P < 0.0001 vs. clones from NOD mice). Unexpectedly, some rag2+ 8.3-CD8+ clones also lost NRP-A7 tetramer-reactivity with time while maintaining high levels of total TCR on the surface (Fig. 1F). However, unlike the NRP-A7 tetramer-negative clones from wild-type NOD mice, the NRP-A7 tetramer-negative clones from rag2+ 8.3-NOD mice bound NRP-V7 tetramer, which contains a higher avidity ligand of NRP-A7-reactive TCRs than NRP-A7 tetramer (Fig. 1F). This result suggested that, in the presence of RAGs, repeated stimulations of 8.3-TCR-transgenic clones with peptide-pulsed DCs were able to cause significant reductions in their avidity for peptide/MHC without abrogating their ability to recognize higher avidity mimics of their target antigen. In fact, staining of rag2+ 8.3-CD8+ clones with a pool of 15 mAbs against Vβ elements other than the transgenic Vβ8.1 element indicated that they co-expressed endogenous TCRs (Fig. 1F; also, see Fig. 3B). Neither clones propagated from rag2−/− 8.3-NOD mice nor splenic CD8+ T cells from rag2+ 8.3-NOD mice stained with these mAbs (Fig. 1F), indicating that endogenous TCRβ expression as detected with the mAb panel was specific, RAG-dependent, and induced in vitro. Importantly, naïve 8.3-CD8+ T cells, rag2+ 8.3-CD8+ clones, and rag2−/− 8.3-CD8+ clones transcribed RAG2 and/or RAG1 mRNA shortly after stimulation with NRP-A7-pulsed DCs (within 3 h; Fig. 1G). Experiments using a low avidity mimic of NRP-A7 that functions as a partial agonist (NRP-I4) yielded similar results (not shown), indicating that the ability of NRP-A7 to induce the reexpression of RAGs by 8.3-CD8+ T cells was not because of its superagonistic properties (9, 13). RAG reexpression in response to NRP-A7 stimulation was transient, however, as RAG mRNAs were invariably absent 48 h later (not shown). Thus, stimulation of NRP-A7-reactive CD8+ cells with peptide-pulsed DCs triggered the expression of RAGs and newly rearranged TCR genes, “dilution” of transgenic TCRs on the cells' surface, and a reduction in the cells' avidity for NRP-A7/Kd.
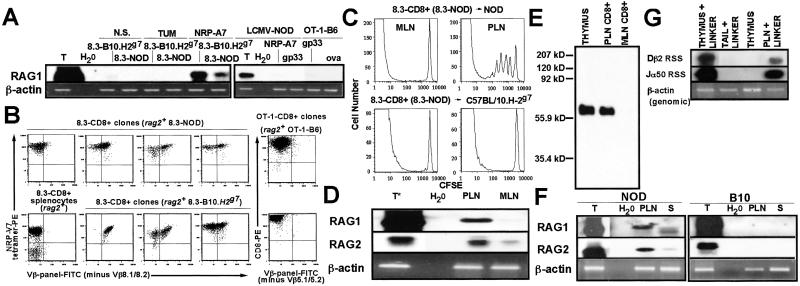
Fig 3.
Expression of RAGs by NRP-A7-reactive CD8+ T cells in vitro and in vivo. (A) Expression of RAG1 mRNA by 8.3-, OT-1-, and LCMV-TCR-transgenic CD8+ T cells upon in vitro stimulation with NRP-A7, ova, or gp33-peptide-pulsed DCs (3 h), respectively. Negative control peptides: TUM, gp33, and NRP-A7, respectively. (B) 8.3-CD8+ clones from 8.3-B10.H2g7 mice express endogenous TCRs upon repeated stimulation with NRP-A7-pulsed DCs. Panels correspond to splenic 8.3-CD8+ cells from a rag2−/− 8.3-NOD mouse (control), a Vβ panel− clone from the spleen of a rag2+ 8.3-NOD mouse, and Vβ panel+ clones from the spleens of rag2+ 8.3-NOD or 8.3-B10.H2g7 mice (experiments independent of those shown in Fig. 1). (C) Proliferation of naïve, CFSE-labeled 8.3-CD8+ T cells in the PLNs of NOD but not of B10.H-2g7 mice. (D) Transcription of RAGs by PLN 8.3-CD8+ T cells. CD8+ cells were purified from the PLNs or MLNs of 3–10 8.3-NOD mice per experiment. Thymus (T) RNA was prepared from crude thymus lysates. RT-PCR products [5% for the thymus RAG2 RT-PCR shown (T*) and 15% for all other RT-PCR products] were probed with RAG1 and RAG2 cDNAs. (E) Immunoprecipitation Western blot for RAG2. RAG2 was immunoprecipitated from purified 8.3-CD8+ T cells or whole thymus (250 μg of protein). (F) RAG transcription in PLN and splenic (S) T cells from NOD mice (3–10 mice per experiment). (G) RSS-specific DNA breaks at Dβ and Jα loci in PLN 8.3-CD8+ cells.
NRP-A7-Reactive T Cell Clones Become Polyclonal upon Repeated Stimulation with Peptide-Pulsed DCs.
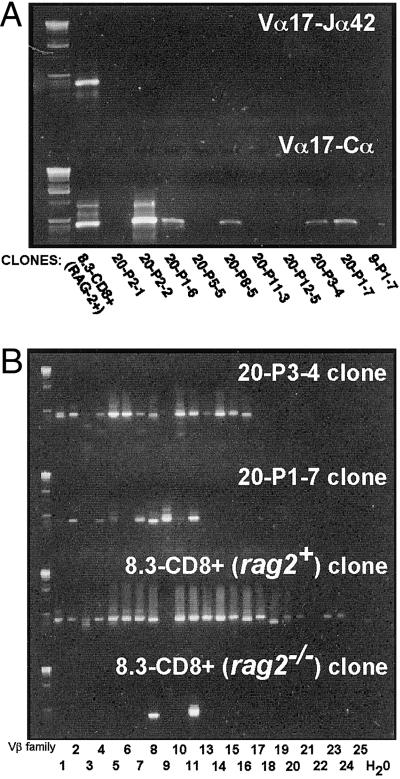
To confirm that tetramer-negative clones propagated from NOD mice had in fact ceased to transcribe Vα17-Jα42 mRNA, we generated TCRα-specific cDNA libraries from NRP-A7 tetramer-negative NOD clones and NRP-A7 tetramer-negative rag2+ 8.3-CD8+ clones. Amplification of each library with Vα17-Jα42-specific primers indicated that, unlike rag2+ 8.3-CD8+ T cell clones, 9 of 10 clones derived from wild-type NOD mice did not transcribe Vα17-Jα42 mRNA (Fig. 2A Upper). Four of these 9 NOD-derived clones, however, transcribed Vα17 elements rearranged to other Jα elements, as determined by amplification of cDNAs with Vα17- and Cα-specific primers (Fig. 2A Lower). PCR analyses of the TCRβ cDNAs of two of these clones with Vβ family- and Cβ-specific primers revealed usage of most Vβ family members, suggesting that these clones had become highly polyclonal (Fig. 2B). Sequencing studies of cloned PCR products from 20-P3–4 cells confirmed the target specificity of most amplification primers (see Fig. 6, which is published as supporting information on the PNAS web site). Sequence analyses of cDNAs from TCR-specific cDNA libraries derived from 5 of these T cell clones confirmed that they transcribed multiple TCRα and TCRβ rearrangements (see Fig. 7, which is published as supporting information on the PNAS web site). Unlike rag2−/− 8.3-CD8+ clones, and in agreement with the anti-Vβ staining results described above (Fig. 1F), rag2+ 8.3-CD8+ clones also transcribed multiple Vβ elements (Fig. 2B, bottom two panels).
Fig 2.
TCR repertoire of clones arising from tetramer-positive precursors, as analyzed by RT-PCR. (A) NRP-A7 tetramer-negative clones do not transcribe Vα17-Jα42 mRNA (Upper), but a subset does transcribe at least some Vα17+ TCRα rearrangements (Lower). (B) NRP-A7 tetramer-negative clones express multiple Vβ elements. TCRβ cDNAs of clones were amplified with Vβ- and Cβ-specific primers. The Vβ11-binding primer crossreacted with Vβ8 in this experiment.
To confirm that our sorting protocols did not deliver multiple cells per well, we sorted a 1:2 mixture of Thy1.1+ and Thy1.2+ 8.3-CD8+ T cells, stimulated the cells as above, and determined how many cultures contained Thy1.1+ and/or Thy1.2+ cells. Of 37 cultures analyzed, 36 contained Thy1.1−/Thy1.2+ (n = 22) or Thy1.1+/Thy1.2− cells (n = 14), and only 1 contained both. This low frequency of double-positive cultures was significantly lower than that expected for a sorting rate of 2 cells per well (P < 0.015) and was compatible with a rate of 1.12 cells per well. The presence of multiple different TCRβ cDNAs along with the loss of tetramer reactivity and/or the expression of endogenous TCRβ chains in many of the clones that were followed in several independent experiments (also see below) is incompatible with lack of clonality being the result of low sorting efficiency. Furthermore, because most 8.3-CD8+ splenocytes exclusively express the transgenic TCRβ chain, the appearance of nontransgenic TCRβ chains on the surface of ≈40–98% of the cells contained in ≈50% of daughter “clones” (see below) cannot be explained by low sorting efficiency. We therefore conclude that individual cells within most, if not all, the rag2-competent “clonal” NOD T cell populations studied above had randomly rearranged their endogenous TCR genes in response to antigen, and evolved into polyclonal mixtures of cells that were devoid of NRP-A7-reactive cells.
Reexpression of RAGs by CD8+ T Cells in Vitro Is Not Unique to NOD-Derived T Cells and Is Not Linked to the H-2g7 Haplotype.
This ability of NOD CD8+ T cells to transiently reexpress RAG mRNAs in vitro in response to TCR ligation was not an exclusive property of T cells maturing in the NOD background, as it also occurred in NRP-A7-stimulated CD8+ T cells from 8.3-B10.H-2g7 mice (Fig. 3A). In fact, NRP-V7 tetramer-binding “clones” from 8.3-B10.H-2g7 mice (7/19) already contained significant numbers of cells expressing endogenous TCRβ chains after two restimulations (Fig. 3B shows several examples). Attempts to investigate the ability of peripheral T cells from wild-type NOD, B10.H-2g7, DBA/2 and BALB/c mice to reexpress RAGs in response to active immunization with peptide were not informative because treatment of all these mice with adjuvant alone (MPL+TDM) was sufficient to induce the appearance of RAG mRNA/protein-expressing CD8+ T cells to the LNs and spleen (not shown). Because certain adjuvants can trigger the migration of immature B cells from the bone marrow to the spleen (22), we cannot exclude the possibility that presence of RAG-expressing CD8+ cells in the LNs of MPL+TDM-treated mice was also caused by an influx of recent thymic emigrants (still expressing RAGs).
We next investigated whether this ability of CD8+ cells to reexpress RAGs was linked to the H-2g7 haplotype or whether it was a unique property of NRP-A7-reactive T cells. This investigation was carried out by determining the presence of RAG mRNAs in antigen-stimulated CD8+ T cells isolated from OT-1 TCR-transgenic B6 (H-2b) and LCMV TCR-transgenic NOD (H-2g7) mice. OT-1 mice express a Kb-restricted TCR that recognizes an ovalbumin-derived peptide (15). LCMV-NOD mice express a Db-restricted TCR that recognizes the LCMV gp33 peptide (16). As shown in Fig. 3A, negative control peptide-stimulated CD8+ T cells from both types of mice expressed trace amounts of RAG1 mRNA, presumably because of presence of recent thymic emigrants in the splenic T cell preparations. In contrast, RAG1 mRNA was consistently absent in OT-1- and LCMV-TCR transgenic T cells that had been cultured with ova- or gp33-pulsed DCs (for 3, 24 and 48h; Fig. 3A and data not shown). Furthermore, none of 15 OT-1 clones that were examined after two restimulations with peptide-pulsed DCs contained significant numbers of cells expressing nontransgenic TCRβ chains (Fig. 3B shows two examples). These results indicated that reexpression of RAGs by peripheral T cells in response to antigen is not an H-2g7-linked phenomenon, or a unique property of NOD T cells.
NRP-A7-Reactive T Cells Spontaneously Reexpress RAGs in Vivo.
It was important to investigate whether NRP-A7-reactive CD8+ T cells could spontaneously reexpress RAGs in vivo. CFSE-labeled 8.3-CD8+ T cells proliferate in the PLN but not MLN of NOD hosts (Fig. 3C Upper), indicating that they undergo autoantigen-induced activation in the PLNs. We thus compared the presence of RAG transcripts in CD8+ cells purified from the PLN and MLN of 8.3-NOD mice. Transcripts for both RAG1 and RAG2 were clearly up-regulated in PLN CD8+ T cells, when compared with MLN CD8+ cells (Fig. 3D). Western blot analysis of RAG2 immunoprecipitates confirmed the presence of RAG2 protein in PLN 8.3-CD8+ cells (Fig. 3E). RAG transcripts and RAG2 protein were also present in the PLNs of wild-type NOD mice, but were absent in the PLNs of B10 mice (Fig. 3F and data not shown), which do not develop islet inflammation. This finding confirmed that reexpression of RAGs is not a peculiarity of TCR-transgenic T cells. Reexpression of RAGs in PLN T cells triggered V(D)J recombination, as DNA from PLNs of 8.3-NOD mice contained recombination signal sequence (RSS) breaks at TCR-Dβ and Jα loci, as determined by linker-ligation PCR (Fig. 3G). Therefore, NOD CD8+ T cells reexpress RAGs upon antigen-induced activation in vivo.
Expression of Endogenous TCRs on in Vivo-Activated, PLN-Associated 8.3-CD8+ T Cells.
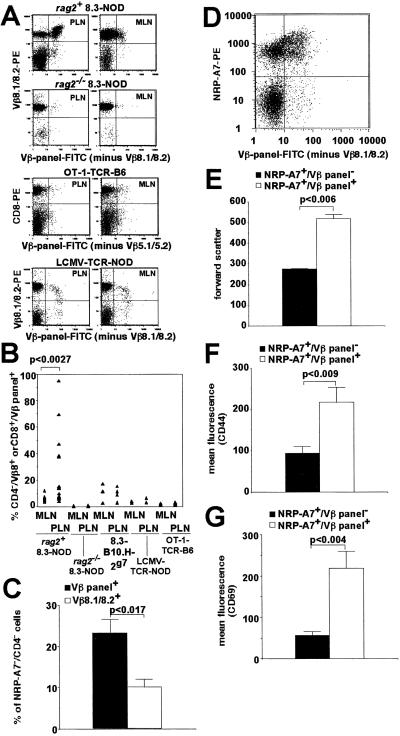
CFSE-labeled 8.3-CD8+ T cells remain quiescent in the PLNs of B10.H-2g7 mice which, like B10 mice, do not develop diabetes (Fig. 3C Lower). To investigate the functional consequences of RAG reexpression in vivo, we assessed the presence of endogenous TCRβ chains on the Vβ8.1/8.2+ CD8+ T cells contained in the MLNs and PLNs of 8.3-NOD and 8.3-B10.H-2g7 mice. Significant percentages of PLN Vβ8.1/Vβ8.2+ CD8+ cells from 8.3-NOD mice stained brightly with a pool of 15 anti-Vβ-specific mAbs (referred to as Vβ panel+; Fig. 4A). This population of cells expressing endogenous TCRβ genes was significantly greater than that found in the MLNs (P < 0.0027; Fig. 4B), and it probably required antigen-driven activation because it was not seen in the PLNs of 8.3-B10.H-2g7, LCMV- or OT-1-TCR-transgenic mice (Fig. 4B). This phenomenon required RAG expression, because antigen-driven activation of 8.3-CD8+ T cells in the PLNs of rag2−/− 8.3-NOD mice (which also develop diabetes) did not result in endogenous TCRβ chain expression (Fig. 4 A and B).
Fig 4.
Expression of secondary TCR rearrangements by 8.3-CD8+ T cells in vivo. (A and B) RAG-dependent expression of secondary TCRβ chains on PLN 8.3-CD8+ cells. Data correspond to PLN and MLN cells from 8.3-NOD (n = 17 and 15, respectively), rag2−/− 8.3-NOD (n = 5 and 6), 8.3-B10.H2g7 (n = 5 and 4), OT-1-B6 (n = 3), and LCMV-NOD mice (n = 3; ≥10 weeks of age). Each triangle corresponds to a different sample. (C) Average percentage of tetramer-negative cells within CD4−/Vβ panel+ and CD4−/Vβ8.1/8.2+ PLN cells of 8.3-NOD mice (n = 5 mice). (D) Representative tetramer reactivity of CD4−/Vβ panel+ and CD4−/Vβ panel− PLN cells. (E–G) Tetramer-positive/Vβ panel+ cells are larger (E) and express higher levels of CD44 (F) and CD69 (G) than tetramer-positive/Vβ panel− cells. Data correspond to eight (E and F) and seven (G) 8.3-NOD mice per group.
To ascertain whether these endogenous TCR chains can edit the antigenic reactivity of the transgenic TCR, we compared the percentages of tetramer-negative cells contained in the CD4−/Vβ panel+ vs. CD4−/Vβ8.1,8.2+ populations (to exclude the CD4+ T cells that in 8.3-NOD mice are selected on endogenous TCRs). Interestingly, the PLN CD4−/Vβ panel+ population contained significantly more tetramer-negative cells than the CD4−/Vβ8.1,8.2+ population (Fig. 4C), suggesting that expression of endogenous TCRs abrogated the tetramer-binding ability of some cells. On the other hand, the CD4−/Vβ panel+ T cells that bound tetramer were larger, and expressed higher levels of CD44 and CD69 than the tetramer-positive T cells that were Vβ panel− (P < 0.009; Fig. 4 D–G). These results confirmed that expression of endogenous TCRs in vivo was associated with T cell activation.
Discussion
The results of this study demonstrate that NRP-A7-reactive CD8+ T cells maturing in at least two different genetic backgrounds (NOD and B10.H-2g7) can transiently reexpress RAGs. Most importantly, the data show that continuous stimulation of these CD8+ T cells with antigen in vitro, or in vivo at a site of chronic autoimmune inflammation (the PLNs), promotes the de novo formation of additional TCR rearrangements in a RAG-dependent manner. Although it has been shown that peripheral immature B cells can express RAGs (6, 23–27), it is generally believed that mature T or B cells cannot (2). An exception to this concept is the description of rare V(D)J recombination events in B6 mice expressing a transgenic TCRβ chain, in which superantigen-mediated deletion of transgene-positive CD4+ T cells resulted in the expression of RAG mRNAs and endogenous TCRβ chains exclusively in TCRβ-transgene-negative CD4+ T cells (21). Likewise, RAG1 or RAG2 mRNAs (but rarely both) were detected in 3 of 11 human CD4+ T cell clones with defective surface TCR expression (28) and, just recently, in superantigen-treated TCRα knockin mice (29). However, unlike the tolerogen-induced reexpression of RAGs described in CD4+ T cells (21, 28, 29), the reexpression of RAGs that we see in NRP-A7-reactive CD8+ T cells is dissociated from genetic manipulations and tolerogenic stimuli and seems to be a frequent event, at least in vivo.
Several lines of evidence indicate that reexpression of RAGs in NRP-A7-reactive CD8+ T cells is induced by antigen. In vivo, RAGs were almost exclusively expressed by CD8+ T cells residing in the PLNs, which is where naïve autoreactive CD8+ T cells first encounter β cell autoantigens. Furthermore, expression of nontransgenic TCR chains on NRP-A7-reactive, TCR-transgenic T cells in vivo was associated with signs of T cell activation. In agreement with this observation, reexpression of RAG mRNAs by CD8+ T cells in vitro occurred in an antigen-specific manner, indicating that TCR signaling is at least one contributing factor. Semiquantitative RT-PCR studies suggest that RAG reexpression in vitro is not as frequent as it is in vivo (unpublished data), suggesting that the local inflammatory environment in the PLNs of NOD mice is a major factor. These observations are in contrast to the expression of RAGs that we saw in CD8+ T cells isolated from adjuvant-treated mice, which was systemic, dissociated from T cell activation, and possibly caused by adjuvant-induced migration of immature thymocytes to the periphery (22).
Although the mechanisms underlying this phenomenon remain to be determined, they seem to be different from those responsible for the expression of RAGs described in peripheral B cells (6, 23–27) and chronically stimulated CD4+ T cells (21). Thus, expression of RAGs in peripheral B cells is restricted to immature IgMlow B cells (2) and ceases upon antigen-induced activation (2, 26, 27). Furthermore, tolerogen-induced reexpression of RAGs in CD4+ T cells is mediated by B cells and is thought to occur in germinal centers (21). Because CD8+ T cells do not normally enter germinal centers (30), reexpression of RAGs in NRP-A7-reactive CD8+ cells is probably induced in the lymph node paracortex, possibly by antigen-loaded interdigitating DCs. The fact that neither OT-1- nor LCMV-TCR-transgenic CD8+ T cells (maturing in B6 or NOD backgrounds, respectively) reexpressed RAGs upon antigenic stimulation indicated that this phenomenon is not a general property of NOD T cells nor of T cells restricted by NOD MHC class I molecules, but rather is a unique property of certain T cell clonotypes. We speculate that this property is imprinted during thymocyte development, perhaps by T cell extrinsic factors.
Because CD8+ T cells from 8.3-B10.H-2g7 mice can also reexpress RAGs in response to antigenic stimulation, at least in vitro, this phenomenon alone cannot account for the autoimmune proclivity of the NOD mouse. Nevertheless, because RAG reexpression and TCR revision have the potential to compromise an individual's natural resistance to autoimmunity afforded by the processes of thymic tolerance, it is tempting to speculate that these processes contribute to the persistence and/or progression of chronic autoimmune phenomena. Reexpression of RAGs in certain PLN CD8+ T cells as a result of chronic stimulation with pancreatic autoantigens, for example, might promote the generation of additional autoreactive T cells (i.e., epitope spreading) and the development of other autoimmune responses (31). Because TCR rearrangement is a random process, it is likely that TCR revision in PLN CD8+ T cells results in the generation of significant numbers of T cells that can no longer recognize the original autoantigenic peptide, or even self-MHC class I molecules. As a result, only a very small fraction of all of the T cells that reexpress RAGs in vivo are expected to survive. Nonetheless, the local inflammatory microenvironment that promotes the generation of these T cells might shelter them from the processes of peripheral immunological tolerance and even perhaps foster the selection of the few clonotypes that might still recognize self-peptide/MHC complexes or complexes other than those that triggered reexpression of RAGs. In this regard, it is tempting to speculate that autoantigen-driven selection of T cells expressing new TCR rearrangements contributes to the avidity maturation of the NRP-A7-reactive subpopulation (9). This speculation is not incompatible with the observation that NRP-A7-reactive CD8+ T cells lose tetramer reactivity upon repeated antigenic stimulation in vitro because, unlike in vivo-activated T cells, in vitro-activated T cells can survive for extended periods of time in the presence of rIL-2. Thus, although rearrangement of new TCR genes by NRP-A7-reactive T cells in vivo is likely to result in death by neglect, it has the potential to “modify” the affinity of the T cells' TCRs for self-MHC. Alternatively, RAG-dependent diversification of the peripheral CD8+ T cell repertoire in response to chronic antigenic stimulation has nothing to do with autoimmunity, and has evolved as a mechanism of tolerance or as a means for the immune system to be able to fight mutants arising during microbial or viral infections (32, 33).
Supplementary Material
Acknowledgments
We thank L. Allen, S. Bou, A. Cignac, and M. Deuma for animal care, T. Utsugi for providing rIL-2, L. Wicker for providing B10.H2g7 mice, O. Bathe for OT-1 B6 mice, D. Serreze for LCMV-NOD mice, and R. Tan and Y. Yang for feedback. This work was supported by a Group Grant from the Canadian Institutes of Health Research and grants from the Juvenile Diabetes Research Foundation (JDRF) and the Canadian Diabetes Association (CDA). P. Serra was funded by the Alberta Heritage Foundation for Medical Research (AHFMR), A.A. by AHFMR and JDRF, and J.Y. by the CDA. P. Santamaria is a Senior Scholar of the AHFMR.
Abbreviations
RAG, recombination-activating gene
TCR, T cell antigen receptor
NOD, nonobese diabetic
LCMV, lymphocytic choriomeningitis virus
DC, dendritic cell
MLN, mesenteric lymph node
PLN, pancreatic lymph node
PE, phycoerythrin
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fugmann S., Lee, A., Shokett, P., Villey, I. & Schatz, D. (2000) Annu. Rev. Immunol. 18 495-527. [DOI] [PubMed] [Google Scholar]
- 2.Nagaoka H., Yu, W. & Nussenzweig, M. (2000) Curr. Opin. Immunol. 12 187-190. [DOI] [PubMed] [Google Scholar]
- 3.Turka L., Schatz, D., Oettinger, M., Chun, J., Gorka, C., Lee, K., McCormack, W. & Thompson, C. (1991) Science 16 778-781. [DOI] [PubMed] [Google Scholar]
- 4.Borgulya P., Kishi, H., Uematsu, Y. & Boehmer, H. v. (1992) Cell 69 529-537. [DOI] [PubMed] [Google Scholar]
- 5.Monroe R., Seidl, K., Gaertner, F., Han, S., Chen, F., Sekiguchi, J., Wang, J., Ferrini, R., Davidson, L., Kelsoe, G. & Alt, F. (1999) Immunity 11 201-212. [DOI] [PubMed] [Google Scholar]
- 6.Yu W., Nagaoka, H., Jankovic, M., Misulovin, Z., Suh, H., Rolink, A., Melchers, F., Meffre, E. & Nussenzweig, M. C. (1999) Nature 400 682-687. [DOI] [PubMed] [Google Scholar]
- 7.Nagata M., Santamaria, P., Kawamura, T., Utsugi, T. & Yoon, J.-W. (1994) J. Immunol. 152 2042-2050. [PubMed] [Google Scholar]
- 8.Serreze D., Chapman, H., Varnum, D., Gerling, I., Leiter, E. & Shultz, L. (1997) J. Immunol. 157 3978-3986. [PubMed] [Google Scholar]
- 9.Amrani A., Verdaguer, J., Serra, P., Tafuro, S., Tan, R. & Santamaria, P. (2000) Nature 406 739-742. [DOI] [PubMed] [Google Scholar]
- 10.Santamaria P., Utsugi, T., Park, B., Averill, N., Kawazu, S. & Yoon, J. (1995) J. Immunol. 154 2494-2503. [PubMed] [Google Scholar]
- 11.Verdaguer J., Yoon, J.-W., Anderson, B., Averill, N., Utsugi, T., Park, B.-J. & Santamaria, P. (1996) J. Immunol. 157 4726-4735. [PubMed] [Google Scholar]
- 12.DiLorenzo T., Graser, R., Ono, T., Christianson, G., Chapman, H., Roopenian, D., Nathenson, S. & Serreze, D. (1998) Proc. Natl. Acad. Sci. USA 95 12538-12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson B., Park, B. J., Verdaguer, J., Amrani, A. & Santamaria, P. (1999) Proc. Natl. Acad. Sci. USA 96 9311-9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verdaguer J., Schmidt, D., Amrani, A., Anderson, B., Averill, N. & Santamaria, P. (1997) J. Exp. Med. 186 1663-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogquist K., Jameson, S., Heath, W., Howard, J., Bevan, M. & Carbone, F. (1994) Cell 76 17-27. [DOI] [PubMed] [Google Scholar]
- 16.Serreze D., Johnson, E., Chapman, H., Graser, R., Marron, M., DiLorenzo, T., Silveira, P., Yoshimura, Y., Nathenson, S. & Joyce, S. (2001) Diabetes 50 1992-2000. [DOI] [PubMed] [Google Scholar]
- 17.Altman J. D., Moss, P. A., Goulder, P. J., Barouch, D. H., McHeyzer-Williams, M. G., Bell, J. I., McMichael, A. J. & Davis, M. M. (1996) Science 274 94-96. [DOI] [PubMed] [Google Scholar]
- 18.Lutz M. B., Kukutsch, N., Ogilvie, A. L., Rossner, S., Koch, F., Romani, N. & Schuler, G. (1999) J. Immunol. Methods 223 77-92. [DOI] [PubMed] [Google Scholar]
- 19.Li Y.-S., Hayakawa, K. & Hardy, R. (1993) J. Exp. Med. 178 951-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlissel M., Constantinescu, A., Morrow, T., Baxter, M. & Peng, A. (1993) Genes Dev. 7 2520-2532. [DOI] [PubMed] [Google Scholar]
- 21.McMahan C. & Fink, P. (1998) Immunity 9 637-647. [DOI] [PubMed] [Google Scholar]
- 22.Gärtner F., Alt, F., Monroe, R. & Seidl, K. (2000) J. Exp. Med. 192 1745-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han S., Zheng, B., Schatz, D., Spanopoulou, E. & Kelsoe, G. (1996) Science 274 2094-2097. [DOI] [PubMed] [Google Scholar]
- 24.Hikida M., Mori, M., Takai, T., Tomochika, K., Hamatani, K. & Ohmori, H. (1996) Science 274 2092-2094. [DOI] [PubMed] [Google Scholar]
- 25.Han S., Dillon, S. R., Zheng, B., Shimoda, M., Schlissel, M. S. & Kelsoe, G. (1997) Science 278 301-305. [DOI] [PubMed] [Google Scholar]
- 26.Hertz M., Kouskoff, V., Nakamura, T. & Nemazee, D. (1998) Nature 394 292-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meffre E., Papavasilious, F., Cohen, P., de Bouteiller, O., Bell, D., Karasuyama, H., Schiff, C., Banchereau, J., Liu, Y. J. & Nussenzweig, M. C. (1998) J. Exp. Med. 188 765-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lantelme E., Palermo, B., Granziero, L., Mantovani, S., Campanelli, R., Monafo, V., Lanzavecchia, A. & Giachino, C. (2000) J. Immunol. 164 3455-3459. [DOI] [PubMed] [Google Scholar]
- 29.Huang C.-Y., Golub, R., Wu, G. & Kanagawa, O. (2002) J. Immunol. 168 3259-3265. [DOI] [PubMed] [Google Scholar]
- 30.MacLennan I. (1994) Annu. Rev. Immunol. 12 117-139. [DOI] [PubMed] [Google Scholar]
- 31.Atkinson M. A. & Leiter, E. H. (1999) Nat. Med. 5 601-604. [DOI] [PubMed] [Google Scholar]
- 32.Klenerman P. & Zinkernagel, R. (1998) Nature 394 482-486. [DOI] [PubMed] [Google Scholar]
- 33.Wilson J., Ogg, G., Allen, R., Goulder, P., Kelleher, A., Sewell, A., O'Callaghan, C., Rowland-Jones, S., Callan, M. & McMichael, A. (1998) J. Exp. Med. 188 785-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.