Abstract
Diet is a major environmental source of proinflammatory AGEs (heat-generated advanced glycation end products); its impact in humans remains unclear. We explored the effects of two equivalent diets, one regular (high AGE, H-AGE) and the other with 5-fold lower AGE (L-AGE) content on inflammatory mediators of 24 diabetic subjects: 11 in a 2-week crossover and 13 in a 6-week study. After 2 weeks on H-AGE, serum AGEs increased by 64.5% (P = 0.02) and on L-AGE decreased by 30% (P = 0.02). The mononuclear cell tumor necrosis factor-α/β-actin mRNA ratio was 1.4 ± 0.5 on H-AGE and 0.9 ± 0.5 on L-AGE (P = 0.05), whereas serum vascular adhesion molecule-1 was 1,108 ± 429 and 698 ± 347 ng/ml (P = 0.01) on L- and H-AGE, respectively. After 6 weeks, peripheral blood mononuclear cell tumor necrosis factor-α rose by 86.3% (P = 0.006) and declined by 20% (P, not significant) on H- or L-AGE diet, respectively; C-reactive protein increased by 35% on H-AGE and decreased by 20% on L-AGE (P = 0.014), and vascular adhesion molecule-1 declined by 20% on L-AGE (P < 0.01) and increased by 4% on H-AGE. Serum AGEs were increased by 28.2% on H-AGE (P = 0.06) and reduced by 40% on L-AGE (P = 0.02), whereas AGE low density lipoprotein was increased by 32% on H-AGE and reduced by 33% on L-AGE diet (P < 0.05). Thus in diabetes, environmental (dietary) AGEs promote inflammatory mediators, leading to tissue injury. Restriction of dietary AGEs suppresses these effects.
Medical nutrition therapy plays an important role in the management of diabetes and its complications. Current dietary strategies center on nutrients, or caloric restriction, but not on risk-associated processing methods (1–3). An unrecognized risk factor for diabetic complications is the heat-generated group of advanced glycation end products (AGEs) which form in common foods during the spontaneous reactions between reducing sugars and proteins or lipids (4–9).
AGEs are best known as endogenous products of nonenzymatic glucose–protein interactions, which include multiple highly reactive structures (7–9). Only a few in vivo AGE structures have been linked to diabetic complications. Common among them are the AGE precursor, methylglyoxal (MG), the late oxidative product ∈N-carboxymethyl-lysine (CML), pentosidine, pyrraline, and others (10–12). Free NH2-containing lipids are also subject to glycation, which is linked to simultaneous lipid peroxidation via reactive oxygen species generation (13, 14), a typical product of which is also CML. In contrast to the in vivo AGEs, dietary AGE proteins and AGE lipids form much more rapidly in the presence of heat and in far greater concentrations. AGEs, in addition to their chemical reactivity, increase cell-oxidant stress and nuclear factor-κB transcriptional activation, promoting inflammatory mediators [e.g., tumor necrosis factor α (TNFα), IL-1β, IL-6, and vascular adhesion molecule (VCAM)-1] (15–18) and supporting the hypothesis that the low-grade inflammation associated with diabetes and its complications may be in part AGE-mediated. AGE infusion in nondiabetic rabbits promotes atheroma-like lesions (15), whereas, in contrast, inhibition of AGE formation retards vascular complications in diabetic animals (19–23).
The nature of pathogenic AGEs is not known as yet (10–12), and multiple derivatives other than those of MG or CML moieties have yet to be identified. Immunoreagents to MG derivatives and CML-like products, however, have presented invaluable means for animal and clinical correlations (10, 11, 24, 25). It thus has been confirmed that two-thirds of orally absorbed dietary AGEs (≈10% of the amount ingested) are retained in tissues in bioreactive forms (26, 27). Evidence supporting pathogenicity of food AGEs in animal models has also emerged; in marked contrast to the severe vascular occlusive lesions forming in diabetic apolipoprotein E-deficient mice or the diabetic glomerulopathy in nonobese diabetic and db/db mice fed standard chow, marked protection against both pathologies was observed in animals fed a low-AGE (L-AGE) diet (28, 29) despite hyperlipidemia and hyperglycemia. In both instances, reduction of serum AGE (sAGE) and tissue AGE levels were significant.
In this article we demonstrate that modulation of oral AGE intake in diabetic subjects triggers changes in markers of inflammation, linked to diabetes and vascular dysfunction.
Methods
Study Subjects/Clinical Protocol.
Two dietary studies were carried out in a total of 24 nonsmoking, diabetic subjects recruited at the Mount Sinai School of Medicine: a 2-week randomized crossover study (n = 11) and a 6-week randomized parallel group study (n = 13). Relevant characteristics are outlined in Table 1. Study subjects were in good glucose control (HbA1c 7.5 ± 0.7%) and had normal renal function. Patients were excluded if they had HbA1c ≥9%, had frequent hypoglycemia, had a cardiac event within the preceding 3 months, were on glucocorticoids or anticoagulants (other than aspirin), had serum creatinine ≥176.8 μmol/liter, or had food allergy or religious restrictions. Four patients from the second study were treated with statins (Lipitor) and two with aspirin; these ended up equally divided between the two treatment groups. Only one subject had a history of established coronary artery disease. The protocols were approved by the Institutional Review Board, and all subjects provided informed consent.
Table 1.
Baseline patient characteristics
| 2-week study (crossover), n = 11 | 6-week study, n = 13 | |
|---|---|---|
| Age, years | 52 ± 5 | ∼62 |
| Type 1/type 2 diabetes | 2:9 | 4:8 |
| Body mass index | 28 ± 3.5 | 29.5 ± 3 |
| HbA1c, % | 7.8 ± 0.7 | 7.2 ± 0.65 |
| Serum creatinine, μmol/liter | 61.88 ± 17.68 | 81.3 ± 30.9 |
| Insulin/oral medications/diet only | 4:6:0 | 5:5:3 |
Data are shown as means ± SEM. There were no significant differences between groups at baseline.
The crossover study consisted of 2 weeks on one diet, a 1- to 2-week washout period, and 2 weeks on the alternate diet. Subjects were admitted to the clinical research center (CRC) for the initial 36 h and returned daily for the collection of blood and urine samples. Adherence assessment was based on a daily food log. Subjects were provided with packaged meals for breakfast, lunch, dinner, and snacks. Fasting and 4-h blood samples were obtained on the first day for postprandial sAGE. In addition, 13 patients were studied for a period of 6 weeks under the same regimen but randomized to either the high-AGE (H-AGE, n = 6) or L-AGE diet (n = 7). After an initial overnight hospitalization, patients returned to the CRC three times per week for blood or urine collection and to receive prepared meals.
In the >200 foods assessed, protein-associated AGEs were tested by competitive ELISA (24, 25), whereas lipid AGEs were tested by a direct ELISA (refs. 13 and 14; select items are shown in Table 2). The ELISA uses an mAb (4G9 mAb, Alteon, Ramsey, NJ) that reacts strongly with CML-modified BSA (23 CML-Lys/mol BSA) and AGE-BSA, weakly with MG-BSA (21.8 MG-Arg/mol BSA), and not with native BSA or human serum albumin. Meals were planned based on the aggregate AGE value of food components. In addition, select food categories were tested for MG derivatives by anti-MG 3D11 mAb (Table 2; ref. 25). This anti-MG-ovalbumin mAb (3D11 mAb) is reactive with MG-BSA (at 4 nmol/ml) and AGE-BSA (at ≈500 μg/ml) but not with CML-BSA, BSA, or ovalbumin (25).
Table 2.
Relative concentrations of CML and MG derivatives in foods correlate with AGE bioreactive properties
| CML, units/mg | MG, nmol/mg | GSH, % of control | TNF-α, ng/mg protein | Intermolecular crosslink formation | |
|---|---|---|---|---|---|
| Food categories | |||||
| Chicken (broiled cubes) | 73.8 ± 12 | 2.4 ± 0.8 | 19.1 ± 10 | 8.1 ± 0.2 | 110 |
| Tuna (broiled) | 31.8 ± 4.2 | 2.5 ± 0.8 | 37 ± 6.7 | 6 ± 0.4 | 9.2 |
| Egg yolk (boiled 12 min) | 18.0 ± 3.2 | 0.9 ± 0.6 | 45 ± 8 | 3.6 ± 0.4 | 22.8 |
| White bread (toasted) | 1.0 ± 0.4 | 0.7 ± 0.1 | 87 ± 10 | 5.4 ± 0.6 | 3.3 |
| Pasta (boiled 12 min) | 2.4 ± 0.3 | 0.6 ± 0.2 | 90 ± 12 | 1.5 ± 0.1 | 2.8 |
| AGE standards | |||||
| AGE-BSA | 76 ± 17 | 0.12 ± 0.04 | 35.8 ± 11 | 30.6 ± 11 | 91 |
| CML-BSA | 109 ± 18 | ND | 69.1 ± 41 | 8.5 ± 6.1 | 2 |
| MG-BSA | 47 ± 5 | 5.25 ± 1.6 | 21.6 ± 2 | 43.3 ± 20 | 55 |
| BSA | 1 ± 0.18 | ND | 85 ± 10 | 1.5 ± 0.5 | 1 |
Average content of AGEs in common representative foods is shown from triplicate measurements based on CML- or MG-sensitive competitive ELISA using 4G9 or 3D11 mAbs as described (24, 25). Where appropriate, CML values reflect the aggregate of protein- and lipid-associated immunoreactivities. ND, not determined.
Bioreactive properties: oxidant stress, cytokine induction, and crosslink formation. Fresh extracts of food samples were AGE-enriched by AGE-affinity chromatography (25, 36) and kept at −80°C. Aliquots (≈10 AGE units per sample by 4G9 mAb) were tested in culture (human umbilical vein endothelial cells for glutathione or T1B-186 for TNFα) (1 × 106 cells per well) in parallel with standard AGE-BSA, BSA, CML-BSA, and MG-BSA (100 ug per sample) (25, 32, 33). Glutathione, shown as percentage change relative to control medium (100%), and TNFα (ng/mg cell protein) were measured in cell lysates. High molecular mass complex formation by [125I]fibronectin 70-kDa fragments is shown as n-fold high molecular mass increase above native BSA.
Culture medium alone.
Two study diets were designed to have similar content of calories, protein, carbohydrate, and fat but differ by ≈5-fold in AGE content, based on CML content (4G9 mAb), by varying the cooking time and temperature. Both diets were compatible with the National Cholesterol Education Program Step 1 diet (2) and the American Diabetes Association (30) and contained 50–55% carbohydrate, 20% protein, <30% fat (< 10% saturated fat), and <300 mg cholesterol. ESHA 7.4 food-processor software (Salem, OR) was used to calculate nutrient content of the diets. Energy requirements were calculated individually based on the Harris Benedict equation, and 3-day nonconsecutive food diaries were analyzed to determine habitual energy intake. These data were used to design meal plans to maintain the subjects' weight. All meals were prepared in the clinical research center.
Clinical Tests.
HbA1c was measured by ion-exchange HPLC (Bio-Rad Variant) and serum fructosamine by a colorimetric method (Cobas Integra, Roche Diagnostics). Plasma or serum levels of C-reactive protein (CRP) were measured by immuno-nephelometry (IMMAGE Nephelometer, Beckman Coulter). TNFα mRNA levels in mononuclear cells were measured by RT-PCR as described (31), and TNFα protein and VCAM-1 were measured by ELISA kits (BioSource International, Camarillo, CA and R & D Systems).
sAGE levels were measured by CML-sensitive ELISA (4G9) (24, 25). Fresh plasma low density lipoprotein (LDL)-apolipoprotein B and lipid components were separated by standard procedures (13, 14); AGE-modified LDL components were determined by competitive or direct ELISA (4G9) as described (13, 14). Synthetic CML-BSA and MG-BSA were provided by Y. Al-Abed (Picower Institute, New York).
Bioreactive properties of food-derived AGEs were determined in parallel with standard AGE-BSA, MG-BSA, and CML-BSA (100 μg/ml) as described (25) based on (i) glutathione-depletion assay using human umbilical vein endothelial cells (OXIS International, Portland, OR) (25, 32–34), (ii) TNFα induction in T1B-186 cells using established ELISA, and (iii) intermolecular cross-linking assay (25, 35). Differences in bioreactivity levels among major food groups were generally consistent with differences in AGE immunoreactivity (Table 2).
Data Analysis.
Results from the 2-week crossover study were based on data obtained on the final day of each treatment period and were adjusted for differences in baseline values. For crossover studies ANOVA was used to compare results from the two treatment periods. Unpaired, two-tailed t test was used to analyze the difference between fasting and postprandial sAGE levels. For the 6-week study, the percent change from baseline values was compared between the L-AGE and H-AGE diet groups and analyzed by the Mann–Whitney test. Differences between baseline and 6 weeks within each treatment group were compared by using the Wilcoxon rank sum test. Mann–Whitney nonparametric analysis was used for differences between grouped sets of data; differences were considered significant at P < 0.05.
Results
Diets and Subjects.
The study's dietary goals, which were to design meals with reduced or enhanced AGE content, were achieved while maintaining the required balance in macro- and micronutrients. The mean daily AGE content of the L-AGE diet was 3.67 ± 1.2 × 106 AGE units/day ± SD compared with 16.3 ± 3.7 × 106 AGE units/day ± SD in the H-AGE diet.
Based on testing of >200 common foods by the CML-sensitive ELISA (select items are shown in Table 2), several patterns emerged. Foods from animal sources that were high in protein and lipid content (meat, cheese, and egg yolks) had the highest values of either CML or MG derivatives and the highest prooxidant, cell-activating potency (Table 2; ref. 25).
During the 2-week study, there were no significant differences in glucose control, cholesterol, triglycerides, body weight, or blood pressure (Table 3).
Table 3.
Effects of different dietary AGE consumption on metabolic variables during 2-week and 6-week studies
|
|
2-week crossover study | 6-week study | ||||||
|---|---|---|---|---|---|---|---|---|
| H-AGE | L-AGE | |||||||
| H-AGE | L-AGE | P | Baseline | 6 weeks | Baseline | 6 weeks | P | |
| Body weight, kg | 81.7 ± 4.2 | 81.2 ± 4.2 | 0.2 | 89.1 ± 7 | 88.9 ± 6.8 | 72 ± 7.2 | 70.4 ± 7.5 | 0.02 |
| Systolic blood pressure, mmHg | 126 ± 3.9 | 133 ± 4.5 | 0.2 | 125.2 ± 3.7 | 127 ± 2 | 127.1 ± 3 | 122 ± 6 | 0.3 |
| Diastolic blood pressure, mmHg | 73 ± 3 | 72 ± 2.6 | 0.6 | 60.7 ± 4 | 63 ± 2 | 67.6 ± 5 | 64 ± 5 | 0.4 |
| Fasting glucose, mmol/liter | 7.5 ± 0.7 | 8.1 ± 0.4 | 0.5 | 6.5 ± 1.2 | 8.1 ± 1.1 | 7.0 ± 1 | 5.6 ± 0.5 | 0.02 |
| Fructosamine, mmol/liter | 319 ± 11 | 325 ± 13 | 0.3 | 299.3 ± 21 | 324 ± 15 | 345 ± 20.3 | 281 ± 13 | 0.8 |
| Cholesterol, mmol/liter | 4.6 ± 0.2 | 4.5 ± 0.3 | 0.4 | 4.97 ± 0.6 | 4.6 ± 0.6 | 4.4 ± 0.2 | 4.4 ± 0.2 | 0.2 |
| LDL cholesterol, mmol/liter | 2.7 ± 0.1 | 2.6 ± 0.2 | 0.1 | 2.86 ± 0.2 | 2.7 ± 0.5 | 2.4 ± 0.1 | 2.5 ± 0.2 | 0.1 |
| HDL cholesterol, mmol/liter | 1.3 ± 0.07 | 1.2 ± 0.1 | 0.1 | 1.53 ± 0.15 | 1.4 ± 0.2 | 1.38 ± 0.1 | 1.3 ± 0.1 | 0.2 |
| Triglycerides, mmol/liter | 1.2 ± 0.1 | 1.3 ± 0.1 | 0.4 | 1.25 ± 0.1 | 1.2 ± 0.1 | 1.15 ± 0.08 | 1.1 ± 0.1 | 0.9 |
Results are expressed as means ± SEM. HDL, high density lipoprotein.
P values refer to the significance between last day of the H-AGE and L-AGE diets.
H-AGE, n = 6; L-AGE, n = 7. P values refer to significance of percentage change (from baseline to 6 weeks) of H-AGE versus L-AGE.
Despite the small reductions in fasting plasma glucose and body weight seen at the end of the 6-week L-AGE versus H-AGE diet (P = 0.02), blood pressure, HbA1c, total and LDL cholesterol, high density lipoprotein cholesterol, and triglycerides remained similar in both studies (Table 3).
sAGE Components.
Two-week study.
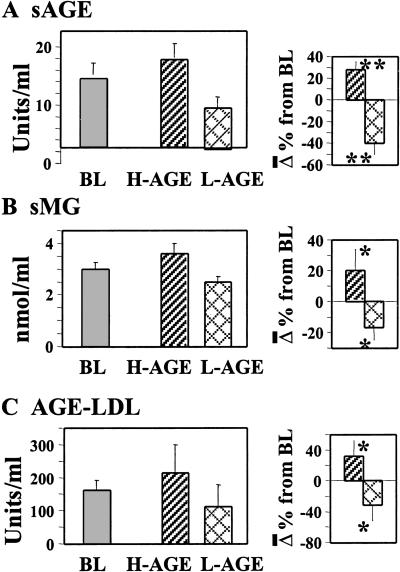
Changes in fasting sAGE levels were seen in all subjects: sAGE increased on the H-AGE diet (from 7.9 ± 2.1 to 13.0 ± 6 AGE units/ml, P = 0.02) and declined on the L-AGE diet (from 10.9 ± 3.2 to 7.7 ± 2.4 AGE units/ml, P = 0.01) (Table 4). Also, AGE-modified LDL cholesterol changed significantly within 2 weeks on either diet (P = 0.03) (Table 4), although AGE-apolipoprotein B changes did not reach significance at 2 weeks (P = 0.08). Total 24-h urinary excretion of low molecular weight AGE during the 2-week study was significantly reduced on the L-AGE diet compared with that seen with the H-AGE (P = 0.01) diet, as was the AGE/creatinine ratio (P = 0.02) (Table 4). There was a small but significant increase in sAGE levels 4 h postprandially on the H-AGE (P < 0.001) but not the L-AGE (P = not significant) diet (Table 5).
Table 4.
Changes in inflammatory markers correspond to changes in sAGE (2-week crossover study)
| H-AGE | L-AGE | P | |
|---|---|---|---|
| sAGE, units/ml | 13.0 ± 6 | 7.7 ± 2.4 | 0.02 |
| AGE LDL, units/ml | 168 ± 57 | 120 ± 54 | 0.03 |
| Urine AGE, units × 10−3/24 h | 30.4 ± 12 | 15.26 ± 10 | 0.01 |
| AGE/creatinine, units/mg | 22 ± 8 | 13 ± 6 | 0.02 |
| TNFα/β-actin | 1.42 ± 0.5 | 0.91 ± 0.5 | 0.05 |
| VCAM-1, ng/ml | 1,108 ± 429 | 698 ± 347 | 0.01 |
| CRP, mg/dl | 6.0 ± 8.6 | 4.1 ± 4.8 | 0.38 |
Values (mean ± SD) were obtained at end of the H- and L-AGE diet study periods.
TNFα mRNA from subjects' peripheral mononuclear cells was tested by RT-PCR; data are shown as mean ± SEM of TNFα/β-actin ratio.
Table 5.
Pre- and postprandial sAGE levels
| L-AGE diet | H-AGE diet | |
|---|---|---|
| Preprandial, AGE units/ml | 10.3 ± 3 | 8.1 ± 2 |
| Postprandial, AGE units/ml | 9.9 ± 2 | 9.7 ± 2† |
sAGE levels (mean ± SD) were measured on day 1 prior to and 4 h after meal (dinner).
, P = 0.001.
Six-week study.
After 6 weeks of the randomized parallel group diet study, fasting sAGE levels were reduced by 40% on the L-AGE diet and increased by 28.2% on the H-AGE diet from baseline values (P = 0.02) (Fig. 1A). In this study serum immunoreactive MG derivatives were also tested and found to be increased by 20% on the H-AGE diet and reduced by 16% on the L-AGE diet (P = 0.007; Fig. 1B). After 6 weeks, AGE-LDL cholesterol increased by 32% on H-AGE and decreased by 33% from baseline on L-AGE diet (P = 0.05; Fig. 1C). As with the 2-week study, baseline serum protein or plasma LDL lipid-associated AGE values in this study did not vary significantly between study groups with regard to both CML and MG derivatives.
Fig. 1.
Circulating AGE levels correspond to dietary AGE intake: a randomized 6-week study. Fasting sAGE levels were determined in 13 diabetic patients (H-AGE, n = 6; L-AGE, n = 7) by CML-sensitive (A) (4G9 mAb) or an MG derivative-sensitive ELISA (B) (3D11 mAb). (C) Plasma AGE-LDL lipid levels were assessed by direct ELISA (4G9). Values are shown at baseline (BL) and the end of H-AGE or L-AGE; data are shown as mean ± SEM of triplicate measurements. The percentage of change between H-AGE and L-AGE are shown (Right). *, P < 0.05; **, P < 0.01.
Vascular Markers.
Two-week study.
Peripheral blood mononuclear TNFα mRNA levels were found reduced on the L-AGE diet compared with the H-AGE diet (P = 0.05; Table 4), whereas serum VCAM-1 was reduced by ≈50% by L-AGE diet compared with H-AGE diet (P = 0.01; Table 4).
Six-week study.
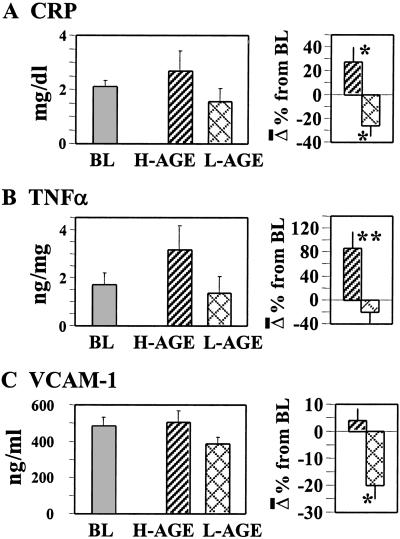
After longer exposure to the two diets, serum CRP levels were found increased by 35% from baseline after the H-AGE diet and decreased by 20% after the L-AGE diet (P = 0.01; Fig. 2A). Peripheral blood mononuclear TNFα protein levels increased significantly (by 86.3%) on the H-AGE diet (P = 0.006) compared with the 20% decrease seen after the L-AGE diet (Fig. 2B); similarly, on the L-AGE diet serum VCAM-1 levels decreased by 20% (P = 0.03; Fig. 2C). No significant differences were present at baseline between study groups.
Fig. 2.
Changes in circulating inflammatory markers during a 6-week exposure to H-AGE versus L-AGE diet: a randomized study. Serum CRP (A), peripheral blood mononuclear cell-derived TNFα (B), or serum VCAM-1 values (C) were obtained at baseline (BL) or at the end of H-AGE or L-AGE periods; data are shown as mean ± SEM of triplicate measurements (n = 13 patients: H-AGE, n = 6; L-AGE, n = 7). The percent changes between H-AGE and L-AGE are displayed (Right). *, P < 0.05; **, P < 0.01.
Discussion
The current study demonstrates that circulating glycotoxins (sAGE) can be modulated in human diabetes by altering dietary AGE intake. sAGE changes are followed by parallel changes in levels of inflammatory molecules (CRP, TNFα, and VCAM-1), all three of which are established markers of diabetes and vascular disease. These American Heart Association- and American Diabetic Association-approved diets were not enriched in fat or carbohydrate (as are Western diets) and were balanced for vitamin content including antioxidant supplements (25). Subjects were under fair glycemic control; only one patient had an established history of coronary artery disease, and no subjects had clinically evident renal disease. Significant changes in sAGE occurred within a short period (2 weeks), which paralleled AGE consumption and were sustained for the 6 weeks of study. Therefore, these changes could not be attributed to alterations in glycemic control nor to energy intake.
Unlike other dietary interventions, the manipulation applied here involved normal procedures of meal preparation (e.g., temperature or time) and not of principal nutrients. Partial degradation of heat-labile vitamins such as thiamine, folate, and B6, known for antioxidant and anti-AGE properties (34, §), was compensated for by standard supplementation (34).
Although hyperglycemia remains a major contributor to diabetic complications, based on the Diabetes Control and Complications Trial and United Kingdom Prospective Diabetes Study trials (30, 36, 37), sustained euglycemia without additional measures (e.g., control of weight gain, cholesterol, or smoking) has yet to be proven effective (1–3).
Herein, glucose and lipid abnormalities were unremarkable over the course of the studies. The findings therefore were independent of dietary fat or carbohydrate and pointed to other factors, namely exogenously supplied dietary AGEs. Changes in CRP are found to mirror sAGE, corresponding to dietary intake, indicating that exogenous oxidant stress-promoting AGEs may contribute to the diabetes-related inflammatory state (23, 38, 39). CRP exhibits a significant association with diabetes and related vascular mortality (40–43). Thus, it is conceivable that the frequent intake of dietary AGE promotes a sustained low-grade inflammatory state. In this sense, other markers of immune response such as cytokines and adhesion molecules are inducible by AGEs via reactive oxygen species production and nuclear factor κB activation (7–14, 44), properties which are also exhibited by dietary AGEs (25). These reactive molecules (ranging between 12 and 22 million AGE units in a “healthy meal”), which are “seeding” the systemic circulation regularly, could, together with hyperglycemia, contribute to the subtle inflammatory state associated with diabetes (23, 41–43, 45).
The identity of pathogenic AGEs remains largely undefined. However, an interesting correlation between postprandial hyperglycemia and serum levels of MG and 3-deoxyglucosone (46) or a transient increase in the production of reactive oxygen species after a meal in diabetic subjects have been reported (47). In this study, postprandial AGE levels correspond to the amount of AGEs ingested (26, 27). Furthermore, the steady-state AGE levels follow a pattern that is consistent with the amount ingested and moves synchronously with the inflammatory mediators. These data may provide a further link between sustained tissue vulnerability and vascular dysfunction (42, 43, 45, 48–50), lasting well beyond the window of postprandial glycemia or lipidemia.
Subjects fed oxidized lipids have been reported to have elevated atherogenic oxidized lipids in postprandial chylomicrons, especially in the presence of diabetes (51, 52). These reports, given the typical Western dietary patterns, may point indirectly to enhanced interactions between dietary lipids and AGEs. Thus, the correlation between dietary glycoxidant and lipoxidant substance influx and inflammatory markers offers a hitherto unrecognized explanation for the higher incidence of vasculopathy.
In our studies, AGE-modified LDL fluctuated significantly in response to AGE intake. A positive correlation between circulating AGE-LDL, vascular tissue AGE, and lesion severity has been shown in nondiabetic patients with occlusive vascular disease (53). A rise in AGE-LDL due to impaired LDL-receptor clearance of LDL (14) would favor tissue AGE-lipid accumulation and inflammatory response. Thus, the concordance between sAGE, AGE-LDL, and inflammatory markers in a diabetic population largely free of vascular disease may provide an additional link between food-derived reactive AGEs and diabetic atherogenesis.
In summary, dietary AGEs are significant contributors to sAGEs in humans. Sustained reduction in AGEs intake may result in effective suppression of inflammatory molecules in diabetes, implying eventual prevention or delay of atherosclerosis. Further clinical studies are needed to establish this modality as a nonpharmacological intervention for diabetic macrovascular disease.
Acknowledgments
We thank Dr. Yousef Al-Abed (Picower Institute) for preparation and characterization of synthetic CML and MG protein preparations. We are grateful to Ina Katz for providing invaluable editorial assistance. This work was supported by National Institutes of Health Grant AG09453 (to H.V.).
Abbreviations
AGE, advanced glycation end product
MG, methylglyoxal
CML, ∈N-carboxymethyl-lysine
TNFα, tumor necrosis factor α
VCAM, vascular adhesion molecule
L-AGE, low AGE
sAGE, serum AGE
H-AGE, high AGE
CRP, C-reactive protein
LDL, low density lipoprotein
This paper was submitted directly (Track II) to the PNAS office.
Lin, J., Alt, A., Liersch, J., Reinhard, G. B., Brownlee, M. A. & Hammes, H.-P. (2000) Diabetes 49,A143 (abstr.).
References
- 1.American Diabetes Association (2001) Diabetes Care 24, Suppl. 1 44-47. [PubMed] [Google Scholar]
- 2.National Cholesterol Education Program (1994) Circulation 89 1333-1445. [DOI] [PubMed] [Google Scholar]
- 3.Lauber R. P. & Sheard, N. F. (2001) Nutr. Rev. 59 298-306. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien J. & Morrissey, P. A. (1989) Crit. Rev. Food Sci. Nutr. 28 211-248. [DOI] [PubMed] [Google Scholar]
- 5.Maillard L. C. (2002) C. R. Acad. Sci. 1912 154-166. [Google Scholar]
- 6.Lee T. C., Kimiagar, M., Pintauro, S. J. & Chichester, C. O. (1981) Prog. Food Nutr. Sci. 5 243-256. [PubMed] [Google Scholar]
- 7.Brownlee M., Cerami, A. & Vlassara, H. (1988) N. Engl. J. Med. 318 1315-1321. [DOI] [PubMed] [Google Scholar]
- 8.Baynes J. W. & Thorpe, S. R. (2000) Free Radical Biol. Med. 28 1708-1716. [DOI] [PubMed] [Google Scholar]
- 9.Ruderman N., Williamson, J. & Brownlee, M., (1992) Hyperglycemia, Diabetes and Vascular Disease (Oxford Univ. Press, Oxford).
- 10.Oya T., Hattori, N., Mizuno, Y., Miyata, S., Maeda, S., Osawa, T. & Uchida, K. (1999) J. Biol. Chem. 274 18492-18502. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda K., Higashi, T., Sano, H., Jinnouchi, Y., Yoshida, M., Araki, T., Veda, S. & Horiuchi, S. (1996) Biochemistry 35 8075-8083. [DOI] [PubMed] [Google Scholar]
- 12.Requena J. R., Ahmed, M. U., Fountain, C. W., Degenhardt, T. P., Reddy, S., Perez, C., Lyons, T. J., Jankins, A. J., Baynes, J. W. & Thorpe, S. R. (1997) J. Biol. Chem. 272 17473-17479. [DOI] [PubMed] [Google Scholar]
- 13.Bucala R., Mitchell, R., Arnold, K., Innerarity, T., Vlassara, H. & Cerami, A. (1995) J. Biol. Chem. 270 10828-10832. [DOI] [PubMed] [Google Scholar]
- 14.Bucala R., Makita, Z., Koschinsky, T., Cerami, A. & Vlassara, H. (1993) Proc. Natl. Acad. Sci. USA 90 6434-6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlassara H., Fuh, H., Donnelly, T. & Cybulski, M. (1995) Mol. Med. 1 447-456. [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt A. M., Hori, O., Chen, J. X., Li, J. F., Crandall, J., Zhang, J., Cao, R., Yan, S. D., Brett, J. & Stern, D. (1995) J. Clin. Invest. 96 1395-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vlassara H., Brownlee, M., Manogue, K. R., Dinarello, C. A. & Pasagian, A. (1998) Science 240 1546-1548. [DOI] [PubMed] [Google Scholar]
- 18.Esposito C., Gerlach, H., Brett, J., Stern, D. & Vlassara, H. (1998) J. Exp. Med. 170 1387-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brownlee M., Vlassara, H., Kooney, A., Ulrich, P. & Cerami, A. (1986) Science 232 1629-1632. [DOI] [PubMed] [Google Scholar]
- 20.Lonn E., Yusuf, S., Dzavic, V., Doris, C. I., Yi, Q., Smith, S., Moore-Cox, A., Bosch, J., Riley, W. A. & Teo, K. K. (2001) Circulation 103 919-925. [DOI] [PubMed] [Google Scholar]
- 21.Che W., Asahi, M., Takahashi, M., Kaneto, H., Okado, A., Higashiyama, S. & Taniguchi, N. (1997) J. Biol. Chem. 272 8453-8459. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa T., Edelstein, D., Du, X. L., Yamagishi, S., Matsumura, T. & Kaneda, Y. (2000) Nature 404 787-790. [DOI] [PubMed] [Google Scholar]
- 23.Du X. L., Edelstein, D., Rossetti, L., Fantus, I. G., Goldberg, H., Ziyadeh, F., Wu, J. & Brownlee, M. (2000) Proc. Natl. Acad. Sci. USA 97 12222-12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitsuhashi T., Vlassara, H., Founds, H. W. & Li, Y. M. (1997) J. Immunol. Methods 207 79-88. [DOI] [PubMed] [Google Scholar]
- 25.Cai W., Gao, Q., Zhu, L., Peppa, M., He, C. & Vlassara, H. (2002) Mol. Med. 8 337-346. [PMC free article] [PubMed] [Google Scholar]
- 26.Koschinsky T., He, C. J., Mitsuhashi, T., Bucala, R., Liu, C., Buenting, C., Heitmann, K. & Vlassara, H. (1997) Proc. Natl. Acad. Sci. USA 94 6474-6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He C., Sabol, J., Mitsuhashi, T. & Vlassara, H. (1999) Diabetes 48 1308-1315. [DOI] [PubMed] [Google Scholar]
- 28.Lin R. Y., Reis, E., Dore, A., Lu, M., Ghodsi, N., Fallon, J. T., Fisher, E. A. & Vlassara, H. (2002) Atherosclerosis 162 303-311. [DOI] [PubMed] [Google Scholar]
- 29.Feng Z., He, C., Cai, W., Hattori, M., Steffes, M. & Vlassara, H. (2002) Diabetes Metab. Res. Rev. 18 224-237. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association (2001) Diabetes Care 23, Suppl. 1 64-65. [Google Scholar]
- 31.Yang C. W., Vlassara, H., Peten, E. P., He, C. J., Striker, G. E. & Striker, L. J. (1994) Proc. Natl. Acad. Sci. USA 91 9436-9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson M. E. (1989) in Glutathione: Chemical, Biochemical and Medical Aspects, eds. Dolphin, D., Poulson, R. & Avramovic, O. (Wiley, New York), pp. 339–365.
- 33.Chu F. F. (2000) Cytogenet. Cell Genet. 66 96-98. [DOI] [PubMed] [Google Scholar]
- 34.Karmas E. & Harris, R. S., (1995) Nutritional Evaluation of Food Processing (Van Nostrand Reinhold, New York).
- 35.Mitsuhashi T., Li, Y. M., Fishbane, S. & Vlassara, H. (1997) J. Clin. Invest. 100 847-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diabetes Control and Complications Trial Research Group (1993) N. Engl. J. Med. 329 977-986. [DOI] [PubMed] [Google Scholar]
- 37.U.K. Prospective Diabetes Study Group (1998) Lancet 352, 837–853.
- 38.Golovchenko I., Goalstone, M. L., Watson, P., Brownlee, M. & Draznin, B. (2000) Circ. Res. 87 746-752. [DOI] [PubMed] [Google Scholar]
- 39.Koenig W., Sund, M., Fröhlich, M., Fischer, H. G., Löwel, H., Döring, A., Hutchinson, W. L. & Pepys, M. B. (1999) Circulation 99 237-242. [DOI] [PubMed] [Google Scholar]
- 40.Stec J. J., Silbershatz, H., Tofler, G. H., Matheney, T. H., Sutherland, P., Lipinska, I., Massaro, J. M., Wilson, P. F. W., Muller, J. E. & D'Agostino, R. B., Sr. (2000) Circulation 102 1634-1638. [DOI] [PubMed] [Google Scholar]
- 41.Jager A., van Hinsbergh, V. W. M., Kostense, P. J., Emeis, J. J., Yudkin, J. S., Nijpels, G., Dekker, J. M., Heine, R. J., Bouter, L. M. & Stehouwer, C. D. A. (1999) Arterioscler. Thromb. Vasc. Biol. 19 3071-3078. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Moran M. & Guerrero-Romero, F. (1999) J. Diabetes Complications 13 211-215. [DOI] [PubMed] [Google Scholar]
- 43.Morigi M., Angioletti, S., Imberti, B., Donadelli, R., Micheletti, G., Figliuzzi, M., Remuzzi, A., Zoja, C. & Remuzzi, G. (1998) J. Clin. Invest. 101 1905-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jinnouchi Y., Sano, H., Nagai, R., Hakamata, H., Kodama, T., Suzuki, H., Yoshida, M., Veda, S. & Horiuchi, S. (1998) J. Biochem. (Tokyo) 123 1208-1217. [DOI] [PubMed] [Google Scholar]
- 45.Panés J., Kurose, I., Rodriguez-Vaca, M. D., Anderson, D. C., Miyasaka, M., Tso, P. & Granger, D. N. (1996) Circulation 93 161-167. [DOI] [PubMed] [Google Scholar]
- 46.Beisswenger P. J., Howell, S. K., O'Dell, R. M., Wood, M. E., Touchette, A. D. & Szwergold, B. S. (2001) Diabetes Care 24 726-732. [DOI] [PubMed] [Google Scholar]
- 47.Ceriello A., Bortolotti, N., Motz, E., Crescentini, A., Lizzio, S., Russo, A., Tonutti, L. & Taboga, C. (1998) Diabetes Care 21 1529-1533. [DOI] [PubMed] [Google Scholar]
- 48.Shige H., Ishikawa, T., Suzukawa, M., Ito, T., Nakajima, K., Higashi, K., Ayaori, M., Tabata, S., Ohsuzu, F. & Nakamura, H. (1999) Am. J. Cardiol. 84 1272-1274. [DOI] [PubMed] [Google Scholar]
- 49.Ceriello A., Taboga, C., Tonutti, L., Giacomello, R., Stel, L., Motz, E. & Pirisi, M. (1996) Diabetologia 39 469-473. [DOI] [PubMed] [Google Scholar]
- 50.Pirags V., Assert, R., Haupt, K., Schatz, H. & Pfeiffer, A. (1996) Exp. Clin. Endocrinol. Diabetes 104 431-440. [DOI] [PubMed] [Google Scholar]
- 51.Staprans I., Rapp, J. H., Pan, X. M., Kim, K. Y. & Feingold, K. R. (1994) Arterioscler. Thromb. 14 1900-1905. [DOI] [PubMed] [Google Scholar]
- 52.Staprans I., Hardman, D. A., Pan, X. M. & Feingfold, K. R. (1999) Diabetes Care 22 300-306. [DOI] [PubMed] [Google Scholar]
- 53.Stitt A. W., He, C. J., Friedman, S., Scher, L., Rossi, P., Ong, L., Founds, H., Li, Y. M., Bucala, R. & Vlassara, H. (1997) Mol. Med. 3 617-627. [PMC free article] [PubMed] [Google Scholar]