Abstract
Gestational diabetes coincides with elevated circulating progesterone levels. We show that progesterone accelerates the progression of diabetes in female db/db mice. In contrast, RU486, an antagonist of the progesterone receptor (PR), reduces blood glucose levels in both female WT and db/db mice. Furthermore, female, but not male, PR−/− mice had lower fasting glycemia than PR+/+ mice and showed higher insulin levels on glucose injection. Pancreatic islets from female PR−/− mice were larger and secreted more insulin consequent to an increase in β-cell mass due to an increase in β-cell proliferation. These findings demonstrate an important role of progesterone signaling in insulin release and pancreatic function and suggest that it affects the susceptibility to diabetes.
Pregnancy is associated with progressive deterioration of glucose sensitivity to insulin action, termed insulin resistance, as well as an increase in nutrient-stimulated insulin secretion (1). Hepatic glucose production is also reported to increase by 15–30% to meet placental and fetal needs (2). These modifications culminate in late pregnancy by a 50–70% reduction in insulin-stimulated glucose uptake in women (2). In 7% of all pregnancies, which represents 200,000 cases annually in the United States alone (3), insulin secretion cannot adapt sufficiently, and gestational diabetes mellitus develops (4).
Modifications in the hormonal status during pregnancy are in part responsible for the alterations in glucose homeostasis. Changes in glucose tolerance and β-cell responsiveness to glucose during pregnancy are concomitant with increases in circulating cortisol, prolactin, placental lactogen, estrogen, and progesterone levels, which all have been shown to modulate glucose metabolism to some extent (2). During pregnancy, progesterone levels increase to favor embryo implantation and blunt ovulation. Traditionally, the association of progesterone levels with gestational diabetes has been primarily attributed to its negative effects on insulin sensitivity. Pregnant rats infused with progesterone are more insulin resistant than placebo-treated rats (5), perhaps because of reductions in GLUT4 expression and glucose uptake in skeletal muscle (6, 7), as well as in white adipose tissue (8, 9). More recently, it has been shown that acute progesterone treatment can enhance insulin secretion to adapt for the decrease in insulin sensitivity (10). However, β-cell function appears to be reduced on chronic exposure to progesterone in vivo (10–12).
Here, we first demonstrate a role of progesterone in female, diabetic db/db mice, in which treatment with either progesterone or RU486, which act as agonist and antagonist of the progesterone receptor (PR), respectively, interferes with glucose homeostasis. We furthermore used PR-knockout (PR−/−) mice to determine the exact role of the progesterone-signaling pathway on glucose metabolism in vivo. Compared to WT animals, female PR−/− mice had an improved glucose tolerance due to an enhanced insulin secretion primarily caused by an increase in β-cell mass subsequent to β-cell proliferation. These findings demonstrate the important contribution of progesterone to glucose homeostasis.
Materials and Methods
Animals.
The generation of PR WT (+/+) and knockout (−/−) mice has been described (13). These mice were maintained in a hybrid C57BL/6J/129 simian virus background (75%/25%, respectively). C57BL/6J mice were purchased from The Jackson Laboratory. Only age-matched (10–16 weeks old) mice were used, unless otherwise stated. Animals were maintained in a temperature-controlled (23°C) facility with a 12-hr light/dark cycle according to the European Union guide for use of laboratory animals. Mice had ad libitum access to water and regular rodent chow (DO4, Usine d'Alimentation Rationelle, France).
In Vivo Evaluation of Glucose Homeostasis.
Overnight fasted mice were injected with glucose (2 g/kg) as described (14). Blood was obtained by tail vein incision, and glycemia was then recorded at indicated times before and after the injection. Glucose was measured by the Maxi Kit Glucometer 4 (Bayer Diagnostic, Puteaux, France). Insulin was measured by ELISA (Crystal Chem, Chicago). Progesterone levels were assessed by RIA (Beckman Coulter).
Pancreatic Islet Studies.
Small pieces of pancreas were digested by collagenase (3 mg/ml) and isolated in oxygenated Krebs–Ringer buffer (134 mM NaCl/4.7 mM KCl/1.2 mM KH2/1.2 mM MgSO4/1 mM CaCl2/10 mM Hepes/20 mM haHCO3/5.5 mM glucose). Approximately four islets per condition were hand-picked and exposed to glucose at the indicated concentrations in the presence or absence of diazoxide (10–6 M), KCl (30 mM), or arginine (20 mM). Insulin released in the medium was measured 30 min later. Data were corrected on a per islet or per milligram of protein basis. Other pieces of pancreas were digested as described above. The isolated islets were homogenized in a solution (pH 7.4) containing 20 mM Hepes, 250 mM sucrose, 4 mM EDTA, and protein inhibitors. Homogenates were kept at −20°C until processing by Western blot.
Histological Studies.
Sections of pancreas were fixed in either Bouin's solution or 10% formaldehyde. They were then either stained with hematoxylin/eosin for analysis of islet size and number, or incubated with specific Abs directed against insulin or glucagon (15), BrdUrd (Dako), or annexin (Boehringer Mannheim).
Statistical Analysis.
Data are presented as means ± SEM. Group means were compared by factorial ANOVA. Upon significant interactions, differences between individual group means were analyzed by Fisher's probable least-squares difference (PLSD) test. Differences were considered statistically significant at P < 0.05.
Results
Progesterone Modulates Glucose Homeostasis in Vivo.
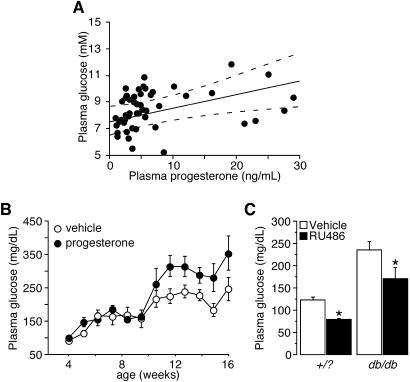
We initially correlated circulating glucose and progesterone levels in the nonfasted state in normal C57BL/6J female mice studied randomly during their oestrous status. In these conditions, we observed a positive correlation between the two variables (r = 0.622, P = 0.037), suggesting that increased progesterone levels are associated with hyperglycemic states (Fig. 1A). A similar but weaker correlation was also found in male mice (r = 0.293, P = 0.074, data not shown).
Fig. 1.
Progesterone affects glucose homeostasis in vivo. (A) Regression analysis of plasma glucose levels plotted against plasma progesterone levels in 51 female B57CL/6J nonfasted mice. (B) Time course of fasting plasma glucose levels in female db/db mice chronically treated with either vehicle or progesterone. (C) Fasting blood glucose levels in +/? and db/db mice treated daily for 2 weeks with either vehicle or RU486 (30 mg/kg). *, A significant difference compared to the vehicle-treated counterparts (P < 0.05).
To further evaluate the impact of progesterone levels on glycemic control, we chronically treated 4-week-old, female db/db mice with either vehicle or progesterone and monitored their plasma glucose levels weekly until the establishment of frank diabetes. Fasting blood glucose concentrations in both groups increased from 75 to 150 mg/dl between 4 and 9 weeks of age (Fig. 1B). After that, fasting glucose levels further increased to reach a plateau (Fig. 1B). This second increase in plasma glucose is usually associated with compensatory β-cell hyperplasia (16). Interestingly, whereas no difference in glycemia was observed between the two groups during the first rise of blood glucose (between weeks 4 and 9), a dichotomy appeared after 9 weeks of age, where glucose levels increased more in progesterone-treated mice relative to untreated mice (320 vs. 215 mg/dl, P < 0.002, Fig. 1B).
We then tested the hypothesis that blocking the progesterone-signaling pathway by administrating RU486, an antagonist of the PR, would prevent the rise in blood glucose levels after 9 weeks (16) in db/db mice. Female db/db mice (2 months old) were treated daily with either vehicle or RU486 (30 mg/kg) for 2 weeks. Vehicle-treated db/db mice had more severe hyperglycemia compared to vehicle-treated controls (220 vs. 116 mg/dl, P < 0.001, Fig. 1C). In both WT and db/db mice, RU486 treatment significantly decreased blood glucose levels (P < 0.003). Taken together, these findings support the hypothesis that progesterone negatively modulated glucose metabolism in vivo.
Improved Glucose Tolerance in Female PR−/− Mice.
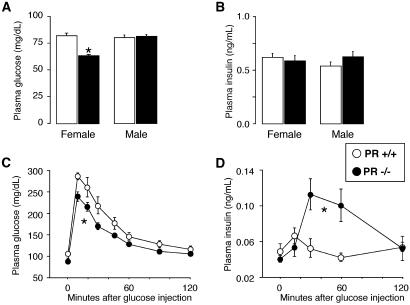
To further evaluate the role of progesterone signaling in glucose homeostasis, we compared glucose and insulin levels in male and female PR+/+ and PR−/− mice after a 6-h fast. Fasting glycemia was lower in female PR−/− mice but not in male PR−/− animals (Fig. 2A), whereas in both genders, no differences in insulin levels were detected between the two genotypes (Fig. 2B). We then performed a glucose tolerance test in overnight fasted female mice. PR−/− mice cleared glucose at a higher rate than PR+/+ mice after glucose injection (2 g/kg, i.p.; P < 0.05, Fig. 2C). Interestingly, PR−/− mice had significantly higher circulating insulin concentrations during the glucose tolerance test compared to PR+/+ mice (P = 0.004, Fig. 2D), suggesting that increased insulin secretion contributed to the faster glucose clearance.
Fig. 2.
Improved glucose tolerance in female PR−/− mice. (A) Plasma glucose concentrations in male and female PR+/+ and −/− mice after a 6-h fast (n = 11–12). (B) Plasma insulin levels in male and female PR+/+ and −/− mice after a 6-h fast (n = 11–12). (C) Glucose tolerance test in overnight fasted female PR+/+ and −/− mice. Mice were injected with glucose (2 g/kg), and glycemia was monitored at indicated time points. (D) Plasma insulin levels measured during the glucose tolerance test in C. *, A significant difference compared to the WT counterparts (P < 0.05). Black symbols represent PR−/− mice and white symbols represent the PR+/+ genotype (n = 10–11 mice).
Pancreatic Islets from Female PR−/− Mice Secrete More Insulin.
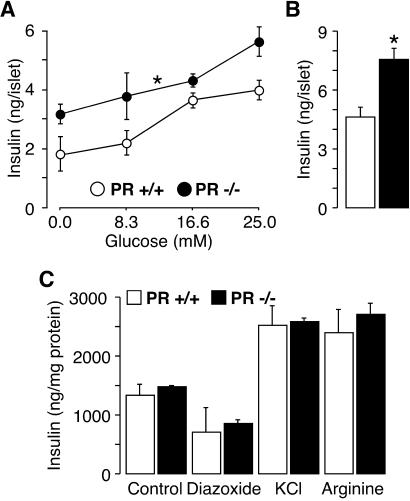
The fact that PR is expressed in pancreatic islets (17, 18) suggests that the potential exists for a direct effect of progesterone signaling on insulin secretion in this tissue. To determine whether the lack of PR directly affects insulin secretion, we isolated pancreatic islets from female PR+/+ and −/− mice by collagenase digestion and measured insulin release in the medium after incubation with secretagogs for 30 min. When expressed on a per islet basis, islets from PR−/− mice secreted more insulin compared to those from WT mice in response to increasing glucose concentrations (P = 0.0162, Fig. 3A) or 20 mM arginine, a non-glucose-like secretagogue (P = 0.0136, Fig. 3B). These differences, however, were no longer detected when insulin release was corrected for protein content. On incubation with 16.6 mM glucose, islets from PR+/+ and −/− mice responded similarly to treatments (when expressed on a per milligram protein basis) with 1 μM diazoxide, a drug acting on the K+-ATP channels, 30 mM KCl, which affects mediators downstream of the K+-ATP channels, or 20 mM arginine (Fig. 3C). Taken together, these findings suggest that higher insulin secretion in response to glucose or arginine in pancreatic islets lacking PR is not due to a specific mechanism in the β-cell, but is rather a consequence of a higher β-cell number within the islet, most likely that of β-cells.
Fig. 3.
Lack of PR results in an enhanced insulin secretion. Isolated islets from PR+/+ and −/− mice were exposed to increasing concentrations of glucose (A), with 20 mM arginine (B), or in the presence of 16.6 mM glucose and 1 μM diazoxide, 30 mM KCl, or 20 mM arginine. Insulin concentration in the medium was measured 30 min later. Data were corrected on a per islet (A and B) or per milligram of protein (C) basis. Experiments were done in triplicate. One representative experiment of two is shown. *, See Fig. 2 for statistical significance.
Lack of PR Increases β-Cell Number in Female Mice.
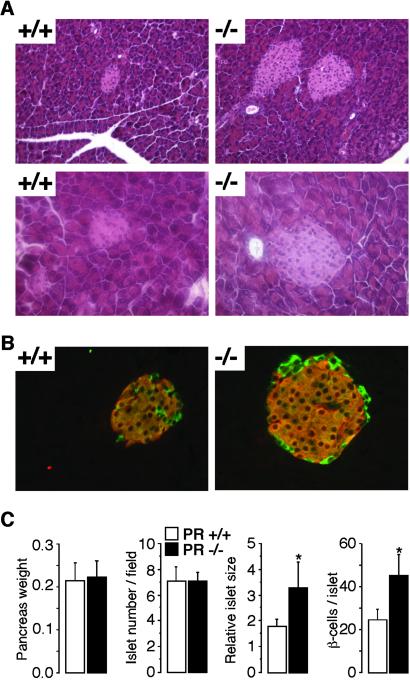
Histological analysis of the pancreas demonstrated that islets from female PR−/− mice were larger compared to those of PR+/+ mice (Fig. 4A), but the number of islets per field was similar between the two groups (Fig. 4C). Likewise, the weight of the pancreas was unchanged in female PR−/− mice (Fig. 4C). Under higher magnification, no apparent difference in cell size within the islet was observed (Fig. 4 A and B), but a clear increase in cell number was noticed (Fig. 4C). We therefore determined possible modifications in the ratio of β-cells/α-cells by staining pancreatic sections with Abs specifically directed against insulin and glucagon (Fig. 4B). Analysis of this double immunofluorescent staining indicated that the lack of PR did not affect the number of α-cells, but rather increased the amount of β-cells (Fig. 4C). These findings suggest that a lack of PR stimulates β-cell hyperplasia rather than affecting β-cell size.
Fig. 4.
Pancreatic islet size is higher in female PR−/− mice. (A) Hematoxylin/eosin staining of pancreas sections from PR+/+ and −/− female mice. [Magnification, ×20 (Upper) and ×40 (Lower).] Five sections cut with a 20-μm thickness interval were studied in five different animals per genotype. (B) Insulin and glucagon fluorescent immunostaining on the adjacent pancreas sections used in A. (Magnification, ×40.) (C) Pancreas weight, size, and number of pancreatic islets, and number of β-cells in female PR+/+ and −/− mice. Counts were done on five sections cut with a 20-μm thickness interval in five different animals per genotype. *, See Fig. 2 for statistical significance.
Increased β-Cell Proliferation in PR−/− Pancreatic Islets.
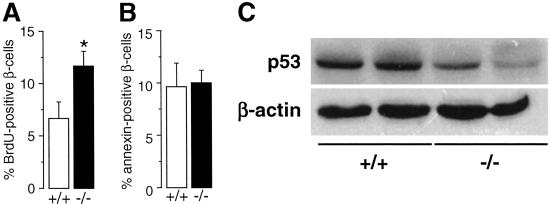
The higher amount of pancreatic β-cells in female mice lacking PR could reflect an increase in the rate of β-cell proliferation or a decrease in β-cell death or apoptosis. To distinguish these possibilities, we injected female, 30-d-old PR+/+ and −/− mice with BrdUrd (50 mg/kg, i.p.) and killed them 3 h later. Compared to WT islets, pancreatic islets from PR−/− mice showed higher BrdUrd immunostaining per β-cell (Fig. 5A). In a separate experiment, no change in β-cell apoptosis was noted in the PR−/− mice as evaluated by immunostaining for annexin, as a marker of cell apoptosis (Fig. 5B). These findings demonstrate that the increase in β-cell number in female PR−/− mice is due to a higher rate of β-cell proliferation.
Fig. 5.
Lack of PR in female mice promotes β-cell proliferation. (A) BrdUrd (blue) and insulin (red) coimmunofluorescent staining of pancreatic sections from female, 30-d-old PR+/+ and −/− mice injected with 50 mg/kg BrdUrd. The pancreas was harvested 3 h after the injection. (B) Annexin (red) and insulin (green) coimmunofluorescent staining of pancreatic sections used in A. The counts of BrdUrd- and annexin-positive β-cells in pancreatic sections described in A and B were done on five sections cut with a 20-μm thickness interval in five different animals per genotype. *, See Fig. 2 for statistical significance. (C) Protein levels of p53 and β-actin in pancreatic islets isolated from female PR+/+ and −/− mice. Homogenates were processed by Western blotting.
To determine the potential mediators of β-cell proliferation in PR−/− mice, we compared protein expression of several cell cycle regulators in isolated pancreatic islets from female PR+/+ and −/− mice. We found no difference in the expression level of p21, p27, cyclin D1, cyclin B1, and cyclin E (data not shown). In contrast, the protein levels of the tumor-suppressor p53 were markedly decreased in PR−/− islets (Fig. 5C), a finding consistent with the higher number of β-cells in these islets. Because p53 has been suggested to be involved in the regulation by progesterone of cell fate in breast cancer (19–21), it is tempting to speculate that the modulation in β-cell number might be caused by a general effect of progesterone on proliferation.
Discussion
Although progesterone has been negatively associated with glycemic control for over 50 years, mostly because of its deleterious effects on peripheral insulin sensitivity during pregnancy, its role on insulin secretion is still unclear. This issue is clinically relevant because a defect in insulin release is a necessary condition for the development of diabetic syndromes such as gestational diabetes. In normal and diabetic db/db mice, our present results show that elevated progesterone levels are associated with hyperglycemia and a higher susceptibility for the development of frank diabetes. The largest differences were observed at a time point concomitant with the stimulation of β-cell hyperplasia in db/db mice between week 9 and 16 (16), suggesting a role for progesterone in this cellular adaptation to hyperglycemia. In contrast, blocking the PR-signaling pathway with RU486 significantly improved plasma glucose levels in db/db mice. Although RU486 also possesses weak anti-glucocorticoid activities that could have contributed to the drug's effects (22), the observations on progesterone treatment suggest that the hormone negatively modulates both glucose tolerance and insulin release.
This hypothesis was further explored by using a mouse model with a targeted deletion of PR and hence a defective progesterone signaling pathway. In female, but not male mice, PR deficiency resulted in an improved glucose tolerance due to a higher insulin secretion. This effect on insulin release likely contributed to the reduced glucose excursion on glucose tolerance test. The enhanced pancreatic function in PR−/− mice was not caused by specific metabolic alterations in the insulin secretion process, but was rather the consequence of a higher number of β-cells in pancreatic islets. Indeed, islets from female PR−/− had approximately twofold more β-cells when analyzed at 12 weeks of age, which was consistent with a twofold higher rate of β-cell proliferation in a BrdUrd incorporation study. Because no concomitant change in β-cell apoptosis was noticed in PR−/− mice, the stimulated insulin release on glucose overload in vivo was therefore most likely due to an increase in β-cell mass. Overall, these studies allow dissociation of the effects of progesterone per se compared to that of other gonadal hormones, which have been reported to interact with and alter progesterone signaling and control of β-cell proliferation (10, 23). They further support earlier in vitro data on the progesterone-mediated reduction in β-cell division (11, 12). Although a direct effect of progesterone action on the β-cell PR is the likely cause for the pancreatic response, we have not ruled out an indirect effect transduced via PR in the CNS.
In pancreatic endocrine tumors, PR immunoreactivity is negatively correlated with the severity of malignancy (25, 26), which is consistent with a negative effect of progesterone signaling on cell proliferation in this tissue. It remains to be confirmed whether the progesterone-signaling pathway affects β-cell proliferation through similar molecular targets as those recently identified in breast carcinoma cells.
Taken together, our present findings demonstrate an important role of the progesterone-signaling pathway in glucose tolerance and pancreatic function through the modulation of β-cell proliferation. It is reasonable to speculate that this effect of progesterone action contributes to gestational diabetes.
Acknowledgments
We acknowledge discussions and support from Jean-Sébastien Annicotte, Marie-France Champy, and Laetitia Piard. This work was supported by grants from Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Hopitaux Universitaires de Strasbourg, Association pour la Recherche sur le Cancer, Association Régionale pour l'Enseignement et la Recherche Scientifique, the European Union (RTD QLG1-CT-1999-00674 and QLRT-2001-00930; Eumorphia), and the National Institutes of Health pursuing an agreement with Baylor College of Medicine (1-P01-DK59820-01) and NIH-HD07857 (to B.W.O.). J.A. is a research director with Centre National de la Recherche Scientifique, and F.P. held postdoctoral fellowships from the Canadian Institutes of Health Research.
Abbreviations
PR, progesterone receptor
References
- 1.Kühl C. (1991) Baillieres Clin. Obstet. Gynaecol. 5 279-292. [DOI] [PubMed] [Google Scholar]
- 2.Butte N. F. (2000) Am. J. Clin. Nutr. 71 1256S-1261S. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association (2002) Diabetes Care 25 S94-S96. [Google Scholar]
- 4.Hellerstrom C., Swenne, I. & Eriksson, U. J. (1985) Diabetes 34 28-31. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez C., Alonso, A., Alvarez, N., Diaz, F., Martinez, M., Fernandez, S. & Patterson, A. M. (2000) J. Endocrinol. 166 283-291. [DOI] [PubMed] [Google Scholar]
- 6.Campbell S. E. & Febbraio, M. A. (2001) Am. J. Physiol. 281 E803-E808. [DOI] [PubMed] [Google Scholar]
- 7.Campbell S. E. & Febbraio, M. A. (2002) Am. J. Physiol. 282 E1139-E1146. [DOI] [PubMed] [Google Scholar]
- 8.Sugaya A., Sugiyama, T., Yanase, S., Shen, X. X., Minoura, H. & Toyoda, N. (2000) Life Sci. 66 641-648. [DOI] [PubMed] [Google Scholar]
- 9.Sutter-Dub M.-T., Kaaya, A., Sfaxi, A.-L., Sodoyez-Goffaux, F., Sodoyez, J.-C. & Sutter, B. C. J. (1988) Steroids 52 583-608. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwenhuizen A. G., Schuiling, G. A., Hilbrands, L. G., Bisschop, E. M. & Koiter, T. R. (1998) Horm. Metab. Res. 30 649-655. [DOI] [PubMed] [Google Scholar]
- 11.Sorenson R. L., Brelje, T. C. & Roth, C. (1993) Endocrinology 133 2227-2234. [DOI] [PubMed] [Google Scholar]
- 12.Kawai M. & Kishi, K. (1999) Eur. J. Endocrinol. 141 419-425. [DOI] [PubMed] [Google Scholar]
- 13.Lydon J. P., DeMayo, F. J., Funk, C. R., Mani, S. K., Hughes, A. R., Montgomery, C. A. J., Shyamala, G., Coneely, O. M. & O'Malley, B. W. (1995) Genes Dev. 9 2266-2278. [DOI] [PubMed] [Google Scholar]
- 14.Rocchi S., Picard, F., Vamecq, J., Gelman, L., Potier, N., Zeyer, D., Dubuquoy, L., Bac, P., Champy, M. F., Plunket, K. D., et al. (2001) Mol. Cell 8 737-747. [DOI] [PubMed] [Google Scholar]
- 15.Pende M., Kozma, S. C., Jaquet, M., Oorschot, V., Burcelin, R., Le Marchand-Brustel, Y., Klumperman, J., Thorens, B. & Thomas, G. (2000) Nature 408 994-997. [DOI] [PubMed] [Google Scholar]
- 16.Gapp D. A., Leiter, E. H., Coleman, D. L. & Schwizer, R. W. (1983) Diabetologia 25 439-443. [DOI] [PubMed] [Google Scholar]
- 17.Pasanen S., Ylikomi, T., Syvala, H. & Tuohimaa, P. (1997) Mol. Cell. Endocrinol. 135 79-91. [DOI] [PubMed] [Google Scholar]
- 18.Winborn W. B., Sheridan, P. J. & McGill, H. C. J. (1987) Pancreas 2 289-294. [DOI] [PubMed] [Google Scholar]
- 19.Formby B. & Wiley, T. S. (1999) Mol. Cell. Biochem. 202 53-61. [DOI] [PubMed] [Google Scholar]
- 20.Medina D., Sivaraman, L., Hilsenbeck, S. G., Conneely, O., Ginger, M., Rosen, J. & O'Malley, B. W. (2001) Ann. N.Y. Acad. Sci. 952 23-35. [DOI] [PubMed] [Google Scholar]
- 21.Sivaraman L., Conneely, O. M., Medina, D. & O'Malley, B. W. (2001) Proc. Natl. Acad. Sci. USA 98 12379-12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada S., York, D. A. & Bray, G. A. (1992) Am. J. Physiol. 262 R1106-R1110. [DOI] [PubMed] [Google Scholar]
- 23.Nieuwenhuizen A. G., Schuiling, G. A., Liem, S. M., Moes, H., Koiter, T. R. & Uilenbroek, J. T. (1999) Eur. J. Endocrinol. 140 256-263. [DOI] [PubMed] [Google Scholar]
- 24.Marchbanks P. A., McDonald, J. A., Wilson, H. G., Folger, S. G., Mandel, M. G., Daling, J. R., Bernstein, L., Malone, K. E., Ursin, G., Strom, B. L., et al. (2002) N. Engl. J. Med. 346 2025-2032. [DOI] [PubMed] [Google Scholar]
- 25.Zamboni G., Bonetti, F., Scarpa, A., Pelosi, G., Doglioni, C., Iannucci, A., Castelli, P., Balercia, G., Aldovini, D., Bellomi, A., et al. (1993) Virchows Arch. A Pathol. Anat. Histopathol. 423 425-431. [DOI] [PubMed] [Google Scholar]
- 26.Viale G., Doglioni, C., Gambacorta, M., Zamboni, G., Coggi, G. & Bordi, C. (1992) Cancer 70 2268-2277. [DOI] [PubMed] [Google Scholar]