Abstract
To define the early events that determine the outcome of acute hepatitis C virus (HCV) infection, we compared the course of viremia with the peripheral and intrahepatic T cell response and intrahepatic cytokine profile in six acutely infected chimpanzees. Three different outcomes were observed after peak viral titers were reached: sustained viral clearance, transient viral clearance followed by chronic infection, and chronic infection that persisted at initial peak titers. The results indicate that HCV spread outpaces the T cell response and that HCV rapidly induces but is not controlled by IFN-α/β; that viral clearance follows the entry and accumulation of HCV-specific IFN-γ-producing T cells in the liver; and that it may not require the destruction of infected cells.
There is a growing consensus that the development of a relatively strong peripheral CD4+ and CD8+ T cell response to hepatitis C virus (HCV) correlates with resolution of the infection (1). However, the early virological and immunological determinants of HCV clearance, persistence, and disease are not well-defined, because most acutely infected patients have not been studied until after the onset of liver disease, at which time the outcome of the infection may already be determined (2–7). We recently studied the virological and immunological features of acute HCV infection prospectively from the time of accidental needlestick inoculation in five health-care workers, and we found that viremia was first detectable several weeks before the appearance of virus-specific T cells in the blood; that viral hepatitis coincided with the onset of a peripheral CD8+ T cell response to HCV; that viral clearance was temporally associated with the production of IFN-γ by those CD8+ T cells; and that it was not accompanied by a surge in liver disease. In contrast, chronic infection developed in two asymptomatic subjects who failed to produce a significant T cell response and in two symptomatic subjects who initially mounted strong T cell responses that ultimately waned (8).
Although these findings provide insight into the viral and immune dynamics that probably determine the outcome of acute HCV infection, for ethical reasons, liver biopsies were not performed in these patients, so we could not address the virus–host interactions at the site of infection. The intrahepatic inflammatory response has been assessed in chronically infected patients (9–13), but those studies were performed long after the persistent infection was well established, so the nature of the infiltrate may have reflected the prolonged infection rather than the initial intrahepatic cellular response. In contrast, the intrahepatic CD8+ T cell response to HCV has been examined in both acutely and chronically infected chimpanzees (14–18), revealing that viral clearance was associated with an early and multispecific intrahepatic CD8+ T cell response to the virus, whereas persistent infection was associated with a weak or narrowly focused response (14) and the emergence of viral escape mutations (16, 18).
Nevertheless, a great deal remains to be learned about the host–virus relationship during HCV infection. In particular, little is known about the relationship between the kinetics of viral spread and the induction of the intrahepatic T cell response to HCV; the efficiency with which HCV-specific T cells home to the liver; how long they survive or how well they function once they arrive; and the role of virus-induced or T cell-derived cytokines in viral clearance has not been defined. Indeed, we do not know whether viral clearance merely reflects the conventional notion of immune destruction of infected cells or whether the virus can also be controlled by noncytolytic effector functions of the immune response. The current study was performed to address these issues.
Materials and Methods
Chimpanzees.
The housing, maintenance, and care of the chimpanzees used in the study were in compliance with all relevant guidelines and requirements. All animals were infected with virus or infectious molecular clones derived from genotype 1a. Chimpanzee 1422 (Ch1422) was inoculated intravenously with 100 μl of serum from a patient with acute fulminant HCV infection (19). Ch1581 was inoculated intravenously with 1 ml of a diluted serum pool from Ch1422 containing one chimpanzee infectious dose (CID) (J.B., unpublished observations). Ch1573 was inoculated intravenously with 2 ml of a monoclonal virus pool containing 64 CID derived from a chimpanzee that was infected with the H77 clone of HCV (20). Ch1558 was transfected intrahepatically with RNA transcribed from a total of 20 μg of plasmid DNA consisting of the H77 clone of HCV lacking the proximal 24 nt of the variable region of the 3′ untranslated region (21). Ch1590 was transfected intrahepatically with RNA transcribed from a total of 20 μg of plasmid DNA consisting of a full length copy of the H77 clone lacking the entire hypervariable region 1 (22). Ch96A008 was inoculated intravenously with 90 ml of plasma from Ch1590 taken 4 wk after inoculation, containing a total of 900 genome equivalents (GE) (22). At baseline and at each week after inoculation, needle liver biopsies and blood samples were obtained.
Peripheral Blood Mononuclear Cells (PBMC) and Liver Biopsy.
Forty milliliters of ACD anticoagulated blood was obtained weekly for isolation of PBMC the next day. Liver tissue was obtained by hepatic needle biopsy. One fragment was put into RPMI medium (GIBCO) containing 10% AB serum; one was fixed in 10% zinc formalin solution for subsequent histological examination; and one fragment was snap frozen for subsequent RNA isolation.
Histological Analysis.
Zinc formalin-fixed paraffin-embedded liver biopsies were sectioned (4 μm), stained with hematoxylin/eosin (23), and analyzed histologically as described (22).
Isolation of Intrahepatic T Cells.
Liver-infiltrating lymphocytes were isolated from 0.5–1 cm of hepatic needle biopsy. The tissue was homogenized in 2–3 ml of Dulbecco's Phosphate-Buffered Saline (GIBCO) by using a Dounce tissue grinder. Cell suspensions were incubated with magnetic beads coupled to anti-CD8 or -CD4 antibodies (Dynabeads, Dynal, Oslo) for 20 min at 4°C, and bound CD8+ or CD4+ T cells were isolated by using a particle magnetic concentrator. The purity of each T cell subset was confirmed by FACS analysis and was always >95%. The isolated intrahepatic CD4+ and CD8+ T cells were then plated into separate wells in 24-well plates (Corning) in 1 ml of 10% human AB+ serum, 100 units/ml IL-2 (Hoffmann–La Roche), 0.04 μg/ml anti-human CD3 monoclonal antibody (Immunotech, Marseilles, France), and 2 × 106 irradiated autologous PBMC as feeder cells. Twice a week, 1 ml of media was exchanged and 100 units/ml IL-2 added. After 2 wk, the expanded T cells were tested for HCV-specific T cell responses (see below).
Recombinant HCV Proteins.
All recombinant HCV proteins (genotype 1a) and control superoxide dismutase protein were kindly provided by M. Houghton (Chiron, Emeryville, CA) (24, 25). These proteins were deduced from strain HCV-1, genotype 1a.
Recombinant Expression Vectors.
Two recombinant vaccinia virus constructs, vHCV 1–1488 and vHCV 827–3011, which encode all HCV proteins according to the sequence of strain H77 (genotype 1a), were used together with a vaccinia virus encoding the T7 RNA polymerase, vTF7 (all generously provided by Charles Rice, Rockefeller University, New York) (26) to induce transient expression of endogenously processed HCV proteins in autologous Epstein–Barr virus (EBV) immortalized B cell lines (B-LCL). B-LCL were infected at a multiplicity of infection of 25 for 1 h at room temperature with vHCV 1–1488 plus vTF7, vHCV 827–3011 plus vTF7, or vTF7 alone. After 1 h, 1 ml of 10% FCS medium was added, and the infected B-LCL were cultured (at 37°C) overnight and then washed once before they were added as stimulators to the intracellular IFN-γ staining assays (see below).
Synthetic Peptides.
A large panel of HCV-derived peptides, whose sequence was based on the HCV-1 strain (genotype 1a) (27) and most of which have been previously identified as cytotoxic T lymphocyte epitopes in infected humans and chimpanzees (6, 9, 14, 15, 26, 28–32), was synthesized, dissolved, and diluted as described (26). The amino acid sequences of the 68 peptides are presented in Table 2, which is published as supporting information on the PNAS web site, www.pnas.org.
Antibodies.
Anti-CD8 FITC, -CD8 phycoerythrin (PE), -CD4 PE, -CD3 APC, -IFN-γ PE, isotype FITC, isotype allophycocyanin, and isotype PE antibodies were obtained from PharMingen and used according to the manufacturer's instructions.
HCV RNA Detection.
HCV RNA was detected in serum by using the RT-PCR (HCV-Monitor Amplification Kit: Roche Diagnostics, Version 2.0), following the manufacturer's instructions. The quantity of HCV RNA in each specimen was expressed in HCV RNA GE per milliliter of serum. In addition, serum HCV RNA was monitored by in-house RT-nested PCR (33). HCV RNA levels in the liver were determined by quantitative real-time PCR by using a BioRad iCycler system. Briefly, 1 μg of total RNA isolated from liver biopsies was reverse transcribed by using random primers and the Taqman Reverse Transcription Reagents from Applied Biosystems according to the manufacturer's instructions. Subsequently, 1/10 of cDNA were subjected to quantitative Taqman PCR (50-μl reaction by using TaqMan Universal PCR Master Mix from Applied Biosystems, 200 nM primer each, and 100 nM TaqMan probe) for HCV by using the primers HCV146U (5′-GTCTGCGGAACCGGTGAG-3′) and HCV201L (5′-GCATTGAGCGGGTTTATC-3′) in the 5′ noncoding region together with TaqMan probe HCV165P (5′-6-FAMd(ACACCGGAATTGCCAGGACGACC)DHQ-1–3′).
HCV Antibody Measurements.
HCV antibody testing was performed with the HCV EIA-2 assay, testing for specificities against C22, C33, and C100 (Abbott).
HCV-Specific Proliferative T Cell Response.
This assay was done on PBMCs as described (8). Polyclonally expanded CD4+ intrahepatic lymphocytes were cultured under the same conditions except they were plated together with 1 × 105 autologous irradiated (3,000 rad) PBMCs as antigen-presenting cells before the proteins were added. The degree of cellular proliferation was expressed as stimulation index (SI) in which the average [3H]thymidine uptake (in cpm) in replicate wells stimulated with each HCV protein was divided by the average [3H]thymidine uptake in replicate wells stimulated with the control protein. A SI of >2.0 was used as a positive cutoff value.
HCV-Specific CD8+ T Cell Responses.
HCV peptide-specific cytotoxic T lymphocyte lines were established from PBMCs by stimulation with pools of synthetic peptides (five peptides per pool at 10 μg/ml of each peptide), as described (29). On day 21, the peptide-stimulated cytotoxic T lymphocyte lines and the unstimulated PBMCs from which the lines were derived were stimulated with individual peptides (10 μg/ml) and analyzed for peptide specific IFN-γ production exactly as described (8).
The presence of HCV-specific CD8+ T cells in polyclonally expanded intrahepatic CD8+ T lymphocytes (see above) was also examined by intracellular IFN-γ staining after 5 h of stimulation with autologous EBV-transformed B-LCL that were infected with recombinant HCV vaccinia viruses vHCV 1–1488 or vHCV 827-3011 together with vTF7 or with vTF7 alone. Peripheral CD8+ T lymphocytes purified from PBMCs obtained at the same time and expanded polyclonally in the same manner as the intrahepatic lymphocytes were also studied to compare the frequency of HCV-specific CD8+ T cells in the peripheral blood and intrahepatic compartments.
The frequency of HCV-specific CD8+ T cells was defined as the percentage of CD8+ T cells that produce IFN-γ in response either to HCV peptide stimulation or to stimulation by B-LCL coinfected with vHCV and vTF7 after subtraction of the IFN-γ+, CD8+ T cells detected after stimulation in the absence of peptide or in the absence of vHCV, respectively.
Target Cell Lines.
Autologous EBV-transformed B-LCL were established from all chimpanzees, as described (34). All target cells were maintained in RPMI with 10% (vol/vol) heat-inactivated FCS (GIBCO).
RNA Isolation and RNase Protection Assay.
Liver RNA isolation and the RNase protection assay were performed exactly as described (23).
Results
Courses of HCV Infection: Sustained Viral Clearance, Transient Viral Clearance, and Persistence.
Viral RNA became detectable within 1 wk by RT-nested PCR in all infected animals and within 3 wk by quantitative PCR, except for Ch1590, which was inoculated intrahepatically with a hypervariable region deleted mutant viral genome (Fig. 1). Importantly, viral titers rose several orders of magnitude very rapidly after their initial appearance. Three distinctly different courses of acute HCV infection were observed: sustained viral clearance (Fig. 1 A and B); transient viral clearance followed by chronic infection (Fig. 1 C and D); and chronic infection that persisted at initial peak titers (Fig. 1 E and F). For ease of discussion, these different outcomes will henceforth be designated sustained clearance, transient clearance, and persistence, respectively.
Fig 1.
Courses of acute HCV infection in chimpanzees after experimental inoculation with HCV. Quantitative HCV RNA levels were expressed as log GEs per milliliter of serum. HCV RNA was also monitored by in-house RT-nested PCR, and results are indicated as + or −. ALT activity was expressed as units per liter. HCV antibody responses reflect the results of an HCV EIA-2 assay as described in Materials and Methods. Liver biopsies were examined for necroinflammatory changes and scored as described (22).
Sustained Clearance.
Ch96A008 and Ch1422 displayed early peak viral titers close to or greater than 105 GE per milliliter that eventually became undetectable and remained undetectable in both animals until at least 68 and 29 wk, respectively (Fig. 1 A and B). In Ch1422, a rapid viral decrease of >3 logarithms during week 10 was associated with a surge in serum alanine aminotransferase (ALT) activity that peaked at 744 units/liter and commensurate histological evidence of necroinflammatory liver disease (Fig. 1B). In contrast, viral clearance occurred in Ch96A008 in the absence of any elevation of serum ALT activity or HCV antibodies, and with much less histological evidence of inflammation (Fig. 1A).
Transient Clearance.
As shown in Fig. 1 C and D, like the previous animals, Ch1573 and Ch1581 displayed early peak viral titers of 1.9 × 105 and 2.5 × 106 GE per milliliter that rapidly decreased by three to four orders of magnitude to become undetectable. Viral clearance was accompanied by a surge in serum ALT activity in Ch1581 (Fig. 1D) but not in Ch1573 (Fig. 1C) and only low-level inflammatory infiltrates in the liver. Unlike the first two animals, however, the virus became detectable again, and it fluctuated thereafter between undetectable and 103.8 GE per milliliter, indicating low-level persistent infection with essentially normal serum ALT activity and minimal intrahepatic inflammation.
Persistence.
As shown in Fig. 1 E and F, Ch1558 and Ch1590 displayed initial peak viral titers of ≈104 GE per milliliter that fluctuated in that range for at least 78 wk thereafter (not shown). It is important to note that in both of these animals, serum ALT values remained normal, although low-level inflammatory infiltrates were detectable occasionally in Ch1558, and no antibodies to HCV were detected until weeks 25 (Ch1558) and 36 (Ch1590) (not shown). The low level of virus replication in Ch1590 during the first 10 wk of infection probably reflects the mutated HCV genome with which it was infected, and the first rise in viremia was associated with the occurrence of several adaptive mutations as described (22).
Peripheral vs. Intrahepatic HCV-Specific CD4+ T Cell Responses to HCV.
As shown in Fig. 2A, a peripheral virus-specific CD4+ T cell response became detectable in all five chimpanzees between 4–5 wk of inoculation except for Ch1590, who became positive 1 wk after infection. In separate studies in which CD4+ and CD8+ T cells were depleted before antigen stimulation, we demonstrated that the proliferative response was limited to the CD4+ T cell subset (data not shown). All of the chimpanzees responded to all of the antigens (Table 1). Therefore, these results indicate that a peripheral virus-specific CD4+ T cell response is induced in all HCV-infected animals, but the outcome of infection appears to be independent of the kinetics, strength, specificity, or diversity of that response.
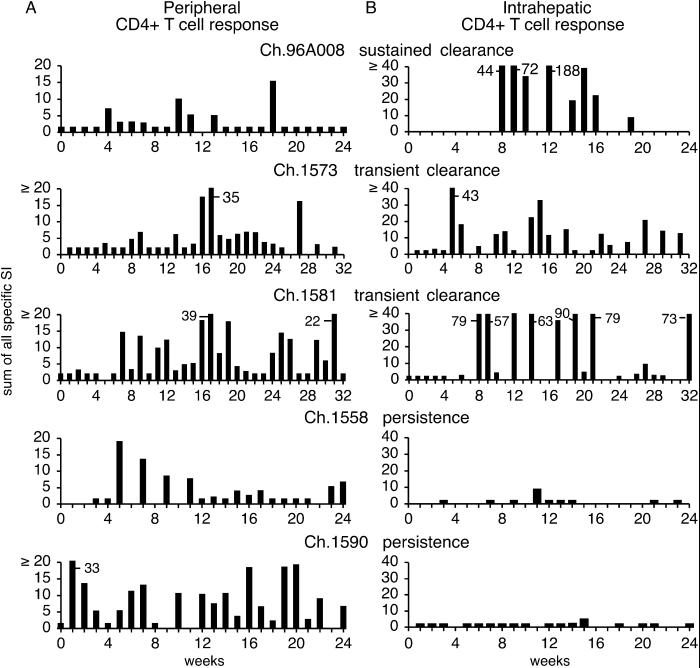
Fig 2.
Peripheral and intrahepatic proliferative CD4+ T cell responses in acutely HCV-infected chimpanzees. The peripheral (A) and intrahepatic (B) responses to core, NS3, NS3–NS4, and NS5 are shown as the sum of all positive stimulation indices. If no response against any protein was detected, the sum was assigned a value of 2 to permit visualization of the results.
Table 1.
Peak peripheral and intrahepatic proliferative CD4+ T cell responses to individual HCV proteins
| Chimp
|
Blood | Liver | ||||||
|---|---|---|---|---|---|---|---|---|
| Core | NS3 | NS4 | NS5 | Core | NS3 | NS4 | NS5 | |
| 96A008 | 9.9 | 2.9 | 5.8 | 6.6 | Neg | 64.4 | 58.0 | 66.0 |
| 1573 | 2.7 | 22.3 | 6.9 | 5.6 | 20.5 | 34.1 | 3.1 | 7.1 |
| 1581 | 9.7 | 12.4 | 9.7 | 19.8 | 10.9 | 27.5 | 33.4 | 62.1 |
| 1558 | 6.7 | 3.9 | 7.8 | 3.3 | Neg | 8.8 | Neg | Neg |
| 1590 | 18.3 | 5.7 | 11.5 | 8.1 | Neg | Neg | Neg | 5.0 |
Expressed as stimulation index.
In contrast, there was a strong correlation between the intrahepatic HCV-specific CD4+ T cell response and the course of infection. A strong and sustained proliferative T cell response was observed in the intrahepatic infiltrate in Ch96A008 that cleared the infection and in Ch1573 and Ch1581 that displayed transient viral clearance (Fig. 2B), and the response was targeted against virtually all of the viral antigens in each of these animals (Table 1). By comparing Figs. 1 and 2, it is evident that the intrahepatic T cell response began shortly before major decreases in viremia in all three animals. Interestingly, the intrahepatic T cell response waned and eventually became undetectable in the animal that cleared the virus (Ch96A008), but it persisted in the animals that transiently cleared the infection (Ch1573 and Ch1581), suggesting ongoing recognition of viral antigen by intrahepatic CD4+ T cells in those animals. In contrast, HCV-specific T cells were virtually undetectable in the liver of persistently infected Ch1558 and Ch1590 (Fig. 2B), and when CD4+ T cell responses were detectable in these animals, they were weak and narrowly focused (Table 1).
Peripheral vs. Intrahepatic HCV-Specific CD8+ T Cell Response to HCV.
The peripheral CD8+ T cell response was tested directly ex vivo by using both a large panel of HCV peptides (6, 9, 14, 15, 26, 28–32) and also by using autologous EBV-B cell lines infected with a recombinant vaccinia virus that expresses HCV residues 1–1488 or 827–3011 to stimulate intrahepatic IFN-γ production. In no instance were HCV-specific IFN-γ-producing CD8+ T cells detected directly ex vivo in any animal, indicating their low frequency in the peripheral blood (not shown). After repeated in vitro stimulation with the panel of the HCV peptides (Table 2); however, peptide-specific CD8+ T cells were sufficiently expanded to be detectable in all of the chimpanzees to varying degrees (Fig. 3A). Ch96A008, which cleared the infection (Fig. 1A), mounted an early and multispecific peripheral CD8+ T cell response to a total of seven peptides (Fig. 3A), and the response persisted for at least 68 wk, the first 23 of which are shown in Fig. 3A. A similar early multispecific peripheral CD8+ T cell response against a total of eight epitopes was also seen in Ch1573 (Fig. 3A), which transiently cleared the virus (Fig. 1C). The CD8+ T cell response persisted together with low levels of virus for the duration of the study. In contrast, the peptide-specific peripheral CD8+ T cell responses were much less vigorous in the three remaining animals (Fig. 3A), including Ch1581, which also displayed transient viral clearance similar to Ch1573. Importantly, class I allele analysis of the chimpanzees (C. Walker, personal communication) revealed that Ch1581 did not share any Patr alleles with Ch1573 or Ch96A008, whereas three of the four Patr A and Patr B alleles were identical in the latter two animals, and either one or two of their alleles was shared by Ch1590 and Ch1558, respectively. Thus, our failure to detect peptide-specific peripheral CD8+ T cells in Ch1581 may reflect a bias in our peptide panel against epitopes that can be recognized by this animal's Patr alleles rather than true nonresponsiveness to the virus. In contrast, because of the similar Patr allelotypes of Ch96A008, Ch1573, Ch1558, and Ch1590, the peripheral nonresponsiveness of the latter two animals to the peptides may reflect differences at the T cell level.
Fig 3.
Peripheral and intrahepatic CD8+ T cell responses in acutely infected chimpanzees. (A) Peripheral CD8+ T cell responses were tested against a large panel of HCV peptides (Table 2). The peptide-specific CD8+ T cell responses are shown at all time points tested, and they are expressed as the percentage of CD8+ T cells that produce IFN-γ in response to the respective peptides. (B) The intrahepatic CD8+ T cell response is shown as the percentage of intrahepatic CD8+ T cells that produce IFN-γ after stimulation with autologous B-LCL that were infected with recombinant vaccinia viruses vHCV 1–1488 or vHCV 827–3011, as described in Materials and Methods.
In striking contrast to our inability to detect HCV-specific CD8+ T cells directly ex vivo in the peripheral blood, intrahepatic CD8+ T cell responses to HCV were detected as early as 4 wk after inoculation in Ch96A008 that cleared the infection and in Ch1573 and Ch1581 that displayed transient viral clearance (Fig. 3B). Importantly, intrahepatic CD8+ T cell responses to HCV were not detected in the two persistently infected animals (Ch1558 and Ch1590) (Fig. 3B). In each responsive animal, the CD8+ T cells appeared in the liver before precipitous declines in viral titer occurred (Fig. 1). It is also important to note that, like the CD4+ T cell response (Fig. 2), the intrahepatic CD8+ T cell response remained detectable in Ch1573 and Ch1581 for at least 48 and 65 wk, respectively (not shown), coincident with persistent low-level viral infection, whereas in Ch96A008, the intrahepatic CD8+ T cell response became undetectable soon after viral clearance, and it has remained undetectable for at least 68 wk (not shown). These results demonstrate that HCV-specific CD8+ T cells are greatly enriched in the liver when compared with the peripheral blood, and they indicate that both initial viral clearance and the subsequent control of HCV infection at greatly reduced titers are associated with the presence of HCV-specific CD8+ T cells in the liver.
Hepatic Cytokine and T Cell RNA During Acute HCV Infection.
As shown in Fig. 4, 2′5′ oligoadenylate synthetase mRNA, a type I IFN-induced gene, appeared in the liver as soon as HCV RNA was detectable in the serum, and the magnitude of its induction closely reflected the level of viremia in all of the animals as well as the level of HCV RNA in the livers of the three animals in which it was tested (Ch96A008, Ch1422, and Ch1558) (data not shown). In contrast to the rapid and universal induction of type I IFN in the liver of these animals, IFN-γ and CD3 mRNA were detectable only in the animals that displayed sustained (Ch96A008 and Ch1422) or transient (Ch1573 and Ch1581) viral clearance in the context of an intrahepatic HCV-specific T cell response (Fig. 4). Importantly, the appearance of these markers occurred late in the infection, and they coincided very well with the intrahepatic CD4+ (Fig. 2) and CD8+ (Fig. 3) T cell responses and with the disappearance of viral RNA in Ch96A008 and Ch1422, or with major decreases in viremia in Ch1573 and Ch1581 (Fig. 4).
Fig 4.
Intrahepatic cytokine profiles in acutely HCV-infected chimpanzees. Liver RNA was tested for the expression of CD3, IFN-γ, and 2′5′ oligoadenylate synthetase. The L32 signal reflects the amount of RNA used in the assay.
Discussion
The results of this study reveal several important aspects of the host–virus relationship during acute HCV infection. First, HCV spreads so rapidly in an infected host that it appears to outpace the immune response by several weeks, perhaps contributing to the tendency of HCV to persist. Second, HCV is a strong inducer of type I IFN, but it is relatively resistant to its antiviral activity, another factor that favors persistent infection. Third, the intrahepatic T cell response to the virus correlates with acute control of the infection, whereas the peripheral T cell response does not. Fourth, major decreases in viral titer in animals that permanently or transiently clear the infection are accompanied by an early, vigorous, multispecific, IFN-γ-producing intrahepatic CD4+ and CD8+ T cell response. Fifth, relatively stable viral titers occur in the absence of an intrahepatic virus-specific T cell response or IFN-γ. Finally, the results suggest that although the infection can be controlled to varying degrees in the context of a destructive immune response to the virus, viral titers can decline more than three orders of magnitude in the absence of biochemical evidence of liver disease and with minimal intrahepatic necroinflammatory changes, suggesting the involvement of noncytolytic T cell effector functions such as the production of antiviral cytokines, as we have previously reported for HBV (23, 35). The recent demonstration (36) that IFN-γ can inhibit amplification of the HCV replicon in Huh-7 cells supports this notion.
Perhaps the single most important observation from this study is that acute control of HCV infection is associated with a vigorous intrahepatic antiviral CD4+ and CD8+ T cell response (Figs. 2B and 3B). The key factor is the intrahepatic accumulation of the T cells, not their induction in the periphery. The results also demonstrate that there is no easily discernible difference in the kinetics, magnitude, or diversity of the intrahepatic CD4+ and CD8+ T cell response between the animal that permanently cleared the infection (Ch96A008) and the animals that only transiently cleared the virus (Ch1573 and Ch1581). Thus, the meaningful relationship is between the vigor of the initial intrahepatic T cell response and the initial decline that leads either to clearance or sustained suppression of viremia at much reduced titers. Interestingly, the vigor of the response does not correlate with the eventual outcome of the infection, which is apparently influenced by other factors in addition to the intrahepatic T cell response (e.g., viral quasispecies, replication efficiency, mutation rate, tissue tropism, etc.) that may determine whether an infection is terminated or merely controlled at low titers relative to initial peak levels. It should also be considered that the difference between sustained and transient viral clearance may simply reflect the sensitivity of the HCV RNA assay rather than biologically different outcomes; i.e., theoretically, Ch96A008 may be persistently infected at subdetectable levels.
It is also important to note that, using the same assay technique, virus-specific CD8+ T cells were detectable in the liver but not in PBMCs obtained at the same time after infection as the intrahepatic lymphocytes (data not shown). However, after in vitro peptide-specific stimulation, virus-specific CD8+ T cells became detectable in the peripheral blood (Fig. 3A). These results imply that virus-specific T cells exist at a very low frequency in the peripheral blood, whereas they preferentially accumulate in the liver, the site of active virus replication. This notion supports recent observations in chronically infected patients that HCV-specific CD8+ T cells were detected at higher frequencies in liver than blood by using soluble tetrameric HLA-A2 molecules (12, 13), and that cloning of intrahepatic HCV-specific cytotoxic T lymphocyte lines from chronically infected humans and chimpanzees is often successful (9, 11, 14–17), whereas antigen-specific expansion is usually required to detect the same HCV-specific responses in the peripheral blood (10). It is also intriguing that only 1–3% of the intrahepatic CD8+ T cells respond to HCV by producing IFN-γ (Fig. 3B). It will be interesting to determine whether this number represents a true estimate of the vigor of the HCV-specific CD8+ T cell response in the liver, or whether it underestimates that response, because most of the HCV-specific T cells that are present in the liver fail to produce IFN-γ in response to antigen stimulation, a condition that has been observed in the peripheral blood of acutely and chronically infected patients (6, 8, 37, 38).
We do not understand why the earliest intrahepatic T cell response is not detectable until 4–8 wk after the onset of viremia. One possibility is that HCV might activate mechanisms that prevent or delay the accumulation of virus-specific T cells in the liver. For example, the strong induction of type I IFN could inhibit IL-12 expression by dendritic cells and monocytes and block IFN-γ production by natural killer (NK) cells (39) that could be required for the recruitment of virus-specific T cells into the liver. Alternatively, it has been recently shown that recombinant HCV E2 protein and anti-CD81 specific antibodies inhibit NK cell proliferation and activation (40, 41). If the intact virus can also inhibit NK cell activation, this could theoretically delay the recruitment of inflammatory cells into the liver because NK cells are a potent source of inflammatory cytokines (39). Another hypothetical mechanism is that HCV may inhibit chemokine production by hepatocytes, which would also delay the entry of inflammatory cells into the liver. Also, it is possible that HCV-infected hepatocytes, liver sinusoidal endothelial cells (42), and dendritic cells (43) that are either potentially infected by HCV or have endocytosed and processed HCV virions could tolerize or delete naive T cells as they pass through the liver or inactivate peripherally primed virus-specific T cells when they arrive. In any event, the challenge is to determine not only what is responsible for the delayed entry, but also how the suppressive effect is terminated when virus-specific T cells finally do accumulate in the liver in animals that control the infection, and why it is not terminated in animals that do not control it.
The effector mechanisms used by the intrahepatic T cells to eliminate or control the virus remain to be determined. It is important to note, however, that the onset of the intrahepatic T cell response in Ch1581 correlated with an increase in serum ALT activity and a major decline in virus titer. Furthermore, a surge in serum ALT activity was associated with a >3-logarithm decline in viremia in Ch1422, suggesting that in both animals cytopathic effector functions of virus-specific T cells were operative. However, clearance of the virus in Ch96A008, the final clearance of the virus in Ch1422 at weeks 18 and 19, and the decrease of viral titers in Ch1573 and Ch1581 occurred in the absence of elevated serum ALT activity, and (except for Ch1422) with minimal histological evidence of liver cell injury, indicating that some mechanism other than the destruction of infected cells might have been responsible for the clearance of the virus in these animals. The most obvious candidates for a noncytopathic antiviral activity would be the IFNs. Type I IFN does not appear to play a role, because HCV rapidly and strongly triggered its expression in the liver in all animals, irrespective of the outcome of infection. These results suggest that, at least in the case of genotype 1a, HCV is a strong inducer of type I IFN, but it is relatively resistant to its antiviral activity. Several potential mechanisms whereby HCV might be able to evade the antiviral effects of IFN have been reviewed recently (44).
In contrast to type I IFN, however, IFN-γ was detectable only in the livers of the chimpanzees that cleared or controlled the virus, raising the possibility that IFN-γ might perform noncytolytic antiviral effector functions during HCV infection, similar to its ability to control HBV replication in the liver of transgenic mice (23) and its association with a decrease in HBV DNA in acutely infected chimpanzees (35). Because the intrahepatic induction of IFN-γ correlated with the virus-specific intrahepatic T cell response in all of the animals, it is likely that IFN-γ was produced by virus-specific T cells that accumulated in the liver, or by inflammatory cells that they activated and/or recruited. Of course, it is also possible that IFN-γ is simply a marker for activated intrahepatic T cells and/or NK cells, and that it plays no direct role in the control of the infection. It is intriguing, however, that recent experiments indicate that IFN-γ efficiently inhibits the replication of an HCV replicon in Huh-7 cells (36). The IFN-γ induced genes and the cellular genes that might control HCV replication are described in the companion report by Su et al. in this issue of PNAS (45).
Supplementary Material
Acknowledgments
We thank Dr. M. St. Claire and M. Shapiro (Bioqual, Incorporated) for animal care; Dr. C. Walker (Ohio State University, Columbus, OH) for Patr allele typing; and R. Koch and R. Engle for excellent technical assistance. We thank Drs. K. M. Chang and L. G. Guidotti for helpful discussions and Dr. H. E. Blum for encouragement and support. This study was supported by Grants AI20001 and CA76403 and by Contracts N01-AI-52705, N01-AI-45180, and N01-CO-56000 from the National Institutes of Health. R.T. was also supported by Grants TH 719/1-1 and TH 719/2-1 (Emmy Noether Program) from the Deutsche Forschungsgemeinschaft (Bonn), and by a postdoctoral training fellowship from the Cancer Research Institute, New York. H.C.S. was supported by a grant from the Fritz Thyssen Foundation. This is manuscript no. 14537-MEM from The Scripps Research Institute.
Abbreviations
HCV, hepatitis C virus
CID, chimpanzee infectious dose
Chn, Chimpanzee n
GE, genome equivalents
PBMC, peripheral blood mononuclear cells
EBV, Epstein–Barr virus
B-LCL, immortalized B cell lines
ALT, alanine aminotransferase
NK, natural killer
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 30, 2002.
References
- 1.Ward S., Lauer, G., Isba, R., Walker, B. & Klenerman, P. (2002) Clin. Exp. Immunol. 128 195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diepolder H. M., Zachoval, R., Hoffmann, R. M., Wierenga, E. A., Santantonio, T., Jung, M. C., Eichenlaub, D. & Pape, G. R. (1995) Lancet 346 1006-1007. [DOI] [PubMed] [Google Scholar]
- 3.Gruner N. H., Gerlach, T. J., Jung, M. C., Diepolder, H. M., Schirren, C. A., Schraut, W. W., Hoffmann, R., Zachoval, R., Santantonio, T., Cucchiarini, M., et al. (2000) J. Infect. Dis. 181 1528-1536. [DOI] [PubMed] [Google Scholar]
- 4.Gerlach J. T., Diepolder, H. M., Jung, M. C., Gruener, N. H., Schraut, W. W., Zachoval, R., Hoffmann, R., Schirren, C. A., Santantonio, T. & Pape, G. R. (1999) Gastroenterology 117 933-941. [DOI] [PubMed] [Google Scholar]
- 5.Lechner F., Gruener, N. H., Urbani, S., Uggeri, J., Santantonio, T., Kammer, A. R., Cerny, A., Phillips, R., Ferrari, C., Pape, G. R. & Klenerman, P. (2000) Eur. J. Immunol. 30 2479-2487. [DOI] [PubMed] [Google Scholar]
- 6.Lechner F., Wong, D. K., Dunbar, P. R., Chapman, R., Chung, R. T., Dohrenwend, P., Robbins, G., Phillips, R., Klenerman, P. & Walker, B. D. (2000) J. Exp. Med. 191 1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Missale G., Bertoni, R., Lamonaca, V., Valli, A., Massari, M., Mori, C., Rumi, M. G., Houghton, M., Fiaccadori, F. & Ferrari, C. (1996) J. Clin. Invest. 98 706-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thimme R., Oldach, D., Chang, K. M., Steiger, C., Ray, S. C. & Chisari, F. V. (2001) J. Exp. Med. 194 1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong D. K., Dudley, D. D., Afdhal, N. H., Dienstag, J., Rice, C. M., Wang, L., Houghton, M., Walker, B. D. & Koziel, M. J. (1998) J. Immunol. 160 1479-1488. [PubMed] [Google Scholar]
- 10.Wong D. K., Dudley, D. D., Dohrenwend, P. B., Lauer, G. M., Chung, R. T., Thomas, D. L. & Walker, B. D. (2001) J. Virol. 75 1229-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koziel M. J., Dudley, D., Wong, J. T., Dienstag, J., Houghton, M., Ralston, R. & Walker, B. D. (1992) J. Immunol. 149 3339-3344. [PubMed] [Google Scholar]
- 12.He X. S., Rehermann, B., Lopez-Labrador, F. X., Boisvert, J., Cheung, R., Mumm, J., Wedemeyer, H., Berenguer, M., Wright, T. L., Davis, M. M., et al. (1999) Proc. Natl. Acad. Sci. USA 96 5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grabowska A. M., Lechner, F., Klenerman, P., Tighe, P. J., Ryder, S., Ball, J. K., Thomson, B. J., Irving, W. L. & Robins, R. A. (2001) Eur. J. Immunol. 31 2388-2394. [DOI] [PubMed] [Google Scholar]
- 14.Cooper S., Erickson, A. L., Adams, E. J., Kansopon, J., Weiner, A. J., Chien, D. Y., Houghton, M., Parham, P. & Walker, C. M. (1999) Immunity 10 439-449. [DOI] [PubMed] [Google Scholar]
- 15.Erickson A. L., Houghton, M., Choo, Q. L., Weiner, A. J., Ralston, R., Muchmore, E. & Walker, C. M. (1993) J. Immunol. 151 4189-4199. [PubMed] [Google Scholar]
- 16.Erickson A. L., Kimura, Y., Igarashi, S., Eichelberger, J., Houghton, M., Sidney, J., McKinney, D., Sette, A., Hughes, A. L. & Walker, C. (2001) Immunity 15 885-895. [DOI] [PubMed] [Google Scholar]
- 17.Kowalski H., Erickson, A. L., Cooper, S., Domena, J. D., Parham, P. & Walker, C. M. (1996) J. Exp. Med. 183 1761-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiner A., Erickson, A. L., Kansopon, J., Crawford, K., Muchmore, E., Hughes, A. L., Houghton, M. & Walker, C. M. (1995) Proc. Natl. Acad. Sci. USA 92 2755-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farci P., Munoz, S. J., Shimoda, A., Govindarajan, S., Wong, D. C., Coiana, A., Peddis, G., Rubin, R. & Purcell, R. H. (1999) J. Infect. Dis. 179 1007-1011. [DOI] [PubMed] [Google Scholar]
- 20.Forns X., Payette, P. J., Ma, X., Satterfield, W., Eder, G., Mushahwar, I. K., Govindarajan, S., Davis, H. L., Emerson, S. U., Purcell, R. H. & Bukh, J. (2000) Hepatology 32 618-625. [DOI] [PubMed] [Google Scholar]
- 21.Yanagi M., St Claire, M., Emerson, S. U., Purcell, R. H. & Bukh, J. (1999) Proc. Natl. Acad. Sci. USA 96 2291-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forns X., Thimme, R., Govindarajan, S., Emerson, S. U., Purcell, R. H., Chisari, F. V. & Bukh, J. (2000) Proc. Natl. Acad. Sci. USA 97 13318-13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guidotti L. G., Ishikawa, T., Hobbs, M. V., Matzke, B., Schreiber, R. & Chisari, F. V. (1996) Immunity 4 25-36. [DOI] [PubMed] [Google Scholar]
- 24.Botarelli P., Brunetto, M. R., Minutello, M. A., Calvo, P., Unutmaz, D., Weiner, A. J., Choo, Q. L., Shuster, J. R., Kuo, G., Bonino, F., et al. (1993) Gastroenterology 104 580-587. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari C., Valli, A., Galati, L., Penna, A., Scaccaglia, P., Giuberti, T., Schianchi, C., Missale, G., Marin, M. G. & Fiaccadori, F. (1994) Hepatology 19 286-295. [PubMed] [Google Scholar]
- 26.Rehermann B., Chang, K. M., McHutchinson, J., Kokka, R., Houghton, M., Rice, C. M. & Chisari, F. V. (1996) J. Virol. 70 7092-7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choo Q. L., Kuo, G., Weiner, A. J., Overby, L. R., Bradley, D. W. & Houghton, M. (1989) Science 244 359-362. [DOI] [PubMed] [Google Scholar]
- 28.Battegay M., Fikes, J., Di Bisceglie, A. M., Wentworth, P. A., Sette, A., Celis, E., Ching, W. M., Grakoui, A., Rice, C. M., Kurokohchi, K., et al. (1995) J. Virol. 69 2462-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerny A., McHutchison, J. G., Pasquinelli, C., Brown, M. E., Brothers, M. A., Grabscheid, B., Fowler, P., Houghton, M. & Chisari, F. V. (1995) J. Clin. Invest. 95 521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang K. M., Gruener, N. H., Southwood, S., Sidney, J., Pape, G. R., Chisari, F. V. & Sette, A. (1999) J. Immunol. 162 1156-1164. [PubMed] [Google Scholar]
- 31.Koziel M. J., Dudley, D., Afdhal, N., Grakoui, A., Rice, C. M., Choo, Q. L., Houghton, M. & Walker, B. D. (1995) J. Clin. Invest. 96 2311-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koziel M. J., Dudley, D., Afdhal, N., Choo, Q. L., Houghton, M., Ralston, R. & Walker, B. D. (1993) J. Virol. 67 7522-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bukh J., Kim, J. P., Govindarajan, S., Apgar, C. L., Foung, S. K., Wages, J., Yun, A. J., Shapiro, M., Emerson, S. U. & Purcell, R. H. (1998) J. Infect. Dis. 177 855-862. [DOI] [PubMed] [Google Scholar]
- 34.Guilhot S., Fowler, P., Portillo, G., Margolskee, R. F., Ferrari, C., Bertoletti, A. & Chisari, F. V. (1992) J. Virol. 66 2670-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guidotti L. G., Rochford, R., Chung, J., Shapiro, M., Purcell, R. & Chisari, F. V. (1999) Science 284 825-829. [DOI] [PubMed] [Google Scholar]
- 36.Frese M., Schwarzle, V., Barth, K., Krieger, N., Lohmann, V., Mihm, S., Haller, O. & Bartenschlager, R. (2002) Hepatology 35 694-703. [DOI] [PubMed] [Google Scholar]
- 37.Gruener N. H., Lechner, F., Jung, M. C., Diepolder, H., Gerlach, T., Lauer, G., Walker, B., Sullivan, J., Phillips, R., Pape, G. R. & Klenerman, P. (2001) J. Virol. 75 5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wedemeyer H., He, X. S., Nascimbeni, M., Davis, A. R., Greenberg, H. B., Hoofnagle, J. H., Liang, T. J., Alter, H. & Rehermann, B. (2002) J. Immunol. 169 3447-3458. [DOI] [PubMed] [Google Scholar]
- 39.Biron C. A. (2001) Immunity 14 661-664. [DOI] [PubMed] [Google Scholar]
- 40.Crotta S., Stilla, A., Wack, A., D'Andrea, A., Nuti, S., D'Oro, U., Mosca, M., Filliponi, F., Brunetto, R. M., Bonino, F., Abrignani, S. & Valiante, N. M. (2002) J. Exp. Med. 195 35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng C. T. & Klimpel, G. R. (2002) J. Exp. Med. 195 43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Limmer A., Ohl, J., Kurts, C., Ljunggren, H. G., Reiss, Y., Groettrup, M., Momburg, F., Arnold, B. & Knolle, P. A. (2000) Nat. Med. 6 1348-1354. [DOI] [PubMed] [Google Scholar]
- 43.Heath W. R. & Carbone, F. R. (2001) Annu. Rev. Immunol. 19 47-64. [DOI] [PubMed] [Google Scholar]
- 44.Tan S. L. & Katze, M. G. (2001) Virology 284 1-12. [DOI] [PubMed] [Google Scholar]
- 45.Su A. I., Pezacki, J. P., Wodicka, L., Brideau, A. D., Supekova, L., Thimme, R., Wieland, S., Bukh, J., Purcell, R. H., Schultz, P. G. & Chisari, F. V. (2002) Proc. Natl. Acad. Sci. USA 99 15669-15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.