Abstract
Chronic exposure to increased force environments (+G) has pronounced effects on the circadian and homeostatic regulation of body temperature (Tb), ambulatory activity (Act), heart rate, feeding, and adiposity. By using the Brn 3.1 knockout mouse, which lacks vestibular hair cells, we recently described a major role of the vestibular system in mediating some of these adaptive responses. The present study used the C57BL/6JEi-het mouse strain (het), which lacks macular otoconia, to elucidate the contribution of specific vestibular receptors. In this study, eight het and eight WT mice were exposed to 2G for 8 weeks by means of chronic centrifugation. In addition, eight het and eight WT mice were maintained as 1G controls in similar conditions. Upon 2G exposure, the WT exhibited a decrease in Tb and an attenuated Tb circadian rhythm. Act means and rhythms also were attenuated. Body mass and food intake were significantly lower than the 1G controls. After 8 weeks, percent body fat was significantly lower in the WT mice (P < 0.0001). In contrast, the het mice did not exhibit a decrease in mean Tb and only a slight decrease in Tb circadian amplitude. het Act levels were attenuated similarly to the WT mice. Body mass and food intake were only slightly attenuated in the het mice, and percent body fat, after 8 weeks, was not different in the 2G het group. These results link the vestibular macular receptors with specific alterations in homeostatic and circadian regulation.
Keywords: het, mice, otoconia, hypergravity, adiposity
Data from previous spaceflight and chronic centrifugation studies have provided evidence for a profound and complex role for gravity in mammalian development, morphology, and physiological function (1, 2). Because living systems have evolved in Earth's static gravitational field, it is perhaps not surprising that both acute and chronic exposure to altered gravitational fields can affect physiological regulation.
Acute responses of mammals to chronic centrifugation include impaired thermoregulation (3–5), loss of circadian rhythmicity (4, 6), altered metabolism (7–11), transient hypophagia (11–13), and a decrease in body mass (bm) (13). After adaptation to chronic increased force environment (+G) exposure, animals exhibit a resumption of normal growth rate (14, 15), increased maintenance energy requirements (11, 14, 15), and altered distribution of bm between fat and fat-free components (13); there is a specific reduction in body fat that is proportional to field strength. This reduction in the body fat component of bm can be quite large; for example, chickens decrease from 30% body fat at 1G to 3% at 3G (14).
We have reported that mice respond similarly to other species exposed to 2G, both acutely and chronically (16). There is a dramatic drop (≈6°C) in body temperature, a loss of circadian rhythmicity, a large drop in activity, a period of hypophagia, a reduction of bm, and an ≈55% reduction of absolute and percent carcass fat by week 8 of 2G exposure (16, 17). Exposure to 2G could initiate these homeostatic, circadian, and autonomic responses via several independent, physiological pathways. Changes in the ambient force environment may be transduced by physiological responses such as fluid volume shifts, changes in intracranial pressure, skeletal muscle loading/unloading, and neurovestibular activation. In turn, these putative afferent mechanisms could contribute to the acute and chronic homeostatic, circadian, and autonomic responses to altered gravitational loads. Little direct evidence exists to support any of these pathways as the primary “transducer” of the ambient force environment.
There exists abundant physiological evidence that the vestibular system plays a large role in maintaining homeostasis during changes both in posture and movement (for review, see ref. 18). However, the static gravitational environment on earth may mask other important aspects of vestibular function. Recently, our laboratory, by using Brn 3.1 knockout mice that lack vestibular and auditory hair cells, demonstrated a significant role for the neurovestibular system in transducing the ambient force environment and mediating the adaptive temperature responses to 2G (19). A distinct limitation with the Brn model, however, was the inability to determine which vestibular receptors, i.e., maculae or ampullae or both, were responsible for the physiological responses to 2G.
The present study tested the hypothesis that vestibular linear acceleration sensory organs are important in the acute and chronic responses to changes in gravitational loading. The head tilt mouse (C57BL/6JEi-het) was used to examine specifically the role of gravity receptors (i.e., the otoconial organs utricule and saccule) in the acute homeostatic and circadian response and chronic metabolic responses to 2G. The head tilt mouse (het/het, abbreviated het), described originally by Sweet (20), is homozygous for a spontaneous mutation located on chromosome 17 (21). The labyrinth and both auditory and vestibular sensory organs seem normal in these animals except that the macular gravity receptors are devoid of otoconia (21). Vestibular responses to linear acceleration are absent in het mice (22), whereas responses to cranial rotation are present (S.M.J., unpublished observations) and auditory function seems to be normal (21, 22). Although freely moving in cages, the animals exhibit relatively normal postures and locomotion except that they may display a head tilt (hence, their name). When briefly placed in a tank of water, however, het mice are unable to properly orient to the gravitational force vector and cannot swim. This observation is in striking contrast to normal mice, which orient well and are strong swimmers under the same circumstances. Collectively, these findings indicate that although auditory and cranial angular acceleration sensors are intact in het mice, gravity receptor function is selectively eliminated.
Our hypothesis would predict that if gravity receptors are the primary mediators of the acute homeostatic and circadian responses and chronic metabolic responses to 2G, then such responses should be reduced significantly or absent in het mice exposed to 2G. To test this prediction, body temperature, circadian rhythms, activity levels, food intake, and body composition were evaluated in the het mouse after chronic exposure to 2G. The results support the hypothesis and evidence a unique linkage between the neurovestibular system and the regulation of homeostasis, circadian rhythms, and body composition.
Methods
Subjects.
The animals used in this study were otoconia-deficient C57BL/6JEi het mice obtained from The Jackson Laboratory. Care of the mice in the experiment met National Institutes of Health standards, as set forth in the Guide for the Care and Use of Laboratory Animals (40) and was approved by the University of California at Davis Institutional Animal Care and Use Committee.
Animals and Biotelemetry.
Sixteen male, adult (28–30 g) het mice (het/het) and 16 male, adult (28–31 g) heterozygote normal littermates (het/+), termed WT mice here (Mus musculus), were implanted i.p. with biotelemetry transmitters (VM-FH disk; Mini-Mitter, Sunriver, OR) to record body temperature (Tb) and activity (Act). The het mutation is recessive where the phenotype is expressed only in homozygote (het/het) animals. All animals were anesthetized (3% isofluorane in pure medical-grade oxygen), a midline celiotomy was performed, and a sterilized transmitter was inserted into the peritoneal cavity by using aseptic techniques. All incisions were sutured and treated with lidocaine and a topical antibiotic. The mice recovered on a heating pad, with Tb constantly monitored by means of a colonic probe. The mice were subdivided into four groups: 2G het experimental (n = 8), 1G het control (n = 8), 2G WT experimental (n = 8), and 1G WT control (n = 8). Individual animals among the het and WT groups were assigned randomly to 1G or 2G.
Housing and Centrifuge.
After 10 days of surgery recovery, the 2G groups were housed on a 4.5-m diameter centrifuge. The 1G groups were housed in an adjacent vivarium room with identical housing and ambient conditions. All animals were housed individually in standard plastic mouse cages with food (Lab Diet 5012, Purina) and water ad libitum. Each cage was placed on top of a telemetry receiver interfaced to a microcomputer data-acquisition system (Datasciences, Minneapolis). The cages were housed inside modules, which provided ventilation, a 24-h light/dark cycle, an ambient temperature of 25 ± 1°C, and visual isolation. The centrifuge modules were mounted with 1 df, thereby ensuring that the net G field was always orthonormal to the cage floor.
Experimental Protocol.
The four groups included het and WT 1G control groups and het and WT 2G groups. The only difference between the 1G and 2G groups (het or WT) was that the 2G groups were exposed to 2G by means of centrifugation during the 8-week experimental period. After a 2-week period of 1G that was used to establish baseline measurements, the 2G het and WT mice were exposed to 2G by means of centrifugation for 8 weeks. Centrifugation was interrupted twice weekly for ≈15- to 20-min periods for a weekly cage change and biweekly food and bm measurements. Tb and Act data were collected continuously at 5-min intervals.
Body Composition.
At the end of the 2G exposure, the mice were removed from the centrifuge and killed immediately. Body composition was determined by the gravimetric method of Bell and Stern (23).
Circadian and Statistical Analyses.
Phase, mean, and amplitude of the Tb and Act circadian rhythms were determined by using a phase-fitting (least-squares harmonic regression analysis) program that used a Fourier-based algorithm (24). The average daily mean, rhythm amplitude (calculated as the mean to maximum of the best-fit Fourier function), and phase (calculated as the time of day of the maximum of the rhythm) of Tb and Act for each group were calculated. Repeated-measures ANOVA was used to compare gravitational conditions [1G (control), early 2G (adaptation), and late 2G (recovery)]. Specific mean comparisons were made by using Tukey's HSD post hoc test (SPSS, Chicago). For body composition, comparisons between the experimental and control groups were analyzed by unpaired t test. An α of <0.05 was used for all tests. All data are reported as mean ± SE.
Results
Tb.
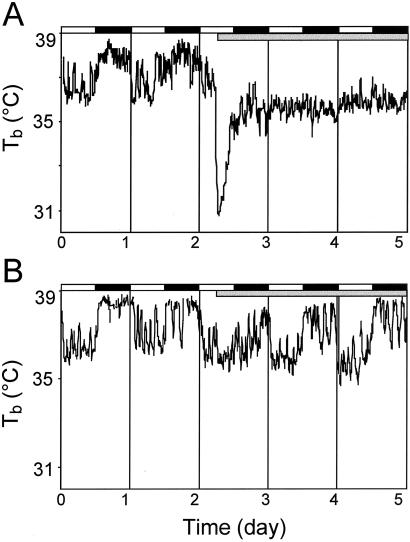
Fig. 1 shows the Tb data for representative individual WT (Fig. 1A) and het (Fig. 1B) mice during 1G and early 2G. During 1G, the circadian rhythm in Tb is robust in both mice. At 2G onset, the WT mouse exhibited a dramatic (≈6.2°C) decrease in Tb. The het mouse, however, evidenced only a slight decrease (≈0.3°C) in Tb.
Fig. 1.
Plots of representative Tb data from WT (A) and het (B) mouse. The onset of 2G is preceded by 48 h of 1G; the WT example demonstrates the typical drop in Tb and loss of circadian Tb rhythmicity. Only a small drop in Tb and small attenuation of the circadian Tb rhythm amplitude are seen in the het example. Gray bar, 2G period.
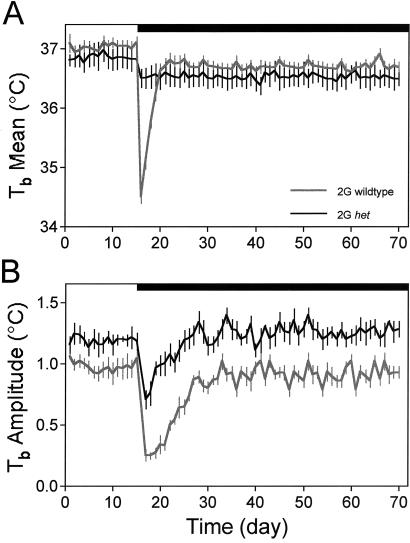
Fig. 2 plots the daily 1G and 2G responses of the Tb mean (Fig. 2A) and Tb circadian amplitude (Fig. 2B) for the 2G het and 2G WT groups. The daily Tb data were compared between 5-day intervals during 1G (days 6–10 of 1G), early 2G (days 2–6 of 2G), and late 2G (days 51–55 of 2G) between the het and WT experimental groups. The 1G 24-h mean Tb was not different between the WTs (37.0 ± 0.2°C) and het mice (36.9 ± 0.2°C). The Tb circadian amplitude at 1G was significantly lower (P < 0.01) for the WTs (0.9 ± 0.1°C) than the het mice (1.18 ± 0.1°C). The average time of day (phase) of the circadian maximum of the two groups at 1G did not differ (23.3 ± 0.1 h vs. 23.3 ± 0.2 h). During early 2G, mean Tb was highly depressed in the WT group, but only slightly in the het group. Mean Tb for the WTs (35.4 ± 0.3°C) during early 2G was significantly lower (P < 0.001) than both mean 1G Tb and early 2G mean Tb for the het mice (36.5 ± 0.1°C). During early 2G, the Tb circadian amplitude was significantly lower (P < 0.001) in the WTs (0.3 ± 0.1°C) compared with 1G and the het group (0.9 ± 0.1°C). Despite the decrease in Tb amplitude in the het mice, the wave form did not change, e.g., lose circadian rhythmicity, as compared with the dramatic loss of rhythmicity in the WTs. During late 2G, both mean Tb and the Tb circadian amplitude recovered to near 1G level. During late 2G, the mean Tb for the WTs (36.7 ± 0.2°C) and for the het mice (36.6 ± 0.3°C) is lower than 1G; however, this difference, as well as the difference between groups, is not significant. During late 2G, the Tb circadian amplitude for the WTs (0.8 ± 0.1°C) is lower than 1G and the Tb circadian amplitude for the het mice (1.3 ± 0.1°C) is higher than 1G, although neither the WT nor het Tb circadian amplitude was significantly different at late 2G compared with 1G.
Fig. 2.
Plots of the Tb mean responses (±SE) for the WT (gray line) and het (black line) groups. (A) Twenty-four-hour Tb averages. (B) Tb circadian rhythm amplitudes. The attenuated Tb amplitude response of the het is another indicator that these mice are refractory to the change in gravity loading and suggests, furthermore, that the hypothalamic circadian clock is affected more in the WT mice. Black bar, 2G period.
There were no differences in the timing of the Tb rhythm between 1G, early 2G, or late 2G in the het mice. Similarly, there were no differences in the circadian phase of the Tb rhythm between 1G and late 2G in the WT mice; however, the circadian phase of the Tb rhythm was significantly different (P < 0.001) between the WT and het mice in early 2G (23.2 ± 0.1 h vs. 21.6 ± 0.1 h) and the WT early 2G compared with WT and het 1G and late 2G (P < 0.001).
Act.
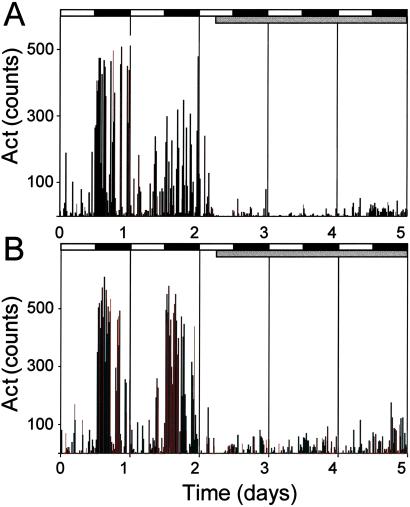
The daily Act data were compared between the same 5-day intervals as Tb. Fig. 3 shows the Act data for individual WT (Fig. 3A) and het (Fig. 3B) mice during 1G and early 2G. During 1G, the circadian rhythm in Act is robust in both mice. At 2G onset, both mice exhibited a dramatic decrease in Act.
Fig. 3.
Plots of representative Act data from a WT (A) and het (B) mouse. The onset of 2G is preceded by 48 h of 1G; the WT example demonstrates the typical drop in Act levels and loss of circadian Act amplitude. A similar response was seen in the het mice in both Act levels and circadian Act amplitude. Gray bar, 2G period.
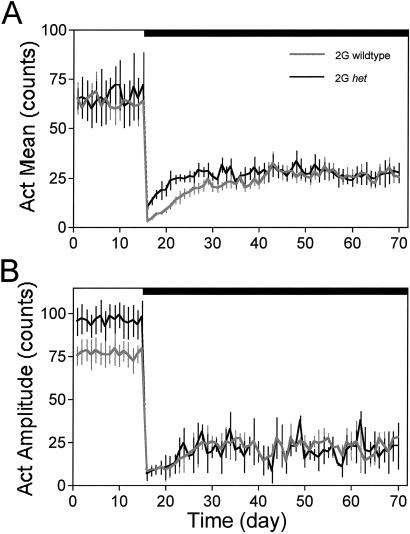
Fig. 4 plots the daily 1G and 2G responses of the Act mean (Fig. 4A) and Act circadian amplitude (Fig. 4B) for the 2G het and 2G WT groups. The 1G 24-h group mean Act was not different between the WT (63 ± 6 counts) and het mice (66 ± 5 counts). The Act circadian amplitude at 1G was significantly lower (P < 0.05) for the WTs (77 ± 5 counts) than the het mice (94 ± 6 counts). At 2G onset, the WT and het mice exhibited a significant decrease from 1G (89% and 75%, respectively; P < 0.001) in Act counts. During early 2G, daily mean Act was highly depressed in both the WT and het 2G groups. Mean Act for the WTs (7 ± 2 counts) during early 2G was significantly lower than both mean 1G Act (P < 0.01) and early 2G mean Act (P < 0.05) for the het mice (18 ± 4 counts). During early 2G, the circadian Act amplitude was significantly lower (P < 0.001) in the WT (10 ± 2 counts) and het groups (9 ± 1 counts) compared with 1G. During late 2G, mean Act had recovered to ≈40% of 1G in both the WT and het mice; it was significantly higher for both groups than during early 2G (P < 0.001). During late 2G, the mean Act for the WTs (26 ± 6 counts) and for the het mice (27 ± 4 counts) was still significantly lower than during 1G (P < 0.001); however, there was no difference between the WT and het groups. During late 2G, the circadian Act amplitude was significantly lower for the WTs (16 ± 2 counts) and het mice (17 ± 3 counts) than during 1G. The timing of the Act rhythm was not calculated because of the low amplitude rhythms during early and late 2G.
Fig. 4.
Plots of the Act mean responses (±SE) for the WT (gray line) and het (black line) groups. (A) Twenty-four-hour Act averages. (B) Act circadian rhythm amplitudes. Black bar, 2G period.
bm and Food Intake.
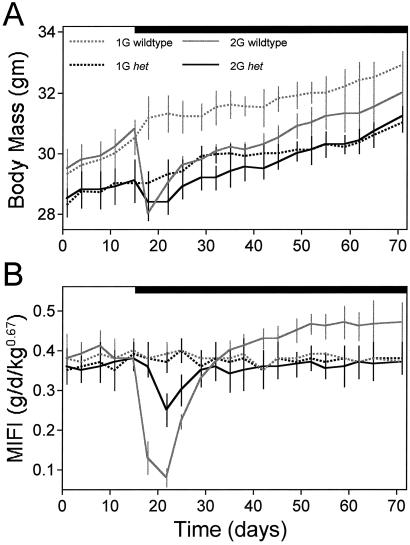
Fig. 5A shows the mean bm responses of all four groups throughout the study. The het mice bm (28.7 ± 0.6 g) tended to be smaller than the WTs (30.0 ± 0.8) during 1G. Three days after 2G onset, both the het and WT mice exhibited a decrease in bm; however, the drop in bm for the het mice (0.7 ± 0.4g) was significantly smaller than for the WTs (2.8 ± 0.2 g). Furthermore, the drop in bm for the het mice was only transient and did not differ significantly from the 1G het counterparts at any time (P > 0.05). In contrast, the drop in bm for the WT mice was sustained and remained significantly lower than the WT 1G counterparts until ≈6 weeks after 2G onset (P < 0.05).
Fig. 5.
(A) Plot of bm (mean ± SE) of the 1G and 2G WT (gray lines) and het (black lines) mice. Black bar, 2G period. At 2G onset, there is a rapid drop (within the first 72 h) in bm in the WT mice. The 2G het mice showed a significantly smaller drop in bm at 2G onset. The 2G WT mice exhibited a lower bm as compared with their 1G counterparts from weeks 1 to 6 of 2G. The 2G het mice never exhibited a lower bm as compared with their 1G counterparts. (B) Plot of MIFI (±SE) of the 1G and 2G WT (gray lines) and het (black lines) mice. The 2G WT mice exhibited the anticipated anorexic response at 2G onset, and, by week 6 of 2G, the 2G WT mice had a significantly higher MIFI than their 1G counterparts. The 2G het mice exhibited only a small, delayed reduction in MIFI at 2G onset, which recovered to 1G levels for the balance of the 2G period.
Fig. 5B shows the feeding responses of all four groups over the duration of the study. Food intake was calculated independent of bm. Mass-independent food intake (MIFI) was calculated as grams of food/bm2/3. The exponent of 2/3 (0.67) is used because intraspecific metabolism scales to bm2/3 (1). The exponent applies to mass, not weight, so it does not change with changes in G. As seen in Fig. 5B, food intake (g/d per bm2/3) was not significantly different between the WT (0.36 ± 0.04 g/d per bm2/3) and het (0.37 ± 0.05 g/d per bm2/3) mice at 1G (P > 0.05). During the first week of 2G, the WTs exhibited a significant (P < 0.001) reduction in food intake (0.15 ± 0.04 g/d per bm2/3) whereas the het mice evidenced a much smaller although still significant (P < 0.05) decrease in food intake (0.30 ± 0.03 g/d per bm2/3). Moreover, the het mice did not decrease food intake until after 4 days at 2G, which is in contrast to the immediate drop in food intake in the WT group. Both the het and WT mice resumed normal (e.g., 1G baseline) food intake within 2 weeks of 2G onset. During the last 2 weeks of 2G (weeks 6–8 of 2G), the WT mice were consuming significantly (P < 0.001) more food (0.46 ± 0.05 g/d per bm2/3) than the 2G het mice (0.36 ± 0.02 g/d per bm2/3) and 1G WTs (0.38 ± 0.04 g/d per bm2/3).
Body Composition.
Table 1 describes the average percent lean mass, body water, and fat content data from the body composition analysis of the four groups. Percent lean mass was significantly higher (P < 0.001) in the 1G het group, 2G het group, and 2G WTs as compared with the 1G WTs. Percent lean mass in the 2G WTs was not significantly different from 1G or 2G het mice. Similar to lean mass, percent water was significantly higher in the 1G het mice, 2G het mice, and 2G WTs as compared with their 1G counterparts. Percent lean mass in the 2G WTs was not significantly different from 1G or 2G het mice. Percent body fat was significantly lower in the het mice relative to the WTs at 1G (P < 0.001). Percent body fat was significantly lower in the 2G WTs relative to the 1G WTs (P < 0.001). Percent body fat was not, however, significantly lower in the 2G het mice relative to the 1G het mice.
Table 1.
Body composition
| % | 1G WT | 2G WT | 1G het | 2G het |
|---|---|---|---|---|
| Lean mass | 83.1 ± 1.2 | 91.5 ± 0.9 | 91.8 ± 0.9 | 92.4 ± 0.8 |
| Water | 57.1 ± 1.1 | 65.1 ± 0.8 | 66 ± 0.5 | 65.9 ± 0.7 |
| Fat | 15.5 ± 1.4 | 8.5 ± 0.6 | 7.4 ± 0.7 | 8.2 ± 0.5 |
, P < 0.001.
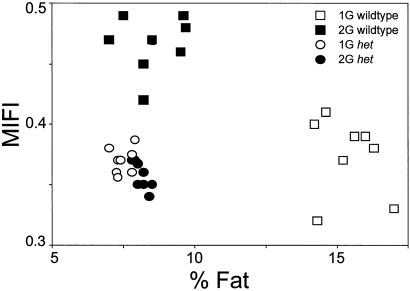
Fig. 6 is a scatter plot that shows the relationship between the last week of MIFI vs. percent body fat for all groups. The plot illustrates that the WT mice demonstrate a metabolic “shift” to a leaner state with a higher energy intake when adapted to 2G. Conversely, het mice do not demonstrate such a metabolic shift at 2G.
Fig. 6.
Plot of MIFI vs. percent body fat. The 2G WTs exhibited the anticipated metabolic shift: an increase in MIFI and a decrease in percent and absolute body fat. The het mice demonstrated a significantly different metabolic relationship at 1G, and this relationship was not altered with chronic 2G exposure.
Discussion
The two general observations of this study were: (i) at 1G, het mice are leaner than WT littermates, with an altered metabolic relationship between food intake and adiposity, and (ii) at 2G, except for Act levels, het mice demonstrated little change in Tb, circadian rhythms, food intake, bm, and adiposity responses compared with WT littermates. These results are consistent with and extend the previous findings in the Brn 3.1 knockout mouse and more recent observations that used surgical, bilaterally labyrinthectomized rats (C.A.F., unpublished observations). Specifically, the findings of this study suggest a significant role for the macular organs in mediating some aspects of homeostatic adaptation to 2G. Collectively, the observations from these three disparate, vestibular models strongly suggest that a vestibular deficit alone is sufficient to attenuate or block centrifugation effects on homeostatic and circadian mechanisms. Because there is mounting clinical evidence that the vestibular system participates in autonomic control (18, 25, 26) and, further, that vestibular dysfunction recently has been implicated in anxiety disorders (26), the findings of this study may have significant biomedical implications. Moreover, by extension, the findings suggest that otolith unloading during spaceflight may contribute to the adaptive and maladaptive physiological changes associated with spaceflight (2).
Tb.
This study clearly demonstrates the important role of the macular receptors in mediating changes in Tb during exposure to altered-force environments. The dramatic loss of Tb (≈6.2°C) in the WT mice reflects, presumably, major changes in the regulation of Tb by means of altering the balance between heat production (HP) and heat loss. In addition, the significantly larger circadian Tb amplitude in the het mice is largely attributable to a lower mean Tb during the rest phase (light period). To date, no heat-balance studies have addressed alterations in Tb regulation, metabolism, or rhythmicity in either microgravity or hypergravity. However, inferentially, previous spaceflight and centrifuge metabolic data from homeotherms suggest that HP is decreased during spaceflight and increased during centrifugation (27). Still, the relative contribution of HP vs. heat loss to these changes is not known. Nevertheless, HP and heat loss are both under homeostatic and circadian control effected by the hypothalamic regulation of the autonomic and neuroendocrine systems. In addition to active physiological mechanisms of HP and heat loss, it has been unclear how changes in physical forces (e.g., hydrostatic pressure and convection) may contribute to the apparent changes in Tb regulation in hypergravity and microgravity. From this study, it is clear that the passive changes contribute very little to the changes in Tb regulation at 2G. The absence of a Tb response in the het mice, similar to the previous observations in the Brn 3.1 mice, provides additional evidence that the physiological effects of centrifugation are not caused by passive changes in such forces.
Circadian Rhythms.
Previous studies have documented a direct effect of G on the expression of circadian rhythms and on the suprachiasmatic nucleus, the circadian pacemaker (6). In this experiment, the dramatic loss of circadian rhythmicity and circadian amplitude in the WTs after 2G exposure is similar to the responses documented in mice and rats (6, 16, 17). We do not believe that the attenuated circadian amplitude and loss of circadian rhythmicity is a “masking” effect, where clock output is obscured by peripheral physiological responses. Two separate lines of evidence support this contention. First, c-Fos expression in the suprachiasmatic nucleus after a 1-h light pulse at circadian time 14–15 is absent in rats exposed to 48 h of 2G (28). This c-Fos response is in contrast to the robust expression of c-Fos in the suprachiasmatic nucleus of rats exposed to an identical 1-h light pulse at the same circadian time at 1G (28). Second, a change in the period of the clock after both 2G and microgravity (e.g., spaceflight) exposure in rats, primates, and beetles (29–31) has been reported. Thus, we feel that the experimental results collectively support a direct effect of G on the circadian clock. That the het mice demonstrated only a small attenuation of Tb circadian amplitude and, further, that the expression of the Tb circadian rhythms was minimally affected in the het mice strongly support a direct modulatory influence of the vestibular system on the circadian pacemaker.
Act.
It seems that activity levels are similar at both 1G and 2G between the het and WT mice. Overall, the similar response between the het and WT mice suggests that the macular receptors do not play a major role in mediating activity levels at 1G or 2G. Moreover, the similar activity levels suggest that other sensory pathways mediate the decrease in activity at 2G. It is not understood why the activity levels should decrease at 2G or which physiological pathways mediate the decrease. Clearly, however, other sensory elements (e.g., proprioceptors, ampullar receptors, etc.) may mediate the decrease in Act levels.
Food Intake.
Food intake is a tightly regulated variable that is controlled by a complex, central signaling network of neuropeptides in the hypothalamus (32, 33). Disruption of this signaling network or the peripheral signals (e.g., leptin, GI hormones) will result in changes in food intake ranging from cessation of eating to massive overeating (32). Food intake in g/d per bm2/3 was similar between the het and WT mice at 1G. However, during the first 2 weeks of 2G and the last 2 weeks of 2G, food intake was significantly different in the WT as compared with 1G and the het mice in both conditions. The acute hypophagia seen in the WT mice is similar to that documented in mice and other species in previous studies (11–13). Moreover, the increased MIFI of the WT mice during late 2G is also similar to that seen in other studies (10, 14, 15).
Body Composition and bm.
After 8 weeks at 2G, the WT mice were significantly leaner than their 1G controls. Interestingly, the het mice at 2G were not significantly leaner than their 1G controls. It thus seems that the neurovestibular system can influence changes in body composition, particularly the mobilization and utilization of fat. This is the first time that rodents exposed to chronic 2G did not exhibit a significant decrease in fat mass. Furthermore, the lower percentage of fat in the 1G het relative to the 1G WTs suggests a unique linkage between tonic vestibular input and adiposity. We do not currently understand this linkage; however, one possibility is that the vestibular system has a tonic inhibitory influence on central sympathetic outflow and, therefore, removal of the inhibition leads to greater global sympathetic outflow and increased lipolysis. The autonomic modulation of metabolic organs (white adipose tissue, liver, and skeletal muscle) is well documented in mammals (34).
Collectively, the food intake and body composition data suggest that the het mice have an altered metabolic relationship between energy intake (feeding) and energy storage (adipose mass) as compared with the WTs at 1G. For example, at 1G, the het mice, as compared with the WTs, maintain a significantly smaller adipose depot despite equivalent caloric intake, suggesting possible alterations in calorie partitioning, substrate utilization, or metabolic rate. Moreover, the available data suggest that the 2G het mice did not have a similar metabolic shift, e.g., increased feeding and decreased percent adipose mass, as the 2G WTs.
The observation that het mice are consistently smaller than their WT littermates suggests a role for vestibular otolith input in bm regulation. Previous studies have demonstrated that bm is inversely related to gravitational load. These studies suggest that this phenomenon is regulated and not merely the result of an inability to acquire food or a metabolic deficit (10). For example, compared with 1G controls, animals adapted to 2G, such as the WT mice in this study, are consistently smaller but with a growth rate similar to the 1G controls. Moreover, fasting G adapted animals leads to additional bm loss, which is recovered upon realimentation. In contrast, animals exposed to the microgravity of spaceflight usually are larger than their respective 1G controls, and, postflight, they decrease bm toward the 1G control levels (35). A regulatory role for the vestibular system also is supported by the 2G bm response of the het mice in this study, which showed a small, transient decrease with a subsequent recovery to the 1G control levels of bm and growth rate. Collectively, these findings suggest that, in addition to other genetic factors, gravity, as perceived by the labyrinth, is a determinant in the set-point of bm. Moreover, the body composition results from this and earlier studies suggest that this G induced regulation of bm is related to altered fat metabolism and storage. Thus, the results from this study suggest that gravity, as perceived by the vestibular system, may influence the growth set-point, e.g., function as an afferent limb in bm regulation.
Vestibulohypothalamic Connection?
Because the neuronal circuits responsible for circadian rhythm genesis, thermal control, feeding, and autonomic function are located, to a large extent, in the hypothalamus, the highly attenuated, physiological responses of the het mice strongly suggest a vestibulohypothalamic linkage. The hypothalamus ensures normal (e.g., feeding, bm, thermoregulation, cardiovascular, fluid balance) and adaptive (e.g., stress, exercise) homeostasis by altering a variety of neural and endocrine effector mechanisms, including the balance among sympathetic and parasympathetic outflow (36). However, the importance and role of vestibular inputs in hypothalamic regulation of homeostasis via the autonomic nervous system during exposure to altered force environments have been largely unrecognized. Despite the paucity of evidence for monosynaptic connections from the vestibular nuclear complex to the hypothalamus, there exists abundant anatomical evidence that the vestibular nuclei project to numerous brainstem autonomic nuclei that, in turn, project to the hypothalamus (37–39). Moreover, a number of these nuclei have been shown to influence homeostatic and circadian function. Such nuclei include the parabrachial, caudal raphe nuclei, solitary nucleus, and the locus coeruleus. In addition, some brainstem information may be routed and processed by limbic structures, e.g., amygdala and infralimbic cortex, before reaching the hypothalamus, and, thus, potential limbic, e.g., “affect,” influences on hypothalamic function should be considered. Limbic modulation of the hypothalamus may be an important component of the response to 2G and the het response, or lack thereof, seemingly would support the putative association of vestibular dysfunction with affective disorders, e.g., anxiety or agoraphobia (for review, see ref. 26). Furthermore, as previously noted, many clinical findings have evidenced an anecdotal linkage between vestibular pathology and hypothalamic/autonomic dysfunction (25). The findings of the present study provide some empirical evidence to support this linkage.
In summary, our findings strongly suggest a unique linkage between the neurovestibular system and homeostatic and circadian regulation. Moreover, this study supports a role for the neurovestibular system in modulating body composition, both at 1G and 2G. This study also provides additional evidence that the physiological effects of centrifugation are largely caused by the increased gravitational component and not by a rotational or angular consequence of centrifugation. Importantly, although we cannot rule out occult deficits in the het mouse with ontogenetic bases, data from our laboratory and others thus far have not demonstrated any other major deficits, developmental or otherwise, in the het mouse. Moreover, recent studies that use adult labyrinthectomized rats have yielded data consistent with the het mouse data from this study (C.A.F., unpublished data). The findings of this study may implicate the vestibular system in a number of clinical syndromes, including circadian dysfunction, sleep–wake disorders, anxiety disorders, space-adaptation syndrome, and autonomic dysfunction.
Acknowledgments
We thank Sue Bennet for performing the body composition analysis. This research was supported in part by National Institutes of Health Grant RO1DC04477 (to S.M.J.), National Aeronautics and Space Administration Grant NAG5-4607 (to T.A.J.), National Space Biomedical Research Institute Grant NCC9-58-2333, and National Aeronautics and Space Administration Grant NAG2-1451 (to C.A.F.).
Abbreviations
MIFI, mass-independent food intake
Tb, body temperature
Act, activity
+G, increased force environment
bm, body mass
HP, heat production
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Smith A. H., (1976) COSPAR: Life Sciences and Space Research XIV (Akademie, Berlin), pp. 91–99.
- 2.Nicogossian A. E., Huntoon, C. L. & Pool, S. L., (1994) Space Physiology and Medicine (Lea and Febiger, Malver, PA).
- 3.Fuller C. A. (1984) Aviat. Space Environ. Med. 55 226-230. [PubMed] [Google Scholar]
- 4.Fuller C. A. (1985) Physiologist 28 S157-S158. [PubMed] [Google Scholar]
- 5.Fuller C. A., Horowitz, J. M. & Horwitz, B. A. (1977) J. Appl. Physiol. 42 74-79. [DOI] [PubMed] [Google Scholar]
- 6.Fuller C. A., Hoban-Higgins, T. M., Griffin, D. W. & Murakami, D. M. (1994) Adv. Space Res. 14 399-408. [DOI] [PubMed] [Google Scholar]
- 7.Daligon B. & Oyama, J. (1975) Am. J. Physiol. 228 742-746. [DOI] [PubMed] [Google Scholar]
- 8.Feller D., Neville, E. & Talarico, K. (1968) Am. J. Physiol. 214 1434-1437. [DOI] [PubMed] [Google Scholar]
- 9.Pace N., Rahlman, D. F. & Smith, A. H. (1980) Physiologist 23 S115-S116. [PubMed] [Google Scholar]
- 10.Smith A., (1973) COSPAR: Life Sciences and Space Research XI (Akademie, Berlin), pp. 201–206.
- 11.Katovich M. & Smith, A. (1978) J. Appl. Physiol. 45 51-55. [DOI] [PubMed] [Google Scholar]
- 12.Warren L. E., Horwitz, B. A., Hamilton, J. S. & Fuller, C. A. (2001) J. Appl. Physiol. 90 606-614. [DOI] [PubMed] [Google Scholar]
- 13.Burton R. & Smith, A. (1996) in Handbook of Physiology, Section 4: Environmental Physiology, eds. Fregly, M. & Blatteis, C. (Oxford Univ. Press, London), Vol. 1, pp. 943–950. [Google Scholar]
- 14.Smith A. & Katovich, M., (1977) COSPAR: Life Sciences and Space Research Volume XV (Permagon, Oxford), pp. 257–261.
- 15.Smith A. H., Burton, R. R. & Kelly, C. F. (1971) J. Nutr. 101 13-23. [DOI] [PubMed] [Google Scholar]
- 16.Murakami D. M. & Fuller, C. A. (2000) J. Grav. Physiol. 7 79-86. [PubMed] [Google Scholar]
- 17.Fuller P. M., Warden, C. H., Barry, S. J. & Fuller, C. A. (2000) J. Appl. Physiol. 89 1491-1498. [DOI] [PubMed] [Google Scholar]
- 18.Yates B. J. & Miller, A. D. (1998) J. Vestib. Res. 8 17-25. [PubMed] [Google Scholar]
- 19.Murakami D. M., Erkman, L., Rosenfeld, M. G. & Fuller, C. A. (1998) J. Grav. Physiol. 5 107-108. [PubMed] [Google Scholar]
- 20.Sweet H. (1980) Mouse Newsletter 63 19. [Google Scholar]
- 21.Bergstrom R. A., You, Y., Erway, L. C., Lyon, M. F. & Schimenti, J. C. (1998) Genetics 150 815-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones S. M., Erway, L. C., Bergstrom, R. A., Schimenti, J. C. & Jones, T. A. (1999) Hear. Res. 135 56-60. [DOI] [PubMed] [Google Scholar]
- 23.Bell G. F. & Stern, J. S. (1977) Growth 42 63-80. [PubMed] [Google Scholar]
- 24.Halberg F., Tong, Y. L. & Johnson, E. A. (1967) in The Cellular Aspects of Biorhythms, ed. von Mayersbach, H. (Springer, Berlin), pp. 20–48.
- 25.Balaban C. D. (1999) Curr. Opin. Neurol. 12 29-33. [DOI] [PubMed] [Google Scholar]
- 26.Balaban C. D. & Thayer, J. F. (2001) J. Anxiety Dis. 15 53-79. [DOI] [PubMed] [Google Scholar]
- 27.Robinson E. L. & Fuller, C. A. (2000) Pflügers Arch. 441 R32-R38. [DOI] [PubMed] [Google Scholar]
- 28.Murakami D. M., Tang, I. H. & Fuller, C. A. (1998) J. Grav. Physiol. 5 71-78. [PubMed] [Google Scholar]
- 29.Fuller, C. A., Murakami, D. M., Hoban-Higgins, T. M., Fuller, P. M., Robinson, E. L. & Tang, I. H. (2002) NASA Special Publications, in press.
- 30.Fuller C. A. (1984) Physiologist 27 S93-S94. [PubMed] [Google Scholar]
- 31.Alpatov A. M., Reitveld, W. J. & Oryntaeva, L. B. (1994) Biol. Rhythm Res. 25 168-177. [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi T., Arase, K. & Bray, G. A. (1988) Int. J. Obes. 12 285-291. [PubMed] [Google Scholar]
- 33.Bray G. A. (2000) Proc. Nutr. Soc. 59 373-384. [DOI] [PubMed] [Google Scholar]
- 34.Nonogaki K. (2000) Diabetologia 43 533-549. [DOI] [PubMed] [Google Scholar]
- 35.Wade C. E., Ortiz, R. M. & Baer, L. A. (2000) Aviat. Space Environ. Med. 71 1126-1130. [PubMed] [Google Scholar]
- 36.Paxinos G., (1995) The Rat Nervous System (Academic, New York).
- 37.Saper C. B. & Loewy, A. D. (1980) Brain Res. 197 291-317. [DOI] [PubMed] [Google Scholar]
- 38.Herbert H., Moga, M. M. & Saper, C. B. (1990) J. Comp. Neurol. 293 540-580. [DOI] [PubMed] [Google Scholar]
- 39.Balaban C. D. & Porter, J. D. (1998) J. Vestib. Res. 8 7-16. [PubMed] [Google Scholar]
- 40.National Academy of Sciences, (1996) Guide for the Care and Use of Laboratory Animals (Natl. Acad. Press, Washington, DC).