Abstract
Optical imaging of intrinsic responses to visual stimuli in extrastriate cortex of owl monkeys provided evidence for the dorsal half of the third visual area, V3. Visual stimuli were used to selectively activate locations in dorsolateral V2 and the rostrally adjoining presumptive V3. Consistent with the proposed retinotopies of dorsal V2 and dorsal V3, small bars of drifting gratings along the horizontal meridian of the contralateral hemifield activated cortex along the V2/V3 border, whereas such stimuli along the vertical meridian activated cortex along the rostral border of V3. Stimuli in limited locations in the lower visual quadrant revealed mirror reversals of retinotopy in dorsal V2 and V3, whereas stimuli in the upper visual quadrant failed to activate either region. Brain sections processed for cytochrome oxidase from the same cases provided architectonic borders of V2 that matched those indicated by the optical imaging. The results support the concept that a narrow dorsal V3 exists in monkeys. V3d borders dorsal V2 and contains a smaller, mirror-image representation of the lower visual quadrant.
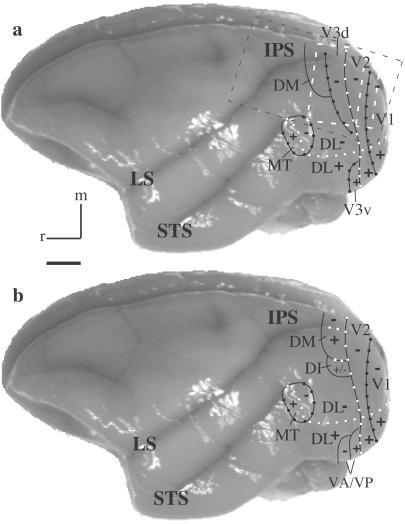
The visual cortex of monkeys and other primates is widely recognized as containing a number of visual areas, each specialized to contribute uniquely to visual function (see refs. 1–3 for review). However, of the several dozen visual areas proposed to exist in monkeys, only three areas, the first and second areas, V1 and V2, and the middle temporal area (MT, V5), are well established as valid subdivisions with known architectonic characteristics, retinotopy, types of modular organization, response characteristics to visual stimuli, and patterns of connections (see ref. 3). Recently we provided architectonic and connectional evidence for the existence of a third visual area, V3 (see Fig. 1a), in prosimian primates (4), New World monkeys (5, 6), and Old World macaque monkeys (7). Although Zeki (8) and Cragg (9) long ago provided connectional evidence for V3 in macaque monkeys, and evidence from microelectrode mapping experiments accumulated for macaques (10, 11) and large New World cebus monkeys (12, 13), that evidence has been open to other interpretations (e.g., see Fig. 1b; see refs. 3 and 7 for review).
Fig 1.
Two interpretations of the organization of extrastriate visual cortex in owl monkeys. Recently, we proposed that a third visual area, V3, with dorsal (V3d) and ventral (V3v) halves borders most of V2 (see a). Traditionally, other areas, including a DM, a dorsointermediate area (DI), and a ventroposterior area (VP), have been placed along the outer border of V2 (see b). Other areas shown include the dorsal lateral (DL), MT, and the ventroanterior (VA) areas. Plus (+) and minus (−) symbols indicate representations of the upper and lower visual quadrants, respectively. Where present, white squares indicate the representation of the HM, whereas black circles indicate the VM. White and black dashed boxes outline the estimated camera views for the optical images acquired for cases 04-24 (Figs. 2–4) and 07-02 (Fig. 5), respectively. IPS, intraparietal sulcus; LS, lateral sulcus; STS, superior temporal sulcus; M, medial; r, rostral. (Scale bar, 5 mm.) See text for references.
The goal of the present study was to address the issue of the validity of V3 with another method of revealing the organization of visual cortex, the use of optical imaging of intrinsic cortical signals evoked by stimuli in different locations in the visual field. In a manner similar to that of functional MRI in humans (e.g., refs. 14–17), optical imaging has the potential of revealing global patterns of retinotopic organization of extrastriate cortex of monkeys. Optical imaging has been used recently to study features of retinotopic organization of V1 in several species of mammals (18–21), but this method has not been widely applied to extrastriate cortex yet. In the present study, we explored the retinotopy of the presumptive dorsal V3 region in owl monkeys using optical images of intrinsic signals. This study was possible in owl monkeys, because these primates have few cortical fissures, and the dorsal V2 and V3 regions are exposed on the caudodorsal surface of the hemisphere. Here we were able to address only the issue of the retinotopy of the accessible dorsal portion of V3. The ventral portion of V3 on the ventral surface of the occipital lobe was not accessible. The optical imaging results were correlated with architectonic features revealed in brain sections cut parallel to the brain surface from the experimental cases. Because cytochrome oxidase (CO) preparations have been widely used to delimit V2 in monkeys (22–26), CO stains were used here to identify V2 and unequivocally distinguish V2 from presumptive V3.
Materials and Methods
Surgical Preparation.
The protocol used in this study was approved by the Vanderbilt University Animal Care and Use Committee. Five adult owl monkeys (Aotus trivirgatus) were prepared for surgery, paralyzed, and anesthetized as described in detail elsewhere (27). Paralysis and anesthesia were maintained by i.v. infusion of vecuronium bromide (0.1–0.2 mg/kg per h) and sufentanil citrate (Sufenta, 12–15 mg/kg per h) mixed in 5% dextrose lactated Ringer's solution delivered at a rate of ≈2.7 ml/h. To ensure that adequate levels of anesthesia were maintained throughout the experiment, heart rate, peak end tidal CO2, and temperature were monitored continuously after paralysis, and the level of anesthetic was increased if necessary. Pupils were dilated with 1% atropine eye drops, and clear gas-permeable contact lenses were used to render the retina conjugate with the viewing screen 28.5 or 57 cm distant. The location of both optic disks and the area centralis were plotted on the screen. The location of these landmarks were used to align the stimuli. An opening was made in the skull over visual cortex and was sealed with 1% agarose under a cover glass.
Optical Imaging.
Intrinsic optical imaging signals from the cortical surface were acquired with the Imager 2001 differential video-enhancement imaging system and VDAQ/NT data-acquisition software (Optical Imaging, Mountainside, NJ). Surface reference images of cortical vasculature were acquired with a 540-nm (green) light. The cortex was illuminated with a 611-nm (red) light during data acquisition and visualized with a tandem lens macroscope attached to a low-noise video camera. The camera was focused at the cortical surface, and the depth of field was subsequently increased by closing the lens diaphragm (aperture).
Visual stimuli were generated by using the VSG stimulus system (Cambridge Research Systems, Rochester, U.K.) and presented on a 21-inch video screen (SONY FD Trinitron, Model GDM-F400) in 120-Hz noninterlaced mode. Stimuli consisted of black rectangular windows and circular patches within which drifting gratings (fundamental spatial frequency, 0.5 cycles per degree; drift velocity, 2 Hz; duty cycle, 20%) were presented at either 0 or 90° orientations (see examples in Figs. 2 and 3). These grating stimuli were presented within either a 2° rectangular window or a 2° patch located at different visual field positions. The mean luminance was set at 36 cd/m2. The rectangular windows extended 38° along the horizontal and 25° along the vertical axis within the lower visual field quadrant. Each grating was moved back and forth along an axis that was orthogonal to the orientation of the grating. The high-contrast gratings of different orientations and control blanks of the same mean luminance were presented in random order across trials. A single trial consisted of (i) the presentation of a stationary grating for 1 sec, (ii) data acquisition during continued presentation of the drifting grating stimulus for 8 sec, and (iii) an interstimulus interval of 10 sec using a blank screen of mean luminance. Stimulus sets were made up of 10–20 trials.
Fig 2.
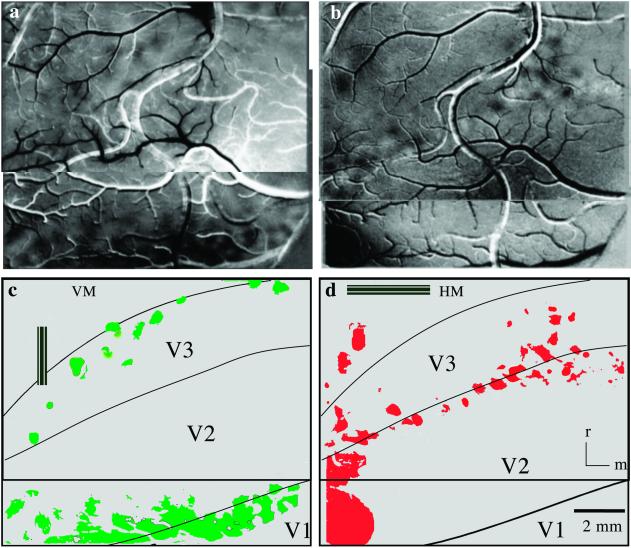
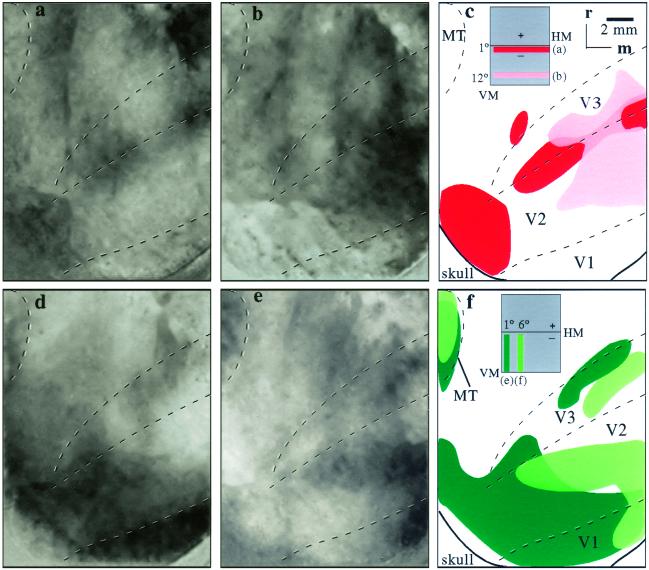
Optical images of cortical activation from visual stimulation of the VM (a) and HM (b) of the contralateral hemifield in monkey 04-24. A single-condition map is shown in a. The foci of activity (dark modules) result from dividing activation from the vertical-bar stimulus containing 90° gratings (see c Inset) by activation from the control blank (see Materials and Methods). A difference image (0° divided by 90°) is presented for the HM stimulus such that the dark foci represent activity following the 0° gratings (see d Inset) and the white foci reflect activity after the 90° gratings. The foci of activity resulting from the stimuli are illustrated below in green for the VM (c) and red for the HM (d), as are the architectonically identified borders of V1, V2, and V3. Images in a and b each represent two camera positions that were largely overlapping. The point at which the two camera positions become distinct is indicated by the horizontal line in the schematics below. See Fig. 1a (white box) for the estimated location of camera view. Rostral (r) and medial (m) directions are indicated. (Scale bar, 2 mm.)
Fig 3.
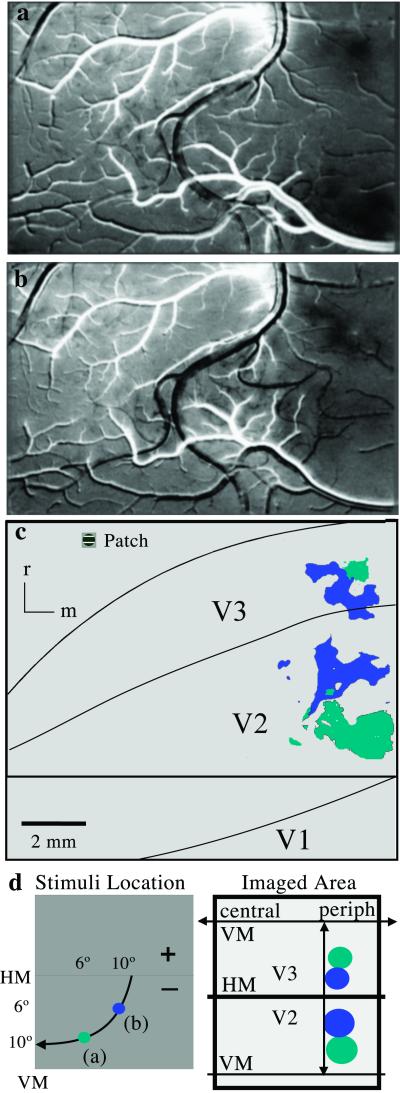
Single-condition maps (0° divided by control blank) of cortical activation resulting from visual stimulation with 2° circular patches presented to different locations in the contralateral lower visual quadrant in monkey 04-24. (a) A circular patch centered 6° from the VM and 10° below the HM (see d) revealed two dark foci of cortical activation. (b) A second patch was centered 10° from the vertical and 6° below the horizontal. (c) The foci of cortical activity from patches a (light blue) and b (dark blue) are shown relative to the architectonic borders of V1, V2, and V3. A representative stimulus patch is shown in the upper left corner. (d Left) Retinotopic locations of the stimuli are shown with an isoeccentricity line (arrow). (d Right) Schematized retinotopic pattern of cortical activation. (Scale bar, 2 mm.)
Video images were acquired at a rate of 30 video frames per sec. All 240 frames acquired for each condition during an 8-sec period were summed to create four separate data frames (activity images) before further analysis. Individual data frames included 744 × 480 pixels, with a resolution of either 87 pixels per mm for the lens combination (50-mm top, 50-mm bottom) or 43.5 pixels per mm for the same lens combination used with the addition of a ×2 converter.
Two approaches were used to create activation maps. By using WINMIX 1.7 software (Optical Imaging), the summed activity images acquired during the presentation of one orientation were divided by the summed activity images acquired during presentation of the orthogonal orientation to create differential maps (28, 29). Alternatively, all activity images associated with stimuli of the same orientation were summed and divided by the “pure blank” obtained by summing the images of the blank control to create single-condition maps (30). Both the differential and single-condition maps then were “clipped” (≈3 deviations around the mean) and scaled in the range of 0–255 gray levels for appropriate display. The resulting images were smoothed by using a low-pass filter with an appropriate Gaussian kernel. No further processing was done on the derived maps (see Figs. 2–4) unless specified (see below and Fig. 5).
Fig 4.
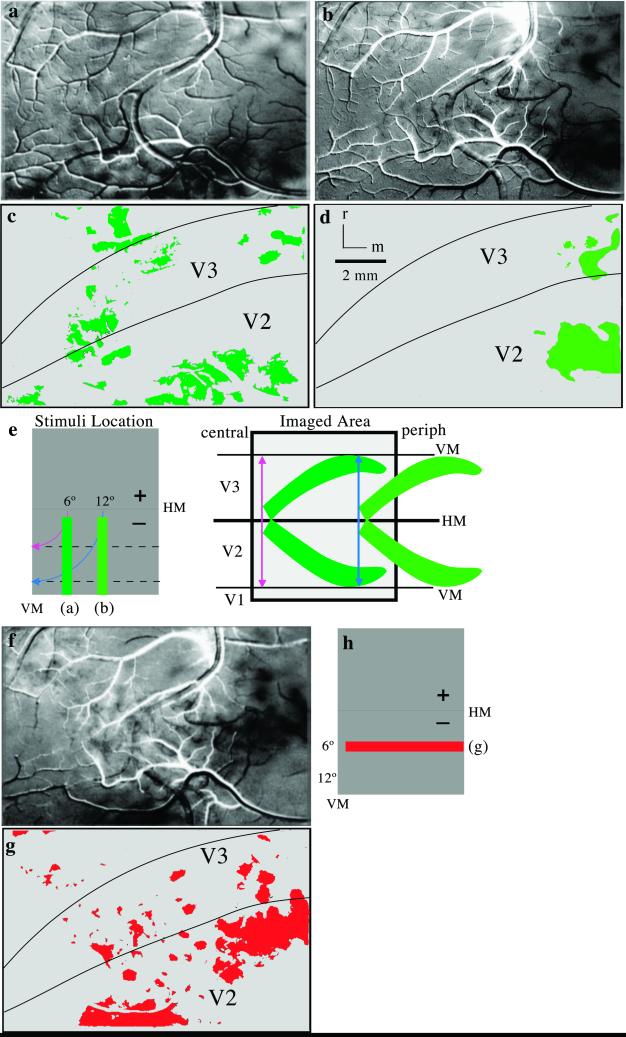
Images of cortical activation resulting from vertical and horizontal bars presented at different locations within the lower visual quadrant in monkey 04-24. A difference image (90/0°) is shown in a, where foci of black (90°) and white (0°) modules were activated by a vertical bar presented 6° away from the VM. Single-condition maps are shown for a vertical bar presented 12° away from the VM (b) and a horizontal bar centered 6° below the HM (f). Colored illustrations of the dark activation foci are shown in c, d, and g along with borders for V2 and V3. (e Left) Retinotopic locations of the vertical-bar stimuli with isoeccentricity lines (blue and pink arrows). (e Right) Schematized retinotopic pattern of activity. (h) Retinotopic location of the horizontal-bar stimulus. (Scale bar, 2 mm.)
Fig 5.
Cortical activation after bar placements in four different locations in the lower visual quadrant in monkey 07-02. All images are single-condition maps (0° per blank for horizontal bars; 90° per blank for vertical bars). The camera view was twice as large as the other cases. Although this allowed for more cortex to be viewed, many more surface blood vessels were included also. Thus, masks were created for the blood vessels, the images were reprocessed (see Materials and Methods), and the top 2.5 mm were cropped. (a and b) Activation after horizontal bars centered at the HM (a) and 12° below the HM (b). (d and e) Activation from vertical bars centered at the VM (d) and 6° from the VM (e). (c and f) Summaries of the cortical activation patterns. (c) Regions of activation are from horizontal bars along the HM (dark red) and 12° into the lower visual quadrant (light red). (f) Regions of activation from vertical bars along the VM (dark green) and 6° into the lower visual field (light green). Retinotopic locations of the bar stimuli are shown in boxes in c and f. The scale is twice as large as the previous images (Figs. 2–4). (Scale bar, 2 mm.) See the black box in Fig. 1a for an estimated location of the camera view.
To reduce vascular artifacts in the case presented in Fig. 5, we acquired a reference image and created a mask blocking out the locations of the major blood vessels in the imaging field of view; this mask was used to selectively filter the raw data from each image. The grayscale value for each pixel in the data images that was located in the blood-vessel mask was replaced by the mean of the grayscale values of the surrounding pixels (29).
To isolate regions of high activation for visual display, the thresholds of some images were adjusted such that only the regions of high activation remained. Regions representing blood vessels then were erased such that only cortical activity was represented. The regions of highest activity were color-coded and displayed beside the original (see Figs. 2–4).
Histological Procedures.
At the termination of each experiment, the monkey was anaesthetized deeply with an overdose of Nembutal (sodium pentobarbital) and perfused transcardially with a saline rinse followed by fixation with 2% paraformaldehyde in 0.1 M phosphate buffer. The brain was removed and the imaged region of cortex was separated from the hemisphere and flattened. The imaged piece of cortex then was frozen and cut with the surface vasculature pattern preserved in the first 100-μm section. Subsequent sections were cut parallel to the surface at 60 μm. The sections were processed for CO (31) to reveal the dense CO bands that characterize V2 and V3 and their borders as described recently for owl monkeys (6).
The image in Fig. 1 of a representative owl monkey brain was captured by using a digital camera (Scion, Frederick, MD) and NIH IMAGE software. The CO image in Fig. 6 was captured by using a Spot 2 camera mounted on a Nikon E800 microscope and acquired through Adobe PHOTOSHOP 6.0.2 software and adjusted for brightness and contrast. None of these images were altered in any other way.
Fig 6.
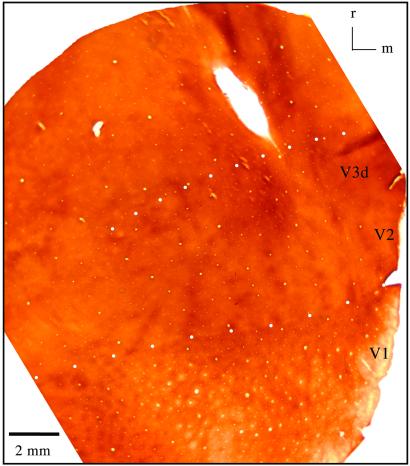
A section of cortex cut parallel to the surface in the region imaged in these experiments. The section was processed for CO. The V1/V2 and outer V3d borders are indicated by white-filled circles. The V2/V3d border is indicated by hollow black squares (monkey 03-19). V2 and V3 have different patterns of dark bands. See text for details. Lateral is on the left. (Scale bar, 2 mm.)
Results
Optical imaging revealed aspects of the retinotopic organization of the dorsal V3 region in five owl monkeys. In each case, the imaging results could be correlated with the CO pattern that identified the V1/V2 boundary and thus enabled the imaging data to be put in register with this border. The CO patterns also allowed a close approximation of the V2/V3 border and often suggested the width of V3. Here we illustrate results primarily from the most extensively studied case (Figs. 2–4). These results were consistent with the data obtained in four other monkeys (see Fig. 5).
The present results support the concept of a dorsal V3 that forms a mirror image of dorsal V2. As expected from previously reported connection patterns and architecture in owl monkeys (6), this dorsal V3 borders much of dorsal V2 and is about half the width of V2 (see Fig. 1a). Dorsal V3 narrows laterally toward the representation of central vision, to the extent that the territories of dorsal and ventral halves of V3 may be separated. Dorsal V3 is devoted exclusively to the lower visual quadrant with central vision lateral, the zero horizontal meridian (HM) along the V2/V3 border, and the vertical meridian (VM) along the rostral border.
The first results we illustrate are the activation patterns evoked by moving gratings within vertical bars along the VM or horizontal bars along the HM (Fig. 2). The presentation of either horizontal or vertical gratings within the vertical and horizontal bars and a blank screen (gray) allowed for two methods of division to reveal differences in cortical activity (see Materials and Methods). For horizontal bars the 0° orientation divided by the blank screen or the 90° orientation resulted in the most robust activation maps. Conversely, for vertical bars the 90° divided by either the blank screen or the 0° orientation showed the most pronounced activation. In effect, the orientation of the grating that matched the orientation of the bar produced greater activation patterns, perhaps because the orthogonal orientation did not stimulate cells with receptive fields larger than 2°. No bias for orientation was seen when using circular patch stimuli (Fig. 3). The VM activation image (Fig. 2a) was created by dividing the 90° stimulus by the blank, gray screen, whereas the HM activation image resulted from the 0° divided by the 90° orientation (Fig. 2b). Thus, the HM activation pattern contains both the 0° (black spots) and 90° (white spots) orientation modules, whereas the VM image contains only the 90° orientation modules. Note that for the colored schematic of the HM activation, only the 0° modules were displayed (Fig. 2d). The images displayed here were the ones that contained the most easily discernable patterns of activation. However, all combinations of images were analyzed and showed similar patterns of activation. That is, the activations related to the HM and VM were in the same cortical locations regardless of whether they were viewed as single-condition or difference maps.
Vertical bars along the VM produced an uneven modular band of activation along the V1/V2 border and the rostral V3 border (Fig. 2 a and c). The image of the brain surface (Fig. 2a) shows the blood-vessel pattern and sequences of small, dark foci representing the intrinsic signal evoked by the vertical window. These blob-like foci are shown schematically (Fig. 2c) with architectonic borders imposed. The uneven appearance of the activity pattern is the result of using stimuli of a fixed orientation, because stimuli of different orientations maximally activated different foci in V1, V2, and V3. The observation relevant to the proposed existence of V3 is that the more rostral band of foci effectively identified the rostral border of V3 in a manner consistent with the border being defined by the representation of the VM. The string of activated foci curves caudally in lateral V3d, consistent with the architectonic evidence that the area narrows laterally but also as a consequence of the representation of central vision in lateral V3d. In central vision, the 2° width of the vertical stimulus becomes significant enough to activate neurons with receptive fields displaced from the VM. Thus, a small circular stimulus (2° in diameter) centered at 1° in central vision of the monkey's lower quadrant also activated the lateral portion of V3 (not shown). The effectiveness of the long vertical window in activating cortex devoted to the VM is demonstrated by the second row of foci of increased activity along the V1/V2 border, because this border has long been known to represent the VM in owl monkeys (32) and other primates. As for V3, the foci cross the width of the lateral part of V2 that is known to be devoted to central vision (33).
Complementary results were obtained when the stimulating horizontal bars were placed along the HM (Fig. 2 b and d). A row of foci of increased activity was located along the V2/V3 border, a region known to represent the HM in owl monkeys (33) and other primates (see ref. 2). For uncertain reasons, the foci tended to scatter somewhat into V2 and V3 medially, but this was not apparent in other cases (see Fig. 5a). However, the activation foci that cross lateral V3d and V2 correspond in location to central vision, where the horizontal bars also would stimulate neurons with receptive fields displaced from the HM. The activation of cortex rostral to lateral V3 (Figs. 2a and 3 a and e) is in the region where central vision appears to be represented in the dorsomedial area (DM; see ref. 3). Connection patterns with V1 suggest that much of the rostral border of V3d is formed by the DM (6).
The activity patterns described above indicate that the caudal and rostral borders of V3 correspond to representations of the HM and VM as expected. Another proposed feature of V3 is that it mirrors V2 in retinotopic organization. By using 2° circular patches of drifting gratings as a stimulus in different locations of the visual hemifield, we were able to assess the retinotopy of V2 and V3 more extensively. An example of the results is shown in Fig. 3, where stimulus patches of drifting gratings were placed in two nearby locations in the lower visual quadrant. In one set of trials the use of a patch stimulus, 10° below the HM and 6° from the VM, activated a larger zone in medial V2 and a smaller zone in medial V3, providing evidence for two separate representations of the same location in the lower visual quadrant (Fig. 3 a and c, light blue). In a second set of trials, stimulus patch b, closer to the HM (6° below the HM and 10° from the VM), activated zones more rostral in V2 and more caudal in V3 (Fig. 3 b and c, dark blue). The displacements of the two sets of activity patterns are consistent with the proposal that V2 and V3 form mirror-image representations. Because the V3 region is narrower and more compressed, the same stimulus patch activated a smaller region of cortex in V3 than in V2 (Fig. 3). These results, together with those obtained when the stimulus patches were placed in other positions in the lower visual quadrant, provided clear evidence that V3 contains a mirror image of the retinotopy of V2.
The retinotopic organizations of V2 and V3 were assessed additionally by placing horizontal and vertical bars in different locations in the visual hemifield (Fig. 4). A vertical bar starting at 1° from the HM extending 28° into the lower visual quadrant, 6° from the VM, activated patches of cortex more laterally along the V2/V3 border and more medially in rostral V3 and caudal V2 as expected for a bar extending from central vision near the HM to less central vision away from the HM (Fig. 4 a and c). Additional patches of activation were just rostral to V3 in an adjoining portion of the DM thought to represent the lower visual quadrant. When the vertical bar was displaced from 6 to 12° from the VM (Fig. 4 b and d), the patches of activity shifted medially, with separate regions of activity in V2 and V3, as expected from the proposed organizations of the two areas. Because the activity zones were just lateral to the lip of the medial wall, the foci of activity in V2 and V3 likely extended more medially out of the viewing region. Similar patterns of cortical activation are shown in a second case where the vertical bars were presented at the VM and 6° from the VM (Fig. 5 d and e). In this case the field of view is magnified such that it is twice as large as shown for the other cases. Hence, an activation of the dorsal portion of the MT (see also Fig. 1) was also captured in the expected location of the VM (34).
In the more extensively examined case (04-24; Figs. 2–4), a horizontal bar spanning 38° of visual angle was placed near the VM and 6° into the lower visual quadrant. The stimulus in central to peripheral vision of the lower quadrant activated foci of cortex distributed lateromedially across portions of V2 and V3 (Fig. 4 f and g). Again, a few foci of cortical activation were found more rostral in the region of the DM. In another case (07-02), comparisons of horizontal bars located at the HM (Fig. 5a) and 6° into the lower quadrant (not shown) revealed similar patterns of activation as bars in these positions in case 04-24 (Figs. 2b and 4f). A bar positioned in a third location 12° below the horizontal activated more medial arrays of patches (Fig. 5b) that likely extended well beyond the field of view onto the medial wall. The activation patterns in V3 were consistent with the proposed retinotopy of V3.
In summary, the results provide compelling evidence that dorsal V3 exists as a narrow, more compressed mirror image of the retinotopy of dorsal V2. All effective stimuli were in the lower visual quadrant, and stimuli in central vision activated the most lateral locations in dorsal V2 and V3. The patchy appearance of the activity peaks is consistent with the premise that orientation-selective neurons are clustered in V2 and V3. Although activity peaks were noted in the regions of the DM and MT, results were not systematically collected from these regions.
The optical imaging results in each case were related to the CO pattern by superimposing blood vessels and other markers in the brain sections with the brain surface images as shown in Fig. 2 a and b. Although the sections were cut as nearly parallel to the brain surface as possible, the borders were represented best by aligning CO patterns from several adjacent brain sections. An example of the CO pattern in a single section is shown in Fig. 6. As in other cases, the V1/V2 border is obvious caudally. From the known width of V2, the rostral V2 border can be estimated closely. In addition, this border was at least approximated in each case by the pattern of CO-dense bands that cross the width of V2 and characterize the field (22–26). The rostral border of V3 was much more difficult to identify with confidence, but wider and less distinct bands than in V2 characterize V3 (5), and they helped us delimit the field in the present cases. In addition, the optical imaging results (e.g., Fig. 2) defined the rostral extent of V3, and this border was consistent with that deduced from connection patterns (6).
Discussion
In the present study we used optical imaging of intrinsic signals in extrastriate cortex of owl monkeys to provide further and highly compelling evidence for dorsal V3. The signals evoked by visual stimuli reflect differences between the absorption patterns of oxygenated and deoxygenated hemoglobin in the visible and near-infrared light range. It is the initial peak of deoxyhemoglobin that has been proposed as the main signal in optical imaging (18, 35–37). Although this signal provides an indirect measure of neural activity, its usefulness in doing so has been demonstrated repeatedly. The great value of the approach is that it provides a global image of the neural activity in a manner that is similar to that obtained by functional MRI in humans (14–17) and more recently by functional MRI in monkeys (38, 39) but with greater spatial resolution.
As a relatively new procedure, optical imaging has not been used yet to study the organization of extrastriate cortex in monkeys outside of well established areas V2 (20, 24, 40) and MT (41). Owl monkeys are ideal subjects for such studies because they have few cortical fissures, and many of the proposed visual areas are in cortex exposed on the brain surface. Our results revealed a narrow, 1.5- to 2-mm-wide dorsal V3 that represents central vision laterally and paracentral and peripheral vision medially toward the medial wall. The full medial extent is yet uncertain, but connection patterns with V1 suggest that V3d terminates at or extends slightly onto the cortex of the medial wall (6). As originally proposed for monkeys (8, 9), the shared border of V3d with V2 represents the HM. However, the width of V3 is approximately half the relative width originally proposed, which is approximately half the width of V2.
Other evidence for V3 in owl monkeys and other primates comes from connection patterns, especially with V1 (4–7, 12, 42). However, several different interpretations of such connection patterns have been possible (see ref. 3). Because the connection patterns were not known completely, and it appeared that the region of ventral V3 did not interconnect with V1, ventral V3 was sometimes assigned to another field, the ventroposterior area. Another problem was that cortex just rostral to V3d, the DM, receives V1 inputs, and thus it was difficult to be certain that V1 connections with V3d were not with adjoining DM. Although it now seems clear that ventral V3 does have V1 connections and that the DM and V3d have separate connection patterns with V1, the present results help distinguish V3d from the DM and establish a retinotopy for V3d that corresponds to the retinotopic pattern of the V1–V3d connections.
Microelectrode recordings also provided valuable information about the retinotopy of extrastriate cortex in monkeys (e.g., refs. 11, 13, 43, and 44). However, results from the V3 region have been interpreted in different ways. This may be because the microelectrode-mapping method is limited by the difficulty of sampling from a large number of recording sites, accurately locating those sites in cortex, obtaining receptive fields with clear and reliably defined locations, and possibly by receptive field scatter for nearby neurons. In addition, the results are obtained subjectively and are unavailable for confirmation by other observers. Nevertheless, a V3d with proportions and a retinotopy that correspond to V3d of the present study has been proposed based on microelectrode recordings in macaques (11) and cebus monkeys (13).
The present study provides visible evidence for the global retinotopy of V3d that is compatible with most of the microelectrode recording evidence and the cortical connection patterns. Although the architectonic appearance of the V3d region has been described in ways that are both compatible and incompatible with the proposed location and extent of V3d (see ref. 3 for review), a weak pattern of CO bands within the region has been described in our recent studies (5–7). The weak CO-banding pattern in V3 suggests that some sort of modular organization exists. This CO pattern was variably apparent in our present preparations. Possibly more compelling architectonic evidence for V3d can be obtained with other histochemical techniques.
Our oriented visual stimuli activated patches of neurons in V3d, and it was clear that the locations of patches varied with stimulus orientation. Thus, many neurons in V3d must be orientation-selective, as microelectrode recordings suggest (10, 45–48). However, the present study was directed toward determining the retinotopy of the V3d region, and the organization of orientation domains was not determined.
The present results do not address the issue of whether V3 is an incomplete visual area, representing only the lower visual quadrant in V3d (see ref. 49) or half of a larger area that includes the ventral region V3v (11). The ventral surface of the cerebral hemisphere simply was not accessible for optical imaging. However, the results from connection patterns strongly support the conclusion that V3v and V3d are separate wings of the same area (5–7). In addition, a visual area that represents only the upper or only the lower visual hemifield would seem highly improbable (50).
Acknowledgments
We are grateful to William Bosking for sharing the analysis software he developed; John Allison and Mary Feurtado for help with animal preparation; and Julia Mavity-Hudson with help in blood-vessel masking. Randolph Blake, Ford Ebner, and Anna Roe provided helpful comments on the manuscript. This research was supported by National Eye Institute Grants EY02686 (to J.H.K.) and EY01778 (to V.A.C.) and Shared Instrumentation Grant S10RR13947 (to V.A.C.). Other support came from National Eye Institute Vision Training and Core Grants EY08126 and HD15052 to the Vanderbilt Vision Center and the Vanderbilt John F. Kennedy Center, respectively.
Abbreviations
Vn, visual area n
MT, middle temporal area
CO, cytochrome oxidase
HM, horizontal meridian
VM, vertical meridian
DM, dorsomedial area
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on May 2, 2000.
References
- 1.Felleman D. J. & Van Essen, D. C. (1991) Cereb. Cortex 1 1-47. [DOI] [PubMed] [Google Scholar]
- 2.Rosa M. G. P. (1997) in Cerebral Cortex, eds. Rockland, K. S., Kaas, J. H. & Peters, A. (Plenum, New York), Vol. 12, pp. 127–203. [Google Scholar]
- 3.Kaas J. H. & Lyon, D. C. (2001) Prog. Brain Res. 134 285-295. [DOI] [PubMed] [Google Scholar]
- 4.Lyon D. C. & Kaas, J. H. (2002) Brain Behav. Evol. 59 114-129. [DOI] [PubMed] [Google Scholar]
- 5.Lyon D. C. & Kaas, J. H. (2001) J. Neurosci. 21 249-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyon D. C. & Kaas, J. H. (2002) J. Comp. Neurol. 449 281-297. [DOI] [PubMed] [Google Scholar]
- 7.Lyon D. C. & Kaas, J. H. (2002) Neuron 33 453-461. [DOI] [PubMed] [Google Scholar]
- 8.Zeki S. M. (1969) Brain Res. 14 271-291. [DOI] [PubMed] [Google Scholar]
- 9.Cragg B. G. (1969) Vision Res. 9 733-747. [DOI] [PubMed] [Google Scholar]
- 10.Zeki S. M. (1978) J. Physiol. (London) 277 273-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gattass R., Sousa, A. P. & Gross, C. G. (1988) J. Neurosci. 8 1831-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sousa A. P. B., Piñon, M. C. G. P., Gattass, R. & Rosa, M. G. P. (1991) J. Comp. Neurol. 308 665-682. [DOI] [PubMed] [Google Scholar]
- 13.Rosa M. G. P., Piñon, M. C., Gattass, R. & Sousa, A. P. B. (2000) Exp. Brain. Res. 132 287-305. [DOI] [PubMed] [Google Scholar]
- 14.Sereno M. I., Dale, A. M., Reppas, J. B., Kwong, K. K., Belliveau, J. W., Brady, T. J., Rosen, B. R. & Tootell, R. B. H. (1995) Science 268 889-893. [DOI] [PubMed] [Google Scholar]
- 15.Shipp S., Watson, J. D. G., Frackowiak, R. S. J. & Zeki, S. (1995) Neuroimage 2 125-132. [DOI] [PubMed] [Google Scholar]
- 16.DeYoe E. A., Carman, G. J., Bandettini, P., Glickman, S., Wieser, J., Cox, R., Miller, D. & Neitz, J. (1996) Proc. Natl. Acad. Sci. USA 93 2382-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tootell R. B. H., Mendola, J. D., Kadjikhani, N. K., Ledden, P. J., Liu, A. K., Reppas, J. B., Sereno, M. I. & Dale, A. M. (1997) J. Neurosci. 17 7060-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosking W. H., Zhang, Y., Schofield, B. & Fitzpatrick, D. (1997) J. Neurosci. 17 2112-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLoughlin N. P. & Blasdel, G. G. (1998) Neuroimage 7 326-336. [DOI] [PubMed] [Google Scholar]
- 20.Blasdel G. & Campbell, D. (2001) J. Neurosci. 21 8286-8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuett S., Bonhoeffer, T. & Hubener, M. (2002) J. Neurosci. 22 6549-6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tootell R. B., Hamilton, S. L. & Silverman, M. S. (1985) J. Neurosci. 5 2786-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krubitzer L. A. & Kaas, J. H. (1990) Visual Neurosci. 15 165-204. [DOI] [PubMed] [Google Scholar]
- 24.Ts'o D. Y., Frostig, R. D., Lieke, E. E. & Grinvald, A. (1990) Science 249 417-420. [DOI] [PubMed] [Google Scholar]
- 25.Malach R., Tootell, R. B. & Malonek, D. (1994) Cereb. Cortex 4 151-165. [DOI] [PubMed] [Google Scholar]
- 26.Sincich L. C. & Horton, J. C. (2002) Science 295 1734-1737. [DOI] [PubMed] [Google Scholar]
- 27.Xu X., Ichida, J. M., Allison, J. D., Boyd, J. D., Bonds, A. B. & Casagrande, V. A. (2001) J. Physiol. (London) 531 203-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blasdel G. G. (1992) J. Neurosci. 12 3115-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosking W. H., Kretz, R., Pucak, M. L. & Fitzpatrick, D. (2000) J. Neurosci. 20 2346-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonhoeffer T. & Grinvald, A. (1993) J. Neurosci. 13 4157-4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong-Riley M. T. (1979) Brain Res. 171 11-29. [DOI] [PubMed] [Google Scholar]
- 32.Allman J. M. & Kaas, J. H. (1971) Brain Res. 35 89-106. [DOI] [PubMed] [Google Scholar]
- 33.Allman J. M. & Kaas, J. H. (1974) Brain Res. 76 247-265. [DOI] [PubMed] [Google Scholar]
- 34.Allman J. M. & Kaas, J. H. (1974) Brain Res. 81 199-213. [DOI] [PubMed] [Google Scholar]
- 35.Blasdel G. G. & Salama, G. (1986) Nature 321 579-585. [DOI] [PubMed] [Google Scholar]
- 36.Grinvald A., Lieke, E., Frostig, R. P., Gilbert, C. & Wiesel, T. N. (1986) Nature 324 351-364. [DOI] [PubMed] [Google Scholar]
- 37.Malonek D. & Grinvald, A. (1996) Science 272 551-554. [DOI] [PubMed] [Google Scholar]
- 38.Disbrow E. A., Slutsky, D. A., Roberts, T. P. & Krubitzer, L. A. (2000) Proc. Natl. Acad. Sci. USA 97 9718-9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Logothetis N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. (2001) Nature 412 150-157. [DOI] [PubMed] [Google Scholar]
- 40.Malach R., Amir, Y., Harel, M. & Grinvald, A. (1993) Proc. Natl. Acad. Sci. USA 90 10469-10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malonek D., Tootell, R. B. & Grinvald, A. (1994) Proc. R. Soc. London 258 109-119. [DOI] [PubMed] [Google Scholar]
- 42.Van Essen D. C., Newsome, W. T., Maunsell, J. H. R. & Bixby, J. L. (1986) J. Comp. Neurol. 244 451-480. [DOI] [PubMed] [Google Scholar]
- 43.Allman J. M. & Kaas, J. H. (1975) Brain Res. 100 473-487. [DOI] [PubMed] [Google Scholar]
- 44.Rosa M. G. P. & Schmid, L. M. (1995) J. Comp. Neurol. 359 272-299. [DOI] [PubMed] [Google Scholar]
- 45.Baizer J. S. (1982) Invest. Ophthalmol. Visual Sci. 23 87-95. [PubMed] [Google Scholar]
- 46.Felleman D. J. & Van Essen, D. C. (1987) J. Neurophysiol. 57 889-920. [DOI] [PubMed] [Google Scholar]
- 47.Gegenfurtner K. R., Kiper, D. C. & Levitt, J. B. (1997) J. Neurophysiol. 77 1906-1923. [DOI] [PubMed] [Google Scholar]
- 48.Adams D. L. & Zeki, S. (2001) J. Neurophysiol. 86 2195-2203. [DOI] [PubMed] [Google Scholar]
- 49.Burkhalter A., Felleman, D. J., Newsome, W. T. & Van Essen, D. C. (1986) Vision Res. 26 63-80. [DOI] [PubMed] [Google Scholar]
- 50.Kaas J. H. (1993) in The Functional Organization of the Human Visual Cortex, eds. Gulyas, B., Ottoson, D. & Roland, P. E. (Pergamon, Oxford), pp. 1–11.