Abstract
The central auditory system of the mustached bat shows two types of reorganization of cochleotopic (frequency) maps: expanded reorganization resulting from shifts in the best frequencies (BFs) of neurons toward the BF of repetitively stimulated cortical neurons (hereafter centripetal BF shifts) and compressed reorganization resulting from the BF shifts of neurons away from the BF of the stimulated cortical neurons (hereafter centrifugal BF shifts). Facilitation and inhibition evoked by the corticofugal system have been hypothesized to be respectively related to centripetal and centrifugal BF shifts. If this hypothesis is correct, bicuculline (an antagonist of inhibitory GABA-A receptors) applied to cortical neurons would change centrifugal BF shifts into centripetal BF shifts. In the mustached bat, electric stimulation of cortical Doppler-shifted constant-frequency neurons, which are highly specialized for frequency analysis, evokes the centrifugal BF shifts of ipsilateral collicular and cortical Doppler-shifted constant-frequency neurons and contralateral cochlear hair cells. Bicuculline applied to the stimulation site changed the centrifugal BF shifts into centripetal BF shifts. On the other hand, electric stimulation of neurons in the posterior division of the auditory cortex, which are not particularly specialized for frequency analysis, evokes centripetal BF shifts of cortical neurons located near the stimulated cortical neurons. Bicuculline applied to the stimulation site augmented centripetal BF shifts but did not change the direction of the shifts. These observations support the hypothesis and indicate that centripetal and centrifugal BF shifts are both based on a single mechanism consisting of two components: facilitation and inhibition.
Keywords: auditory cortex, cochlea, frequency tuning, inferior colliculus, plasticity
The auditory system shows two types of reorganization of cochleotopic (frequency) maps: expanded and compressed. Expanded reorganization results from shifts of the frequency–tuning curves (or receptive fields) of neurons toward the best frequency (BF) of electrically stimulated cochlear hair cells (1–3), the BF of electrically stimulated cortical auditory neurons (4–7), the frequency of repetitively delivered acoustic stimulus (4–6, 8), the frequency of tone bursts paired with electric stimulation (ES) of the basal nucleus (6, 9, 10), or the frequency of a conditioning tone followed by an unconditioned electric leg or foot stimulation (8, 11–13). Expanded reorganization is common in the sensory systems of different species of mammals (14–18), whereas compressed reorganization is not common; it results from shifts of the tuning curves of neurons away from the BF of stimulated cortical neurons and the augmentation of neurons at a core of neural representation of a stimulus (19, 20). The BF shifts resulting in expanded reorganization have been termed centripetal BF shifts and those resulting in compressed reorganization, centrifugal BF shifts (17).
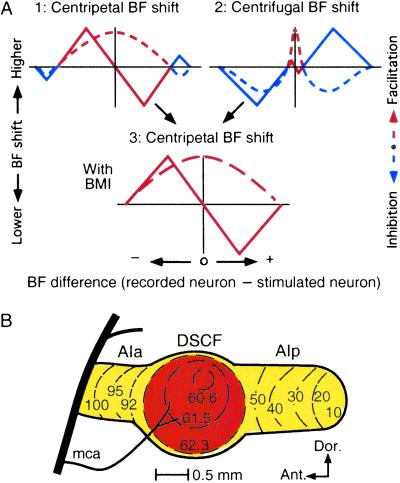
Suga et al. (17) hypothesized that corticofugal facilitation and lateral inhibition respectively evoke centripetal and centrifugal BF shifts of subcortical and cortical auditory neurons, because centrifugal BF shifts were observed at the edge of the area for centripetal BF shifts. If facilitation is strong and widespread to neighboring unmatched neurons in BF, and inhibition is weak, the corticofugal system may evoke centripetal BF shifts. (Neurons with a BF equal to that of stimulated neurons are called matched neurons, and others are called unmatched neurons.) On the contrary, if facilitation is highly focused to matched neurons, and inhibition is strong and widespread to neighboring unmatched neurons, the corticofugal system may evoke centrifugal BF shifts (Fig. 1A 1 and 2) (17). This hypothesis suggests that the two types of reorganization are due not to two completely different mechanisms but rather to a single mechanism consisting of two components: facilitation and inhibition.
Fig 1.
Centripetal and centrifugal BF shifts and the primary AC of the mustached bat. (A) Facilitation (red dashed lines) and inhibition (blue dashed lines) spread along the frequency axis from the location of stimulated neurons whose BF is referenced as 0 kHz. Recorded neurons, whose BFs are higher (+) or lower (−) than the BF of the stimulated ones, shift their BFs toward (solid red lines) or away from (solid blue lines) the BF of the stimulated neurons. It was hypothesized that facilitation and inhibition, respectively, evoke centripetal (A1, red solid lines) and centrifugal (A2, blue solid line) BF shifts (17). If this hypothesis is correct, elimination of inhibition by BMI would change centrifugal BF shifts into centripetal BF shifts (A3, solid red line). Because our present data support this hypothesis, this figure serves as the summary of our results. (B) The AC of the mustached bat over-represents 60.6- to 62.3-kHz sounds in its DSCF processing area (42). The numbers and lines indicate iso-BF lines. AIa and AIp, the anterior and posterior divisions of the AC; mca, middle cerebral artery.
In the big brown bat (Eptesicus fuscus), the centripetal BF shifts of collicular and cortical neurons can be evoked by auditory fear conditioning (8, 11, 12), by a tone burst repetitively delivered to the animal, or by focal ES of cortical auditory neurons (4–6, 8). The amount of BF shift changes as a function of the difference in BF between stimulated and recorded cortical or subcortical neurons or as a function of the difference between the frequency of a stimulus sound and the BF of a recorded neuron. The change is basically the same regardless of the means to evoke BF shifts. ES of cortical auditory neurons is the easiest and most effective means to evoke BF shifts (4–6, 8).
In the mustached bat (Pteronotus parnellii parnellii), sounds at ≈61 kHz are over-represented in the cochlea and the central auditory system (21–23). In the auditory cortex (AC), the large area representing ≈61 kHz is called the Doppler-shifted constant-frequency (DSCF) area (Fig. 1B) (24). Activation of cortical DSCF neurons with electric pulses augments the auditory responses at the BF and sharpens the frequency–tuning curves of matched subcortical DSCF neurons without shifting their BFs. It simultaneously suppresses the auditory responses of unmatched subcortical neurons at their BFs and shifts their BFs (together with frequency–tuning curves) away from the BFs of the activated cortical neurons (20). That is, cortical ES evokes centrifugal BF shifts for compressed reorganization.
The corticofugal system forms multiple feedback loops (25–28), the longest of which modulates the cochlear hair cells through olivocochlear fibers (29). In the mustached bat, a cochlear microphonic (CM) response (field potential) mostly originating from outer hair cells is sharply tuned to ≈61 kHz. A short train of electric pulses delivered to cortical DSCF neurons at a high rate for 3 min evokes a short-term centrifugal BF shift of the contralateral CM (29).
If the hypothesis proposed by Suga et al. (17) is correct, elimination of inhibition from the cortical DSCF area and/or corticofugal system must change centrifugal BF shifts evoked by ES of cortical DSCF neurons to centripetal BF shifts (Fig. 1A3). The aim of the present study is to test their hypothesis. We found that the elimination of inhibition in the AC changed centrifugal BF shifts of cortical and collicular DSCF neurons and cochlear hair cells into centripetal BF shifts as expected and proved that the two types of reorganization are based on a single mechanism consisting of two components: facilitation and inhibition.
Materials and Methods
Methods for surgery, acoustic and electric stimulations, and recording of neural activity were the same as those described in Zhang and Suga (20). Drug applications to cortical areas were performed similarly to those described in Zhang and Suga (30). Acquisition and processing of CM were the same as those described in Xiao and Suga (29). The protocol for this research was approved by the animal studies committee of Washington University in St. Louis.
Eighteen adult mustached bats (Pteronotus parnellii rubiginosus) from Trinidad were used. Under neuroleptanalgesia (Innovar 4.08 mg/kg, CZNTER, Fayetteville, NC), a 1.5-cm-long metal post was glued on the dorsal surface of the bat's skull, and a tungsten-wire electrode (30- to 50-μm tip diameter) was implanted at the intracranial opening of the cochlear aqueduct to record the CM (29, 31). A local anesthetic (lidocaine HCl, Astra USA, Westborough, MA) and antibiotic ointment (Furacin, RXV Products, Porterville, CA) were applied to the surgical wound.
Three to four days after surgery, the awake animal was placed in a polyethylene-foam body mold that was hung with an elastic band at the center of a soundproof room maintained at 31°C. The metal post glued on the skull was fixed to a metal rod with set screws to immobilize the animal's head, and the head was adjusted to face directly at a loudspeaker located 74 cm away. A few holes (50- to 100-μm diameter) were made in the skull covering the AC and inferior colliculus (IC), and a pair of tungsten-wire electrodes (≈7-μm tip diameter; 20–35 μm apart, one proximal to the other) was inserted to a 500- to 700-μm depth in the cortical DSCF area or the posterior division of the AC (AIp) through one of the holes. First, the BFs of cortical DSCF neurons were measured. Then these neurons were electrically stimulated through the above electrodes, or bicuculline methiodide (BMI; an antagonist of inhibitory GABA-A receptors) was applied to the stimulation site, and the effects of the stimulation or BMI were examined on the CM recorded with the implanted electrode or auditory responses of collicular or cortical neurons recorded with a tungsten-wire microelectrode (≈7-μm tip diameter). The effects were evaluated in reference to the relationship in BF between the stimulated cortical neurons and the recorded CM or neurons.
ES of Cortical Neurons.
ES delivered to cortical DSCF or AIp neurons with a pair of tungsten-wire electrodes to examine corticofugal modulation of collicular or cortical neurons was a monophasic electric pulse (0.2 ms long, 100 nA) delivered at a rate of 5 s for 7 min. Such electric pulses delivered at a low rate do not evoke any noticeable change in CM. Therefore, ES to examine the corticofugal modulation of CM was a 6.2-ms-long train of four electric pulses (0.2 ms long, 100 nA, 2.0-ms interval) delivered at a rate of 33/s for 3.0 min (29). The electric pulses were estimated to stimulate neurons within a 60-μm radius around the electrode tip (19).
Acoustic Stimulation.
Acoustic stimuli delivered to the animal were 20-ms-long tone bursts with a 0.5-ms rise–decay time for the studies of neural responses and were 2.0-ms-long tone bursts with a 0.01-ms rise–decay time for the studies of CM. These tone bursts were generated with a voltage-controlled oscillator and an electronic switch and were delivered to the animal at a rate of 5/s from a condenser loudspeaker. The frequency and amplitude of tone bursts were varied manually to audiovisual measurements of the BF and minimum threshold of a given neuron or CM. Then, those parameters were computer controlled to obtain a frequency–tuning or –response curve of the neuron or CM. In computer-controlled frequency and/or amplitude scans, the frequency of a tone burst was varied in 34 0.1-kHz steps for the studies of neurons and CM or 34 1.0-kHz steps for the studies of AIp neurons. The 1.0-kHz step was used for the studies of AIp neurons, because their frequency–tuning curves were not sharp compared with those of DSCF neurons and CM. An identical frequency scan was repeated 50 times for the studies of neural responses but 10 times for the studies of CM. The amplitude of the tone bursts was set at 10 dB above the minimum threshold of a given neuron or CM or varied in 4-, 5-, or 10-dB steps every 50 or 10 frequency scans.
Blocking Inhibition in the AC with BMI.
A glass micropipette (≈10-μm tip diameter) filled with 5 mM BMI (Sigma) in saline was placed at the site where the BF of electrically stimulated cortical DSCF or AIp neurons was measured. Then, 1.0 nl of 5.0 mM BMI was applied to the stimulation site with a Picospritzer II (General Valve, Fairfield, NJ). The Picospritzer was set at 0.67 bar in pressure and 30 ms in duration.
Data Acquisition.
Neural responses.
Action potentials of a single neuron were selected with a time-amplitude-window discriminator (Bak Electronics, Rockville, MD; model DIS-1). The waveform of an action potential of a recorded neuron was stored and displayed on the screen of a digital storage oscilloscope at the beginning of data acquisition. This action potential template was compared with action potentials during data acquisition. The responses of a single collicular or cortical neuron to tone bursts were recorded before and after ES and/or BMI application. Frequency–response curves based on the responses to frequency scans were obtained at the amplitude of the tone bursts fixed at 10 dB above the minimum threshold of a given neuron, which was 24.87 ± 4.18 dB sound pressure level for 186 neurons. Frequency–tuning curves were obtained with multiple frequency scans at different amplitudes. The responses of a single neuron to tone bursts were displayed as an array of peristimulus time (PST) histograms or PST cumulative histograms. The data were stored in the computer hard drive and were used for off-line analysis.
CM responses.
The CM recorded with the implanted electrode was amplified, filtered (40–70 kHz band-passed), and displayed on the oscilloscope screen. The frequency–responses curves of CM were based on the responses to 10 identical frequency scans recorded before and after ES and/or BMI application. The amplitude of the tone bursts in the frequency scan was fixed at 10 dB above the minimum threshold of a given CM, which was 51.05 ± 2.98 dB sound pressure level for four cochleae. The noise level of the recording system was ≈5 μV. Frequency resolution for the measurement of the BF of the CM was 0.10 kHz.
Off-Line Data Processing.
The magnitude of auditory responses of a neuron was expressed by a number of impulses per 50 identical stimuli after the subtraction of background discharges counted in the last block of the frequency scan. The magnitude of a CM response was expressed by a peak-to-peak amplitude of the CM with a computer. It was plotted as the function of the frequencies of tone bursts.
The BF shift (i.e., shift in the frequency–response or –tuning curve) of an auditory neuron or CM evoked by a focal cortical stimulation or a BMI application was considered significant if it shifted back (i.e., recovered) >90%. A t test was used to test the difference between the auditory responses obtained before and after the ES or BMI application. The difference between BF shift-difference curves was also tested with a t test.
Normalization of BFs.
The mean frequency of the second harmonic of the CF component of biosonar pulses emitted by the 18 Trinidadian mustached bats, P. p. rubiginous, at rest [hereafter, resting frequency (RF)] ranged from 57.761 to 59.179 kHz (mean ± SD: 58.280 ± 0.583 kHz). These RFs are different from the mean RF of Jamaican mustached bat, P. p. parnellii (61.21 ± 0.68 kHz, 34 bats) and Panamanian mustached bats, P. p. rubiginous (60.87 ± 0.48 kHz, 77 bats) (32).
In the Jamaican mustached bat, the BF or resonance frequency of the CM was ≈0.2 kHz higher than the RFs of individual bats (33, 34). Accordingly, the cortical frequency map is slightly different between individual bats (35). Therefore, to compare tuning curves across animals, all stimulus frequencies and BFs were normalized according to the following formula (32):
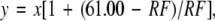 |
where x is the frequency of a stimulus tone or BF; y is its normalized frequency or BF; and RF is the animal's resting frequency. The “61.00” in the formula reflects the mean RF of a large population of mustached bats from Panama and Jamaica.
Results
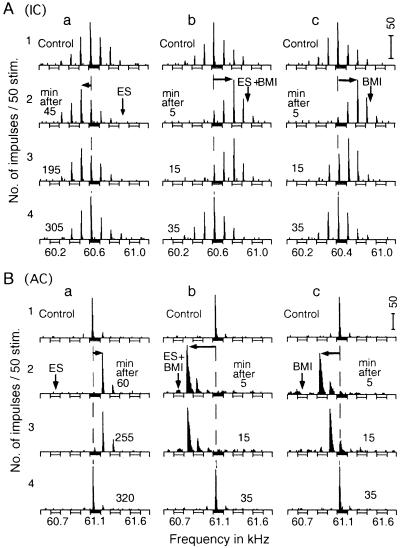
BMI applied to cortical neurons increased background discharges, augmented auditory responses to tone burst stimuli (Figs. 2B and 3A), and evoked BF shifts of unmatched collicular and cortical neurons (Fig. 2). In Fig. 2A, a collicular neuron was tuned to 60.6 kHz (control). When cortical neurons tuned to 60.9 kHz were electrically stimulated, the BF of the collicular neuron shifted from 60.6 to 60.5 kHz. That is, the neuron showed a centrifugal BF shift (Fig. 2Aa2). However, when BMI was applied to the cortical stimulation site immediately before the ES, the collicular BF at 60.6 kHz shifted to 60.8 kHz. That is, the collicular neuron showed a centripetal BF shift (Fig. 2Ab2). Because BMI applied to cortical neurons suppresses inhibitory synapses associated with them, the neurons became more active than in the normal condition. Accordingly, BMI, without ES, evoked the centripetal BF shift, which was larger in amount than the centrifugal BF shift evoked by ES (Fig. 2Ac2).
Fig 2.
Changes in the direction of BF shifts of a collicular and a cortical DSCF neuron of the mustached bat. BMI applied to cortical DSCF neurons changes centrifugal BF shifts (a) evoked by ES of cortical DSCF neurons into centripetal BF shifts (b and c). The arrays of PST histograms display frequency–response curves of a collicular (A) and a cortical (B) DSCF neuron. The vertical and horizontal arrows respectively indicate the BFs of cortical DSCF neurons receiving ES and/or BMI and centrifugal or centripetal BF shifts of the recorded neurons. 1–4: arrays of PST histograms recorded before (control) and after ES and/or BMI applications. The amplitude of tone bursts was set at 10 dB above the minimum threshold of a given neuron. ES, 0.2-ms 100-nA electric pulses delivered at a rate of 5/s for 7.0 min; BMI, 1.0 nl of 5 mM BMI.
Fig 3.
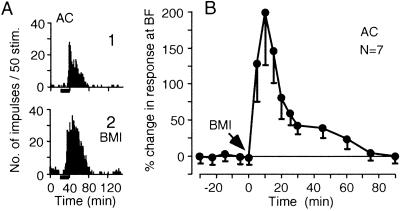
The effect of BMI on the responses of cortical DSCF neurons to tone bursts. (A) PST histograms display the responses of a single cortical neuron to 60.8-kHz 34-dB SPL tone bursts without (1) and with (2) BMI. The short horizontal bar below each histogram indicates a 20-ms-long tone burst. (B) The time course of the augmentation of the auditory responses of seven cortical neurons caused by BMI. Each data point indicates a mean and a standard error (n = 7).
Just as collicular neurons showed centrifugal BF shifts for cortical ES and centripetal BF shifts for BMI, cortical neurons showed the same changes as those of collicular neurons. In Fig. 2B, a cortical neuron tuned to 61.1 kHz showed a centrifugal BF shift for ES of cortical neurons tuned to 60.7 kHz (Fig. 2Ba2). However, it showed a centripetal BF shift for BMI applied to the 60.7-kHz tuned location (Fig. 2Bb2). The centripetal BF shift was two times larger than centrifugal BF shift, and an increase in response to a tone burst was noticeable. Without ES, BMI also evoked a centripetal BF shift of the cortical neuron (Fig. 2Bc2). In both the IC and AC, the recovery of BF shifts was slow for ES but fast for BMI, as described below.
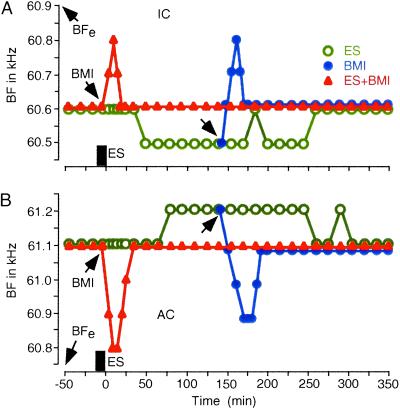
The centrifugal BF shifts of both collicular and cortical neurons developed with a long latency (64.0 ± 18.0 min, n = 75), after cortical ES. The shifts lasted 202.0 ± 68.0 min (n = 75) (Fig. 4, open circles). BMI applied at the beginning of cortical ES evoked short-lasting centripetal BF shifts of collicular and cortical neurons (17.8 ± 6.5 min, n = 73) and prevented the development of centrifugal BF shifts evoked by the ES (Fig. 4, filled triangles). BMI applied during the ongoing centrifugal BF shifts of the collicular and cortical neurons also evoked short-lasting centripetal BF shifts (19.0 ± 6.2 min, n = 9). After the centripetal shifts, the collicular and cortical BF shifts did not return to the centrifugal BF shifts but to the control BFs (Fig. 4, filled circles). The centripetal BF shifts occurred within 5 min after BMI application and disappeared within 35 min. This time course was similar to that of the augmentation of the auditory responses of cortical neurons evoked by BMI (Fig. 3B).
Fig 4.
The time courses of BF shifts of a collicular (A) and a cortical (B) DSCF neuron evoked by ES and/or BMI applied to cortical DSCF neurons. BFe: BF of cortical DSCF neurons receiving ES and/or BMI. ES (rectangle): 7-min-long ES with a 0.2-ms 100-nA electric pulse delivered at a rate of 5/s. BMI (1.0 nl of 5 mM BMI) was applied immediately before or 135 min after ES (arrows).
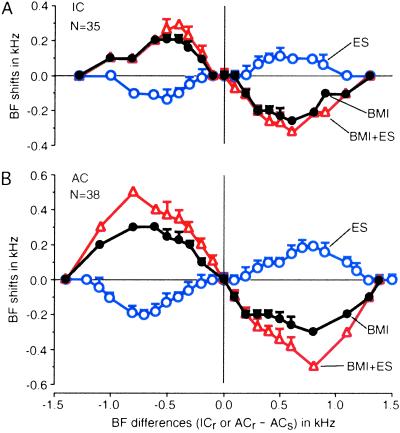
All of the BF shifts measured for 37 collicular and 81 cortical neurons are plotted as a function of differences in BF between recorded collicular or cortical neurons and electrically stimulated or BMI applied cortical neurons (hereafter, BF shift–difference curve; Fig. 5). As reported by Zhang and Suga (20), cortical ES did not shift the BFs of matched collicular neurons whose BFs did not differ more than 0.20 kHz from that of the stimulated cortical neurons. However, it evoked centrifugal BF shifts in unmatched collicular neurons whose BFs differed >0.20 kHz but not more than 1.10 kHz. Within the range of BF differences of 0.20–0.50 kHz, the larger the BF difference, the larger the BF shift was. BF shifts were smaller beyond this range (Fig. 5A, open circles).
Fig 5.
BF shift–difference curves representing the relationship between BF shifts and the differences in BF between recorded neurons (ICr or ACr) and cortical neurons (ACs) receiving ES and/or BMI. A and B, respectively, show the BF shifts of 35 collicular and 38 cortical neurons evoked by ES alone (open circles), BMI alone (filled circles), and BMI + ES (open triangles). Each data point indicates a mean and a standard error. BMI inverted the BF shift–difference curves for ES.
BMI applied to the cortical DSCF neurons immediately before ES inverted the BF shift–difference curve of collicular neurons. The amount of centripetal BF shifts became larger by 0.10 kHz (P < 0.002), and the range of BF differences for centripetal BF shifts became wider by 0.20 kHz (P < 0.01) (Fig. 5A, open triangles). Centripetal BF shifts evoked by BMI applied to cortical DSCF neurons during centrifugal BF shifts were not different from those evoked by BMI applied before the centrifugal BF shifts developed (Fig. 5A, filled circles).
Cortical ES evokes thalamic BF shifts that are slightly larger than the collicular BF shifts (20). Therefore, it was expected that cortical BF shifts would be larger than collicular BF shifts, which was the case (Fig. 5B). ES of cortical DSCF neurons evoked the centrifugal BF shifts of nearby cortical DSCF neurons. These centrifugal cortical BF shifts were significantly larger and more widely spread than the collicular BF shifts (Fig. 5B, open circles). BMI applied to the cortical stimulation site evoked centripetal BF shifts of nearby cortical neurons, which were also larger than those of collicular neurons (Fig. 5B, filled circles and open triangles). The cortical BF shifts at the peak were ≈25% larger than collicular ones regardless of whether the shifts were centrifugal or centripetal.
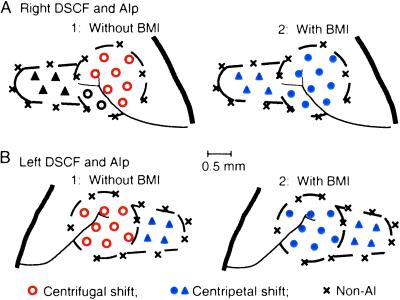
The AIp represents 10- to 50-kHz sounds and is not specialized for fine frequency analysis as the DSCF area (23). ES of cortical AIp neurons evoked centripetal BF shifts of nearby cortical AIp neurons, as reported by Sakai and Suga (36). BMI applied to the stimulation site in the AIp augmented the centripetal BF shifts evoked by the ES but did not change the direction of the BF shifts. In each of five animals, mapping experiments were performed over 2 weeks to further demonstrate that BMI applied to cortical DSCF neurons changed the centrifugal BF shifts of nearby cortical DSCF neurons to centripetal ones, but BMI applied to cortical AIp neurons did not change the direction of the centripetal BF shifts of nearby cortical AIp neurons. Fig. 6 A1 and B1 show the distributions of stimulation sites where ES evoked either centrifugal or centripetal BF shifts in the normal condition, whereas Fig. 6 A2 and B2 show the distribution of BMI applied sites where BMI evoked centripetal BF shifts. It was clear that under BMI, centrifugal BF shifts were not evoked.
Fig 6.
Cortical loci where ES with or without BMI evoked either centrifugal (open circles) or centripetal (filled circles or triangles) BF shifts. (A) The right AC of a mustached bat. (B) The left AC of another mustached bat. The dashed circle and rectangle represent the DSCF and AIp areas, respectively. The thick and thin lines represent the sylvian fossa and a branch of middle cerebral artery. The loci (neurons) evoked centrifugal BF shifts without BMI (open circles) changed into centripetal ones (filled circles) with BMI. Xs, cortical loci where ES evoked no BF shifts of DSCF and AIp neurons.
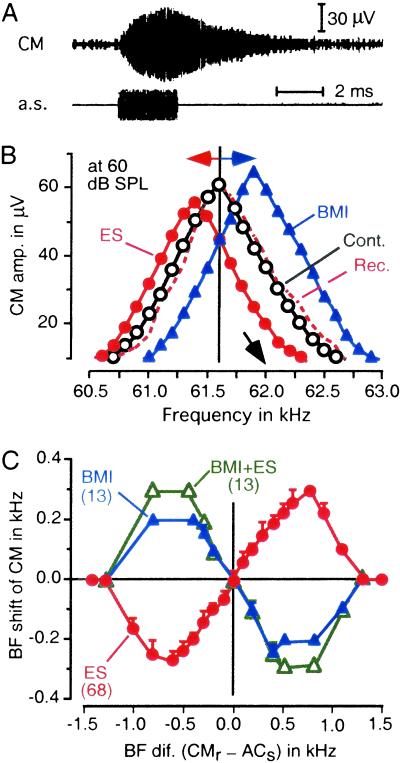
ES of cortical DSCF neurons at a low rate evokes collicular and cortical BF shifts but does not evoke a BF shift of the CM recorded from the cochlea, whereas ES at a high rate evokes a centrifugal BF shift of the contralateral CM (29). As found in the IC and AC, BMI applied to the stimulation site, however, evoked a centripetal BF shift. In Fig. 7B, the BF of the CM was 61.60 kHz; it shifted down to 61.40 kHz for ES of 62.00 kHz tuned cortical neurons and shifted up to 61.90 kHz for BMI applied to the stimulation site. Fig. 7C shows a BF shift–difference curve of the CM. The amount of BF shift was highly specific for BF differences. Within a ±0.50-kHz BF difference, the slope of the BF shift–difference curve was 0.46 for ES, −0.45 for BMI, and−0.62 for BMI + ES.
Fig 7.
BF shifts and BF shift–difference curves of the CMs to 2-ms-long tone bursts. (A) The CM evoked by a 61.6-kHz 60-dB SPL tone burst. The envelope of the CM was different from that of the tone burst because of the sharp tuning of hair cells to a 61.6-kHz sound (21). (B) The frequency–response curve of the CM recorded from a single cochlea (open circles) shows a centrifugal BF shift (filled circles) for ES of 62.0-kHz tuned cortical DSCF neurons but centripetal BF shift (filled triangles) for BMI applied to them. Cont., control; Rec., recovery. (C) BF shift–difference curves of CM for ES alone, BMI alone, and BMI + ES. The data were obtained from four cochleae of three bats. The numbers in parentheses indicate those of cortical loci to where ES and/or BMI were applied. ACs, stimulated cortical DSCF neurons; CMr, recorded CM.
The study of the BF shifts of the CM and those of collicular and cortical neurons was different in the rate of ES. Therefore, the difference in the BF shift for ES between the neural and CM data is not discussed. However, the difference in BF shift for an identical dose of BMI between them is discussed below. The BF shift at a peak evoked by BMI was 0.20 kHz for the cochlea and IC and 0.30 kHz for AC. The number of samples at the peak of the BF shift–difference curve was too small to perform statistical analysis, so that the slopes of the regression lines for BF shifts between −0.50 and +0.50 kHz were calculated. The slope was −0.45 for the cochlea, −0.46 for the IC, and −0.54 for the AC. The slope was statistically different between the AC and others but not different between the cochlea and IC. These observations indicate that almost all collicular BF shifts evoked by BMI are due to the cochlear BF shift, and that ≈75% of the cortical BF shift evoked by BMI is due to the cochlear BF shift.
Discussion
As reviewed in the introduction, expanded reorganization resulting from centripetal BF shifts is common in the mammalian sensory system. In the AC of the gerbil, centripetal BF shifts occur in the elliptical area centered at the electrically stimulated cortical neurons. This elliptical area is surrounded by a zone where centrifugal BF shifts occur (37). Because the amount of centrifugal BF shifts is much smaller than that of centripetal BF shifts, this zone was first unnoticed (36) but later discovered through more extensive studies of BF shifts (37). In the AC of the big brown bat (5, 6) and the AIp of the mustached bat (36), centrifugal BF shifts were found at the edge of the large area where centripetal BF shifts occur. If extensive studies are performed in these animals, a zone for centrifugal BF shifts may be found, as in the gerbil.
Centrifugal BF shifts for compressed reorganization have thus far been found only in the DSCF and frequency modulation (FM)-FM areas of the AC of the mustached bat. As already described, the DSCF area is large and consists of neurons that are extremely sharply tuned to particular frequencies and also tuned in sound amplitude. It is highly specialized for processing biosonar information carried by a sound at ≈61 kHz. The DSCF area has frequency vs. amplitude coordinates (22); the FM-FM area is also large and consists of neurons tuned to particular echo delays (target ranges). It has an echo-delay axis instead of a frequency axis and is specialized for processing target-range information (23, 38, 39). Therefore, the hypothesis has been proposed that compressed reorganization resulting from centrifugal shifts of tuning curves occurs in a highly specialized AC, whereas expanded reorganization resulting from centripetal shifts of the curves occurs in nonspecialized (ordinary) AC (17). This hypothesis remains to be tested with animals other than the mustached bat.
As demonstrated in our present paper, BMI changed centrifugal BF shifts in the DSCF area into centripetal BF shifts but did not change the direction of centripetal BF shifts in the AIp area (Fig. 1A). These observations suggest that the DSCF area specialized for processing sound at ≈61 kHz has the neural circuit for lateral inhibition stronger than that in the AIp area. However, we do not yet know whether inhibitory neurons are more abundant and more developed in the cortical DSCF area than in the AIp area. In the mustached bat, the frequency–tuning curves of peripheral neurons tuned to ≈61 kHz are very sharp without lateral inhibition. The shape of these tuning curves is triangular, so that the higher the stimulus level, the wider the tuning curves (21). In the central auditory system, tuning curves of many neurons change to spindle-shaped due to lateral inhibition, and their width is independent of stimulus levels (22, 32, 35, 40). Neurons with a sharp “level-tolerant ” tuning curve are very common in the DSCF system (41). Cortical ES evokes the sharpening of the frequency–tuning curves of collicular neurons (20).
It remains to be studied whether BMI applied to the AC changes the sharpness of the frequency–tuning curves of collicular neurons. There are a number of questions remaining to be answered. However, it is interesting and important to know that the brain has two types of reorganization for improvement of auditory signal processing, and that the two types of reorganization are based on a single mechanism consisting of two components: facilitation and inhibition.
Acknowledgments
We thank Drs. D. C. Fitzpatrick and K. K. Ohlemiller for comments on our article. Our work was supported by a research grant from the National Institute on Deafness and Other Communicative Disorders (DC00175).
Abbreviations
AC, auditory cortex
AIp, posterior division of the AC
BF, best frequency
RF, resting frequency
BMI, bicuculline methiodide
CM, cochlear microphonic
DSCF, Doppler-shifted constant frequency
ES, electric stimulation
IC, inferior colliculus
PST, peristimulus time
References
- 1.Raggio M. W. & Schreiner, C. E. (1994) J. Neurophysiol. 72 2334-2359. [DOI] [PubMed] [Google Scholar]
- 2.Raggio M. W. & Schreiner, C. E. (1999) J. Neurophysiol. 82 3506-3526. [DOI] [PubMed] [Google Scholar]
- 3.Schreiner C. E. & Raggio, M. W. (1996) J. Neurophysiol. 75 1283-1300. [DOI] [PubMed] [Google Scholar]
- 4.Yan W. & Suga, N. (1998) Nat. Neurosci. 1 54-58. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury S. A. & Suga, N. (2000) J. Neurophysiol. 83 1856-1863. [DOI] [PubMed] [Google Scholar]
- 6.Ma X. & Suga, N. (2001) J. Neurophysiol. 85 1078-1087. [DOI] [PubMed] [Google Scholar]
- 7.Yan J. & Ehret, G. (2001) Neuroreport 12 3313-3316. [DOI] [PubMed] [Google Scholar]
- 8.Gao E. & Suga, N. (1998) Proc. Natl. Acad. Sci. USA 95 12663-12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilgard M. P. & Merzenich, M. M. (1998) Science 279 1714-1718. [DOI] [PubMed] [Google Scholar]
- 10.Bakin J. S. & Weinberger, N. M. (1996) Proc. Natl. Acad. Sci. USA 93 11219-11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao E. & Suga, N. (2000) Proc. Natl. Acad. Sci. USA 97 8081-8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji W., Gao, E. & Suga, N. (2001) J. Neurophysiol. 86 211-225. [DOI] [PubMed] [Google Scholar]
- 13.Weinberger N. M., Javid, R. & Lepan, B. (1993) Proc. Natl. Acad. Sci. USA 90 2394-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buonomano D. V. & Merzenich, M. M. (1998) Annu. Rev. Neurosci. 21 149-186. [DOI] [PubMed] [Google Scholar]
- 15.Rasmusson D. D. (2000) Behav. Brain Res. 115 205-218. [DOI] [PubMed] [Google Scholar]
- 16.Weinberger N. M. (1998) Neurobiol. Learn. Mem. 70 226-251. [DOI] [PubMed] [Google Scholar]
- 17.Suga N., Gao, E., Zhang, Y., Ma, X. & Olsen, J. F. (2000) Proc. Natl. Acad. Sci. USA 97 11807-11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irvine D. R., Rajan, R. & McDermott, H. J. (2000) Hearing Res. 147 188-199. [DOI] [PubMed] [Google Scholar]
- 19.Yan J. & Suga, N. (1996) Science 273 1100-1103. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y. & Suga, N. (2000) J. Neurophysiol. 84 325-333. [DOI] [PubMed] [Google Scholar]
- 21.Suga N. & Jen, P. H. (1977) J. Exp. Biol. 69 207-232. [DOI] [PubMed] [Google Scholar]
- 22.Suga N. & Manabe, T. (1982) J. Neurophysiol. 47 225-255. [DOI] [PubMed] [Google Scholar]
- 23.Suga N. (1984) in The Extent to Which Biosonar Information Is Represented in the Bat Auditory Cortex, eds. Edelman, G. M., Gall, W. E. & Cowam, W. M. (Wiley, New York), pp. 315–373.
- 24.Suga N. & Jen, P. H. (1976) Science 194 542-544. [DOI] [PubMed] [Google Scholar]
- 25.Saldana E., Feliciano, M. & Mugnaini, E. (1996) J. Comp. Neurol. 371 15-40. [DOI] [PubMed] [Google Scholar]
- 26.Bishop A. L. & Henson, O. W., Jr. (1987) Hearing Res. 31 175-182. [DOI] [PubMed] [Google Scholar]
- 27.Huffman R. F. & Henson, O. W., Jr. (1990) Brain Res. Rev. 15 295-323. [DOI] [PubMed] [Google Scholar]
- 28.Warr W. B. (1992) in Neuroanatomy, eds. Webster, D. B., Popper, A. N. & Fay, R. R. (Springer, New York), pp. 410–448.
- 29.Xiao Z. & Suga, N. (2002) Nat. Neurosci. 5 57-63.11753417 [Google Scholar]
- 30.Zhang Y. & Suga, N. (1997) J. Neurophysiol. 78 3489-3492. [DOI] [PubMed] [Google Scholar]
- 31.Henson O. W. & Pollak, G. D. (1972) Physiol. Behav. 8 1185-1187.5074034 [Google Scholar]
- 32.Suga N. & Tsuzuki, K. (1985) J. Neurophysiol. 53 1109-1145. [DOI] [PubMed] [Google Scholar]
- 33.Huffman R. F. & Henson, O. W., Jr. (1993) J. Comp. Physiol. 171 725-734. [DOI] [PubMed] [Google Scholar]
- 34.Henson O. W., Pollak, G. D., Kobler, J. B., Henson, M. M. & Goldman, L. J. (1982) Hearing Res. 7 127-147. [DOI] [PubMed] [Google Scholar]
- 35.Suga N., Zhang, Y. & Yan, J. (1997) J. Neurophysiol. 77 2098-2114. [DOI] [PubMed] [Google Scholar]
- 36.Sakai M. & Suga, N. (2001) Proc. Natl. Acad. Sci. USA 98 3507-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakai M. & Suga, N. (2002) Proc. Natl. Acad. Sci. USA 99 7108-7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suga N. & O'Neill, W. E. (1979) Science 206 351-353. [DOI] [PubMed] [Google Scholar]
- 39.O'Neill W. E. & Suga, N. (1982) J. Neurosci. 2 17-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L., Pollak, G. D. & Resler, C. (1992) J. Neurophysiol. 68 1760-1774. [DOI] [PubMed] [Google Scholar]
- 41.Suga N. (1995) Neurosci. Res. 21 287-299. [DOI] [PubMed] [Google Scholar]
- 42.Suga N., Niwa, H., Taniguchi, I. & Margoliash, D. (1987) J. Neurophysiol. 58 643-654. [DOI] [PubMed] [Google Scholar]