Abstract
Corticotropin-releasing factor (CRF) is a neuropeptide involved in integrating the behavioral, autonomic, and hormonal responses to stress within the central nervous system. Patients suffering from depression have abnormal activity in stress responsive brain regions and elevated cerebrospinal fluid CRF. The DSM-IV criteria for major depressive disorder include behavioral changes such as depressed mood, anhedonia, and psychomotor agitation/retardation. We studied the effects of 434 μg of CRF given intracerebroventricularly over 40 min in group and individually housed monkeys to examine the role of elevated levels of central CRF on behavior. CRF elicited a wide range of behaviors, which fell into three broad categories: anxiety-like, depressive-like, and externally oriented. Externally oriented behaviors decreased, and anxiety-like behaviors increased regardless of how the animals were housed. Interestingly, increased depressive-like behaviors were only observed when the animals were socially housed. In a separate experiment, we examined the effects of the same dose of CRF on the regional cerebral glucose metabolism of lightly anesthetized monkeys by using positron emission tomography and [18F]fluorodeoxyglucose. CRF infusion increased glucose metabolism in the pituitary/infundibulum, the amygdala, and hippocampus. These results indicate that increased central CRF tone affects primate behavior in a context-dependent manner, and that it activates limbic and stress-responsive regions. The fact that intracerebroventricular CRF increases depressive-like behavior in socially housed animals and increases activity in limbic brain regions may help explain the behavioral and metabolic alterations in humans with affective disorders, and this model could therefore have significant value in the development of novel antidepressant treatments.
Corticotropin releasing factor (CRF) is a 41-aa peptide (1) that plays an important role in the mammalian stress response by integrating the behavioral, autonomic, and hormonal responses. CRF and its receptors are widely distributed throughout the brain, but are especially dense in the hypothalamus, where CRF plays a neuroendocrine role, and in extrahypothalamic regions such as the amygdala, the cerebral cortex, and brainstem autonomic nuclei (2, 3), regions implicated in mood and anxiety disorders.
In rodents, intracerebroventricular (ICV) CRF causes physiological and behavioral changes similar to those observed in humans undergoing stress (4, 5). However, the behavior elicited by CRF in rodents is different depending on the context and the dose given. For example, Sutton et al. (5) showed that CRF has “activating” effects in familiar environments, but is anxiogenic in novel environments. In monkeys, ICV CRF also elicits distinct behavioral changes if the animals are tested when restrained compared with when freely moving (6).
For these reasons, attention has focused on the role of CRF in psychiatric disorders related to increased stress or anxiety. Increased cerebrospinal fluid (CSF) CRF has been widely reported in patients suffering from anxiety or affective disorders (7–9), and in some cases, the elevation can be reversed with successful treatment (10, 11). However, alterations in CSF CRF are quite variable, with bipolar patients and some unipolar patients showing no changes (12, 13). This variability in CSF CRF in patients with psychiatric conditions related to stress might stem from several sources, including heterogeneity among patient groups with respect to symptoms, age, sex, medication, or length of illness. However, the variability may also be related to social or behavioral factors that have yet to be investigated.
Imaging studies have shown that patients with anxiety and affective disorders also have distinct alterations in both the structure and the function of limbic brain regions. Patients with unipolar depression have increased blood flow in the amygdala (14), and both bipolar and unipolar depressed patients show increased glucose metabolism in the amygdala (15). Depressed or anxious patients also show decreased hippocampal volume (16, 17), and patients with anxiety disorders consistently show altered blood flow and metabolism in the hippocampus (18, 19).
Along with the biochemical, structural, and metabolic changes in depression and anxiety come the more obvious phenotypic changes. Ultimately, classification of these types of disorders is based on how they present in the clinic, that is, the behavior of these patients based on observation and self-report. The DSM-IV criteria for major depression and anxiety include behavioral changes such as anhedonia, psychomotor agitation or retardation, sleep disturbances, autonomic activation, and altered mood and social behavior. Like the physiological alterations in these patients, the behavioral manifestations are also quite variable across individuals and conditions.
We have used a monkey model to examine the relationship between increased CSF CRF, behavior and brain metabolism, to understand how these three characteristics are altered in patients with anxiety or affective disorders. For the behavioral experiment, we infused either CRF or vehicle ICV, and observed behavior when the animals were in their normal social group, and when they were individually housed. To examine the neural correlates of the behavioral effects of CRF, we used positron emission tomography (PET) and the tracer [18F]fluorodeoxyglucose (FDG) to scan the animals before and after ICV infusion of the same dose of CRF. We expected that CRF would induce anxiety-like and depressive-like behaviors, and that glucose metabolism would be most affected in brain regions related to stress, anxiety and depression and which contain high densities of CRF and its receptors (i.e., limbic regions). By using a repeated-measures design to compare the effects of ICV CRF on behavior and cerebral glucose metabolism, we were able to observe how a neuropeptide with altered activity in anxiety and affective disorders can have differential effects on behavior as a function of environment and increase activity in limbic brain regions. The results indicate that this model may have significant value in the development of novel antidepressant strategies.
Methods
Subjects.
The subjects were 11 young adult rhesus monkeys (Macaca mulatta) of both sexes, weighing 6–10 kg. The animals were reared from birth in peer-only groups, and all subjects had lived in their stable social group for >2 years. None of the females were pregnant. Eight of the subjects lived in social groups of six to seven other monkeys, and the other three subjects were pair housed. Of these, nine animals were studied in the PET experiment, eight were studied in the socially housed situation, and six were studied in the individually housed situation.
Surgical Treatment.
All procedures were approved by the National Institutes of Health Animal Care and Use Committee. Under general anesthesia, each animal had a cannula surgically implanted into the right lateral ventricle by using standard stereotaxic coordinates (18 mm AP, 2–3 mm ML) (20). The cannula was lowered in 1-mm increments until CSF efflux was detected, immobilized with dental acrylic, and attached s.c. via a catheter to an injection port (Access Technologies, Skokie, IL) implanted in the interscapular region. The assembly was filled with sterile Elliot's B solution (artificial CSF) and kept patent by weekly flushing with 1 ml of the same solution. After a 2-week recovery period, the position of the cannula was verified by x-ray. Behavioral testing occurred at least 2 weeks later, and the PET experiment was performed at least 2 months later.
Behavioral Observations.
Forty-eight hours before the behavioral experiment, the study animal was fitted with a nylon jacket to hold the pump used for ICV CRF infusion. On the morning of the experiment, 1 mg of ovine CRF (Bachem) was dissolved in 0.4 ml of sterile water, the solution was filtered (Millipore, Miller GV 0.22 μm), and the volume made up to 1 ml with Elliot's B solution. The study animal was caught, and the area of skin superficial to the injection port was anesthetized with s.c. procaine. A MiniMed portable infusion pump (model 404 SP) was programmed with a 30-min delay to allow the monkey to recover from handling before the start of CRF infusion. The pump was placed in a pocket on the back of the jacket, a 3-ml syringe containing the dose of CRF was inserted into the pump, and a Huber needle was inserted into the injection port. Allowance was made for the void volume in the catheter so that a total of 434 μg (1 ml) of CRF in Elliot's B solution, or Elliot's B solution alone in the control condition, was infused ICV over a 40-min period at a constant infusion rate of 10.8 μg/min. The dose of CRF was chosen based on dose–response curves and behavioral observations in a separate group of animals, which showed that this dose gave a rapid and reliable behavioral response without any adverse effects. The animal was placed back into its social group, or separated from peers and placed in a single cage, and ICV CRF or vehicle infusion began 30 min later. Only group-housed animals were studied in both the social and individual housing conditions.
Two trained observers, blind to the treatment condition, alternated scoring the occurrence and duration of each behavior in 5-min periods over the 40-min observational period (operational definitions of the behaviors are shown in Table 1, which is published as supporting information on the PNAS web site, www.pnas.org). Subjects were treated in a randomized fashion by using a counterbalanced repeated measures design with respect to both housing (single or group) and treatment condition (CRF or vehicle), with at least 2 weeks between treatments.
Data Analysis.
Repeated-measures ANOVA was performed on the mean duration of each behavior during vehicle or CRF infusion. The data were also pooled into three main categories of behaviors (Table 1), and repeated-measures ANOVA was performed on each category during vehicle or CRF infusion. Externally oriented behaviors (environmental exploration, locomotion, vocalization, social contact) were defined as behaviors where the animal was interacting with their environment, whether it was their surroundings or a conspecific. Changes in externally oriented behaviors were not a result of sedation, as the animals remained awake and responsive to investigators. Depressive-like behaviors (huddling, wall-facing, passive behavior) were those behaviors that have been previously recorded in primate models of depression and resemble depressive-like behaviors in humans (i.e., they had face validity) (21). Anxiety-like behaviors (self-grooming, self-clasping, stereotypies, stypics) were those behaviors that were reminiscent of the behavioral effects of ICV CRF in rodents (4, 5).
PET Experiment.
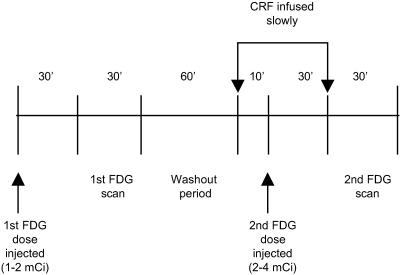
The timeline for the PET experiment is shown in Fig. 1. This method of consecutive FDG/PET scans is based on that of Chang et al. (22). Each animal was fasted overnight. The morning of the experiment, the animal was immobilized with ketamine HCl (10 mg/kg i.m.) and received atropine (0.05 mg/kg i.m.). An intratracheal tube was placed and IV and intraarterial catheters were inserted. The animal was anesthetized with a low dose of sodium pentobarbital (10 mg/kg IV) for transportation from the animal care facility to the PET area. Isoflurane gas was used for further maintenance anesthesia until the end of the study. Temperature was maintained at 37°C, and heart rate and respiration were monitored throughout the experiment. The animal was positioned prone in a stereotaxic frame allowing the acquisition of coronal PET slices of the head and brain.
Fig 1.
Timeline of the PET experiment for each animal studied.
Although the data were collected on either the Scanditronix PC 1024-7B (SC1) or the Scanditronix PC 2049-15B (SC2) positron emission tomographs, each animal was scanned on only one machine. Acquisition was performed in 2D mode. Both scanners have in-plane resolutions of 6.5 mm full width at half maximum (FWHM), but differ in their axial resolution (SC1: 12 mm FWHM; SC2: 6 mm FWHM) and the number of slices acquired (SC1: 7 slices; SC2: 15 slices). Data were obtained at 4 Z levels in the SC1 and 2 Z levels in the SC2. Data from the different scanning levels were interleaved for a total of 28 (SC1) and 30 (SC2) anatomical slices, with a common interslice interval of 3.25 mm (25). Transmission scans were performed for attenuation correction at each of the respective Z levels in each scanner. Comparison of data acquired in another group of monkeys scanned sequentially in each scanner on the same day demonstrated that the glucose metabolic rates obtained in each scanner were highly comparable and within the differences seen in test–retest studies (D.J.D., unpublished observations).
FDG (1–2 mCi in 10 ml of saline; 1 Ci = 37 GBq) was administered as an IV bolus and after a 30 min uptake period, scanning began. Blood sampling started at the time of tracer injection and continued throughout the scan (1.5 ml in heparin-treated tubes at 15-s intervals for the first 2 min, and at 3, 4, 5, 10, 15, 20, 30, 45, and 60 min). Samples were placed on ice until centrifugation and processing. Activity was counted in 100-μl samples of plasma by using a well counter calibrated to the PET scanner to determine the time activity curve. Plasma samples from various time points were also analyzed for ACTH and cortisol (Hazleton Laboratories, Herndon, VA). The first FDG/PET scan began after the 30-min FDG uptake period, and continued over the next 30 min. CRF infusion began 60 min after the end of the first scan (Fig. 1).
The same dose of CRF used in the behavioral studies was administered ICV (434 μg over 40 min) using the same pump. Ten minutes into the CRF infusion, a blood sample was taken to measure the residual activity from the first FDG dose before injection of the second FDG dose (2–4 mCi in 10 ml saline). The same blood sampling protocol was applied. The timing of the second FDG injection was chosen based on preliminary observations in awake, behaving monkeys, where the effects of CRF were noticeable within 5–10 min after the start of CRF infusion.
The plasma time-activity curves and the plasma glucose concentrations, averaged from the 0-, 15-, and 30-min samples, were applied to the raw count images to yield glucose metabolic rates (mg per 100 g of tissue per min). Inasmuch as the kinetic rate and lumped constant constants have not been determined in the anesthetized monkey, we used the values routinely used in human studies (23, 24). This introduces a scaling factor difference but does not affect the within-subjects comparison. In addition, the plasma input function of the second FDG study was corrected for the residual activity from the first FDG study (22).
Data Analysis.
The PET data were analyzed with a VAX computer system and the software program mirage. A template composed of small rectangular regions of interest (ROIs; 4–16 pixels, pixel size = 2 mm), developed by an experienced researcher (D.J.D.) was applied to the 19 consecutive coronal slices containing the brain (see ref. 25 for details). The ROIs were first placed on the set of post-CRF images, which provided better counting statistics and anatomical structure identification. Because the animal was in a stereotaxic frame and did not move between scans, the ROIs were translated without changes onto the animal's pre-CRF images. The data from ROIs from a brain region found in several consecutive frames were averaged. The ROI template included cortical areas (occipital, parietal, temporal, central, frontal, orbital, and cingulate cortices), several subcortical regions (hippocampus, amygdala, caudate, and putamen), as well as midline regions (medulla, thalamus, cerebellum, and a ventral area defined as the pituitary/infundibulum). The pituitary is a very small region in the rhesus monkey brain, and is likely subject to partial volume effects, but in the case of this study, the entire infundibular stalk plus pituitary were easily recognized in all of the animals in the second set of images taken after CRF infusion, and a small ROI was placed in this region to quantify glucose metabolic rate. No correction for partial voluming was applied to the data because this was a within-subjects comparison.
To compensate for variation in glucose metabolic rates across animals, the data were standardized with a Z-score transformation (26), and bilateral differences were tested by using dependent t tests. When no significant differences were found, data from both hemispheres were pooled to give an average glucose metabolic rate. This led to the comparison of 18 regions before and after CRF, and the statistical significance of any changes was tested by using repeated-measures ANOVA.
Results
Effects of CRF on Behavior.
The behavioral effects of ICV CRF were induced very rapidly (within 5 min) after the start of ICV infusion, and continued throughout and beyond the observation period, so the data for each 5-min observational period were pooled over the entire 40 min. Each animal's response to CRF was highly variable. Interrater reliability was 0.85.
Social Group Condition.
Huddling and slouched wall-facing behaviors were pooled because they typically cooccurred, and because of the similarities in their appearance. With huddling, the monkey was crowded with head bent forward below the shoulders. The behavior described as wall facing is shown in Fig. 2. The monkeys that slouched and faced the wall appeared withdrawn, and would have been scored as huddled if the wall had not prevented the head from being lower than the shoulders. Huddling and wall-facing (depressive-like behaviors) increased significantly after CRF (F1,7 = 14.13, P ≤ 0.01). Self-clasping, a typical measure of anxiety in nonhuman primates, also increased significantly after ICV CRF (F1,7 = 9.36, P ≤ 0.02), and locomotion and environmental exploration (externally oriented behaviors) decreased (F1,7 = 18.03, P ≤ 0.004; F1,7 = 10.35, P ≤ 0.02) (data not shown).
Fig 2.
The behavioral effects of ICV CRF in an adult male rhesus monkey when housed in his normal social group. Note the brown nylon jacket the animal is wearing, which contains the pump used to infuse CRF. The animal has withdrawn from his peers and is exhibiting huddling/wall-facing behavior, one of the depressive-like behaviors induced by ICV CRF in socially housed monkeys.
Individually Housed Condition.
Stypic behavior (an anxiety-like behavior) increased significantly after CRF in the lone housing condition (F1,5 = 7.04, P ≤ 0.05), whereas environmental exploration decreased significantly after CRF compared with vehicle (F1,5 = 9.51, P ≤ 0.03) (data not shown).
Social Group Compared with Isolation.
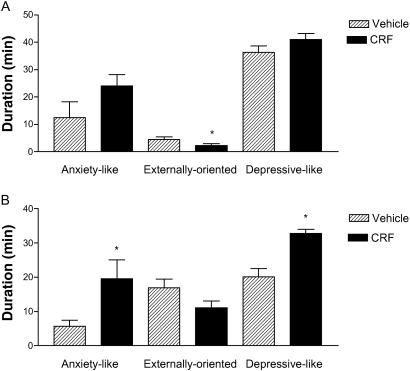
When the behaviors were pooled into anxiety-like, externally oriented, and depressive-like, there were obvious differences between the two housing conditions. In the social situation, both depressive- and anxiety-like behaviors increased after CRF (F1,7 = 24.86, P ≤ 0.002; F1,7 = 5.57, P ≤ 0.05) (Fig. 3), and in the single housing condition, externally oriented behaviors decreased after CRF (F1,7 = 12.90, P ≤ 0.02) (Fig. 3). Fig. 3 shows that although anxiety-like behaviors increased and externally oriented behaviors decreased after CRF in both the social and individual housing conditions, depressive-like behaviors only increased when the animals were socially housed.
Fig 3.
(A) Duration of time spent performing anxiety-like, externally oriented, or depressive-like behaviors during ICV infusion of vehicle or CRF in six animals tested when individually housed. (B) Duration of time spent performing anxiety-like, externally oriented, or depressive-like behaviors during ICV infusion of vehicle or CRF in eight animals tested in a social group. Data are mean ± SEM; *, P ≤ 0.05.
Effects of CRF on Cerebral Glucose Metabolism.
One animal was tested with vehicle-only infusion before the second FDG PET scan as a control for test–retest reproducibility. Glucose metabolic rates in all regions of the brain examined were within 5% between the two PET scans, suggesting the effects in the experimental condition were caused by CRF infusion (data not shown).
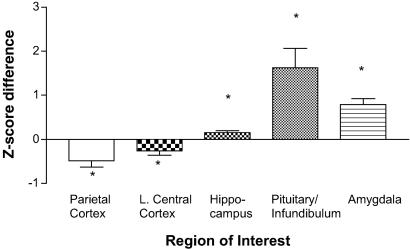
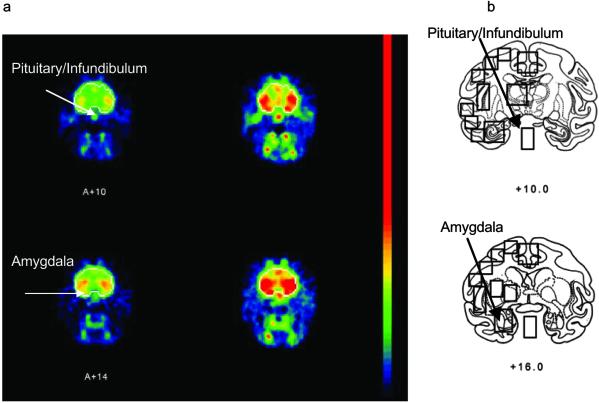
Z scores (standardized glucose metabolic rate) were significantly increased in the hippocampus (F1,8 = 9.26, P ≤ 0.02), pituitary/infundibular region (F1,8 = 12.46, P ≤ 0.01), and amygdala (F1,8 = 35.6, P ≤ 0.0003) after ICV CRF compared with vehicle (Fig. 4). The PET images at the level of the amygdala and pituitary/infundibulum of one representative animal are shown in Fig. 5. Z scores were also significantly decreased in the parietal and left central cortices (F1,8 = 8.15, P ≤ 0.03; F1,8 = 8.36, P ≤ 0.02 respectively) after ICV CRF compared with vehicle (Fig. 4). There were no global changes in cerebral glucose metabolism between the first scan and the second scan.
Fig 4.
The effects of ICV CRF on Z-standardized glucose metabolic rates. Glucose metabolism increased in the pituitary/infundibulum, amygdala, and hippocampus, and decreased in the parietal and left central cortices. Results are presented as difference in Z score. Data are mean ± SEM; *, P ≤ 0.05.
Fig 5.
(a) Coronal FDG/PET images in the nonhuman primate brain before (Left) and after (Right) ICV CRF, showing the significantly increased glucose metabolic rate in the pituitary/infundibular region (Upper) and amygdala (Lower) in one animal. For clarity, the brains are outlined in the images. (b) Stereotaxic brain atlas of the equivalent slice showing several of the ROIs used to calculate glucose metabolic rate.
Effects of CRF on Plasma Glucose and Hormone Levels.
There was no significant increase in plasma glucose concentration during the first and second FDG uptake periods (0–30 min after FDG injection). However, plasma glucose was significantly increased (dependent t test: t1,8 = 2.55, P = 0.03) at 100 min after the start of CRF infusion. For technical reasons, plasma ACTH and cortisol measures could only be taken in five of the nine animals that underwent a PET scan, but both hormones were elevated at the end of the second scan (621% and 57%, respectively).
Discussion
The results of this study indicate that central administration of CRF has distinct effects on both the behavior and regional cerebral glucose metabolism of adult rhesus monkeys.
With respect to behavior, there are three important aspects of our results. First, the behavioral response to ICV CRF was highly variable across individuals in both housing conditions. Each animal responded to ICV CRF by showing only some of the spectrum of behaviors seen across the whole group, though all animals showed at least one example of the typical changes in each of the three behavioral categories. Second, anxiety-like and externally oriented behaviors were similarly affected by ICV CRF (increased anxiety and decreased interaction with the environment) regardless of the housing condition, indicating that these types of behaviors are part of a general response to increased central CRF tone. Finally, depressive-like behaviors (huddling, wall-facing, passivity) were only significantly increased after ICV CRF when the animals were in their normal social group.
Separation from the social group is stressful for nonhuman primates, typically inducing despair behavior in peer-reared monkeys (21), high levels of anxiety, and when alcohol is available, it increases alcohol consumption (27, 28). Baseline anxiety- and depressive-like behaviors were elevated during vehicle infusion in the separated versus social group condition (Fig. 3). This may explain why depressive-like behavior did not increase significantly after CRF when the animals were individually housed. If baseline depressive-like behavior is already elevated because of separation, then the addition of a further depressant (CRF) may not have as strong an effect. Indeed, anxiety- and depressive-like behaviors reached comparable levels after ICV CRF in both housing conditions, indicating that although the animals may have experienced a higher level of stress when separated from their peers, the combination of separation and ICV CRF was not additive. The fact that CRF increased depressive-like behavior only in the socially housed animals indicates that it has differential behavioral effects depending on the social context.
These observations compare favorably with those of the one other study that examined the behavioural effects of ICV CRF in rhesus monkeys. Kalin et al. (6) noted a variable response to ICV CRF across individuals, as well as differential effects on behavior depending on the housing situation; when restrained in primate restraining chairs, the animals showed increased struggling and exploratory behavior, whereas they showed huddling and lying down behavior in their home cage (6). The huddling and lying down behavior described appears similar, if not identical to the depressive-like behaviors we observed in our socially housed animals. However, although housing conditions were different in the two studies, the depressive-like behaviors only occurred when the animals were in their home cages, and could interact with conspecifics.
Glucose metabolism increased in the amygdala, hippocampus, and pituitary/infundibular regions, areas that contain high levels of CRF receptors in the rhesus monkey brain (29). Increased limbic glucose metabolism occurred even though the animals were anesthetized during the PET scan, suggesting receptor activation. Increased pituitary activity was expected because activation of CRF receptors at this site is the initial step in the activation of the hypothalamic–pituitary–adrenal (HPA) axis, the primary target for CRF released in vivo from the hypothalamus, and the body's main endocrine mechanism for dealing with stress. Increased amygdala activity has also been reported in depressed patients (14, 15), as well as in those with anxiety disorders during symptom provocation (30). Increased activity in the amygdala was also expected because of the role it plays in emotional responses in humans and behavioral responses to stress and/or anxiety in animals. Monkeys with amygdala lesions show reduced fear and aggression, increased submission, and hypoemotionality (31–33). The widely accepted explanation for these behaviors is that the amygdala mediates emotional behavior by associating environmental stimuli with affective states. In addition, the amygdala projects to widespread cortical regions in the primate brain (34), and altered amygdalar function after CRF infusion may therefore be partially responsible for the decreased metabolism in the central and parietal cortices.
Like the amygdala, the hippocampus also plays a role in emotional behavior and influences HPA axis function, so increased activity here after ICV CRF was expected. Recently, Kennedy et al. (35) showed that after successful antidepressant treatment with paroxetine, patients with major depression showed decreased hippocampal glucose metabolism, suggesting overactivity within this region in depression. Functional imaging studies in patients with anxiety disorders consistently show altered metabolism and blood flow in the hippocampus (18, 19). The results of our current study suggest that altered hippocampal function in anxiety and affective disorders could be caused by overactivity within CRF neurons.
Taken together, the behavioral and PET data indicate that CRF has general effects on the brain and behavior. Although we are unable to correlate the behavioral results in the awake monkeys with the metabolic results from the PET studies in anesthetized animals, there are several indications that both effects arise from the same mechanism, the activation of the HPA axis. After ICV CRF we observed, regardless of the housing condition, a general behavioral response, which included increased anxiety-like behaviors and decreased externally oriented behaviors. These behaviors are often used as indices of stress and are characterized by increased HPA axis activity. Although we were unable to measure plasma HPA axis hormones during the behavioral study, Kalin et al. (6) reported increased ACTH and cortisol after ICV CRF in their animals. Furthermore, the significant increase in plasma ACTH, cortisol and glucose observed by the end of our PET studies (100 min after CRF) strongly suggests activation of the HPA axis, even in the anesthetized animal. Also, after ICV CRF, glucose metabolism was increased in the pituitary, amygdala, and hippocampus, structures heavily involved in the initial and negative feedback aspects of the HPA response to stress.
The premier finding of this study, however, is that ICV CRF also has very specific, contextually dependent effects, in that it increased depressive-like behaviors only in socially housed monkeys. Body language is an important means of communication for humans, but nonverbal animals are even more dependent on it. The depressive-like behaviors induced by CRF therefore may communicate something to conspecifics about the animal's state. This is a very adaptive mechanism for social animals, and in the presence of a social group, this type of behavior may lead to social support.
This finding emphasizes the fact that if we are to continue studying the role of CRF in the pathophysiology of human psychiatric conditions using animal models, we must do so under the appropriate social contexts. In most studies of the effects of CRF on the behavior of rodents, for example, the peptide is administered to animals in isolation, either in their home cage, or in various testing arenas. We know from the earlier literature that, even in rats, CRF has differential effects on the behavior of the animals depending on whether the environment is familiar or novel (5). The behavioral effects of CRF in rodents may be different yet again if they are studied in social situations. As for nonhuman primates, our results verify the idea that CRF has different behavioral effects in different contexts.
The contextual effect of CRF on behavior also has important implications for the role of CRF in human psychiatric disorders, and may help to explain the variability in CSF CRF and behavior of patients with anxiety or mood disorders. Even in humans, CRF may act differently in different contexts to elicit the behavior that we categorize into anxiety and affective disorders. Individual response to elevated cerebral CRF tone may depend on such factors as support network and social group, past experience, and social dominance; in other words, the individual's context. Basal CSF CRF is elevated in primates that have experienced early life stress (36), and wild baboons that are low in the dominance hierarchy and under persistent social stress show hypercortisolemia, which appears to originate with excess central CRF activity (37). If past or present stress (context) can have long-term effects on the activity of central CRF neurons, then these experiences may also alter the behavioral response to CRF.
Our results provide a much needed addition to the current knowledge of how elevated levels of CRF can affect primate behavior and cerebral glucose metabolism. Thus far, there have been very few reports on the effects of ICV CRF in primates, even though altered CSF CRF is a consistent finding in depressed and anxious patients. This study investigates the effects of ICV CRF in different social contexts in primates, and shows increased glucose metabolism in limbic and stress-responsive regions after ICV CRF. Given the social and behavioral issues and abnormalities in CRF signaling in depressed and anxious individuals, this model could be used as a meaningful research tool in the development of novel antidepressant strategies.
Supplementary Material
Acknowledgments
We thank Mary Girton and Richard Diggs for their expert technical assistance. Special thanks also go out to John Bacher, D.V.M., for surgical assistance. Finally, we thank Dr. Victor Viau for suggestions and enthusiasm for the manuscript. This work was funded by the National Institute of Child Health and Human Development and the National Institute of Mental Health, Bethesda.
Abbreviations
CRF, corticotropin-releasing factor
CSF, cerebrospinal fluid
FDG, [18F]fluorodeoxyglucose
HPA axis, hypothalamic–pituitary–adrenal axis
ICV, intracerebroventricular
PET, positron emission tomography
ROI, region of interest
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Vale W., Spiess, J., Rivier, C. & Rivier, J. (1981) Science 213 1394-1397. [DOI] [PubMed] [Google Scholar]
- 2.Swanson L. W., Sawchenko, P. E., Rivier, J. & Vale, W. (1983) Neuroendocrinology 36 165-186. [DOI] [PubMed] [Google Scholar]
- 3.Van Pett K., Viau, V., Bittencourt, J. C., Chan, R. K. W., Li, H. Y., Arias, C., Prins, G. S., Perrin, M., Vale, W. & Sawchenko, P. E. (2000) J. Comp. Neurol. 428 191-212. [DOI] [PubMed] [Google Scholar]
- 4.Britton D. R., Koob, G. F., Rivier, J. & Vale, W. (1982) Life Sci. 31 363-367. [DOI] [PubMed] [Google Scholar]
- 5.Sutton R. E., Koob, G. F., LeMoal, M., Rivier, J. & Vale, W. (1982) Nature 297 331-333. [DOI] [PubMed] [Google Scholar]
- 6.Kalin N. H., Shelton, S. E., Kraemer, G. W. & McKinney, W. T. (1983) Peptides 4 217-220. [DOI] [PubMed] [Google Scholar]
- 7.Nemeroff C. B., Widerlov, E., Bissette, G., Walleus, H., Karlsson, I., Eklund, K., Kilts, C. D., Loosen, P. T. & Vale, W. (1984) Science 226 1342-1344. [DOI] [PubMed] [Google Scholar]
- 8.Banki C. M., Karmacsi, L., Bissette, G. & Nemeroff, C. B. (1992) J. Affective Disord. 25 39-46. [DOI] [PubMed] [Google Scholar]
- 9.Bremner J. D., Licinio, J., Darnell, A., Krystal, J. H., Owens, M. J., Southwick, S. M., Nemeroff, C. B. & Charney, D. S. (1987) Am. J. Psychiatry 154 624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemeroff C. B., Bissette, G., Akil, H. & Fink, M. (1991) Br. J. Psychiatry 158 59-63. [DOI] [PubMed] [Google Scholar]
- 11.Veith R. C., Lewis, N., Langohr, J. L., Murburg, M., Ashleigh, E. A., Castillo, S., Peskind, E. R, Pascualy, M., Bissette, G., Nemeroff, C. B. & Raskind, M. A. (1992) Psychiatry Res. 46 1-8. [DOI] [PubMed] [Google Scholar]
- 12.Kling M. A., Roy, A., Doran, A. R., Calabrese, J. R., Rubinow, D. R., Whitfield, H. J., Jr., May, C., Post, R. M., Chrousos, G. P. & Gold, P. W. (1991) J. Clin. Endocrinol. Metab. 72 260-271. [DOI] [PubMed] [Google Scholar]
- 13.Geracioti T. D., Loosen, P. T. & Orth, D. N. (1997) Biol. Psychiatry 42 166-174. [DOI] [PubMed] [Google Scholar]
- 14.Drevets W. C., Videen, T. O., Price, J. L., Preskorn, S. H., Carmichael, S. T. & Raichle, M. E. (1992) J. Neurosci. 12 3628-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drevets W. C., Price, J. L., Bardgett, M. E., Reich, T., Todd, R. D. & Raichle, M. E. (2002) Pharmacol. Biochem. Behav. 71 431-447. [DOI] [PubMed] [Google Scholar]
- 16.Sheline Y. I., Wang, P. W., Gado, M. H., Csernansky, J. G. & Vannier, M. W. (1996) Proc. Natl. Acad. Sci. USA 93 3908-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurvits T. V., Shenton, M. E., Hokama, H., Ohta, H., Lasko, N. B., Gilbertson, M. W., Orr, S. P., Kikinis, R., Jolesz, F. A., McCarley, R. W. & Pitman, R. K. (1996) Biol. Psychiatry 40 1091-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semple W. E., Goyer, P., McCormick, R., Morris, E., Compton, B., Muswick, G., Nelson, D., Donovan, B., Leisure, G., Berridge, M., et al. (1993) Biol. Psychiatry 34 115-118. [DOI] [PubMed] [Google Scholar]
- 19.Bisaga A., Katz, J. L., Antonini, A., Wright, E., Margouleff, C., Gorman, J. M. & Eidelberg, D. (1998) Am. J. Psychiatry 155 1178-1183. [DOI] [PubMed] [Google Scholar]
- 20.Snider R. S. & Lee, J. C., (1961) A Stereotaxic Atlas of the Monkey Brain (University of Chicago Press, Chicago).
- 21.Mineka S. & Suomi, S. J. (1978) Psychol. Bull. 85 1376-1400. [PubMed] [Google Scholar]
- 22.Chang J. Y., Duara, R., Barker, W., Apicella, A. & Finn, R. (1987) J. Nucl. Med. 28 852-860. [PubMed] [Google Scholar]
- 23.Sokoloff L., Reivich, M., Kennedy, C., Des Rosiers, M. H., Patlak, C. S., Pettigrew, K. D., Sakurada, O. & Shinohara, M. (1977) J. Neurochem. 28 897-916. [DOI] [PubMed] [Google Scholar]
- 24.Phelps M. E., Huang, S. C., Hoffman, E. J., Selin, C., Sokoloff, L. & Kuhl, D. E. (1979) Ann. Neurol. 6 371-388. [DOI] [PubMed] [Google Scholar]
- 25.Doudet D., Hommer, D., Higley, J. D., Andreason, P. J., Moneman, R., Suomi, S. J. & Linnoila, M. (1995) Am. J. Psychiatry 152 1782-1787. [DOI] [PubMed] [Google Scholar]
- 26.Clark C., Carson, R., Kessler, R., Margolin, R., Buchsbaum, M. S., DeLisi, L., King, C. & Cohen, R. (1985) J. Cereb. Blood Flow Metab. 51 142-150. [DOI] [PubMed] [Google Scholar]
- 27.Higley J. D., Suomi, S. J. & Linnoila, M. (1996) Alcohol Clin. Exp. Res. 20 629-642. [DOI] [PubMed] [Google Scholar]
- 28.Higley J. D., Suomi, S. J. & Linnoila, M. (1996) Alcohol Clin. Exp. Res. 20 643-650. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez M. M., Young, L. J., Plotsky, P. M. & Insel, T. R. (1999) J. Comp. Neurol. 408 365-377. [PubMed] [Google Scholar]
- 30.Tillfors M., Furmark, T., Marteinsdottir, I., Fischer, H., Pissiota, A., Langstrom, B. & Fredrikson, M. (2001) Am. J. Psychiatry 158 1220-1226. [DOI] [PubMed] [Google Scholar]
- 31.Weiskrantz L. (1956) J. Comp. Physiol. Psychol. 49 381-391. [DOI] [PubMed] [Google Scholar]
- 32.Aggleton J. P. & Passingham, R. E. (1981) J. Comp. Physiol. Psychol. 95 961-977. [DOI] [PubMed] [Google Scholar]
- 33.Meunier M., Bachevalier, J., Murray, E. Q., Malkova, L. & Mishkin, M. (1999) Eur. J. Neurosci. 11 4403-4418. [DOI] [PubMed] [Google Scholar]
- 34.Amaral D. G. & Price, J. L. (1984) J. Comp. Neurol. 230 465-496. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy S. H., Evans, K. R., Kruger, S., Mayberg, H. S., Meyer, J. H., McCann, S., Arifuzzman, A. I., Houle, S. & Vaccarino, F. J. (2001) Am. J. Psychiatry 158 899-905. [DOI] [PubMed] [Google Scholar]
- 36.Coplan J. D., Andrews, M. W., Rosenblum, L. A., Owens, M. J., Friedman, S., Gorman, J. M. & Nemeroff, C. B. (1996) Proc. Natl. Acad. Sci. USA 93 1619-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sapolsky R. M. (1989) Arch. Gen. Psychiatry 46 1047-1051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.