Abstract
In humans, failure to express the fragile X mental retardation protein (FMRP) gives rise to fragile X syndrome, the most common form of inherited mental retardation. A fragile X knockout (fmr1 KO) mouse has been described that has some of the characteristics of patients with fragile X syndrome, including immature dendritic spines and subtle behavioral deficits. In our behavioral studies, fmr1 KO mice exhibited hyperactivity and a higher rate of entrance into the center of an open field compared with controls, suggesting decreased levels of anxiety. Our finding of impaired performance of fmr1 KO mice on a passive avoidance task is suggestive of a deficit in learning and memory. In an effort to understand what brain regions are involved in the behavioral abnormalities, we applied the [14C]deoxyglucose method for the determination of cerebral metabolic rates for glucose (CMRglc). We measured CMRglc in 38 regions in adult male fmr1 KO and WT littermates. We found CMRglc was higher in all 38 regions in fmr1 KO mice, and in 26 of the regions, differences were statistically significant. Differences in CMRglc ranged from 12% to 46%, and the greatest differences occurred in regions of the limbic system and primary sensory and posterior parietal cortical areas. Regions most affected are consistent with behavioral deficiencies and regions in which FMRP expression is highest. Higher CMRglc in fragile X mice may be a function of abnormalities found in dendritic spines.
Fragile X syndrome is the most common form of inherited mental retardation, with an estimated frequency in males of 1/4,000 (1). Besides moderate to severe mental retardation, fragile X patients exhibit macroorchidism, an elongated face (2), hyperactivity, autistic-like behavior, and increased sensory sensitivity (3). The disorder is usually caused by a repeat expansion of (CGG)n trinucleotide in the 5′-untranslated region of the fmr1 gene on Xq27.3 (4). The expanded repeat results in transcriptional silencing of the fmr1 gene and, as a consequence, the absence of its protein product, the fragile X mental retardation protein (FMRP) (5, 6). In brain, FMRP is highly expressed in neurons but not in glia (7). It is found in cytoplasm, largely associated with actively translating ribosomes (8), and is localized in dendrites and dendritic spines (7). FMRP has been demonstrated to interact with a subset of mRNAs, including its own and those of other important neuronal proteins such as MAP1Β (9). It has been postulated to function as a translation regulator in vivo (10, 11). The most striking neuropathological feature of fragile X syndrome is the long, thin, and tortuous appearance of cortical dendritic spines (12, 13), a similar morphology to that seen early in development (14). Increased intracranial volume (15), enlarged ventricles, increased volumes of selective subcortical gray matter regions, and decreased size of the posterior cerebellar vermis (16) have also been reported.
How the lack of FMRP results in mental retardation and the function of FMRP in the normal brain are subjects of intense investigation. One approach to these issues is the study of the fmr1 knockout (KO) mouse (17), which does not express FMRP. Male mice hemizygous for the mutation have several characteristics in common with the human syndrome, including enlarged testicles; long, thin dendritic spines (18); some subtle spatial learning abnormalities (19); auditory hypersensitivity; and increased susceptibility to audiogenic seizures (20, 21). The fmr1 KO mouse therefore appears to be a good animal model for the human disorder and permits an assessment of the effect of the fmr1 mutation on a constant genetic background.
Our aim was to measure regional cerebral metabolic rates for glucose (CMRglc) in the fragile X KO (fmr1 KO) mouse to determine whether abnormalities in specific brain regions or networks might affect the cognitive and behavioral abnormalities in this syndrome. We measured CMRglc as an indicator of the level of functional activity in various brain regions. We also did behavioral assessment in our animals to try to understand the correspondents in the mouse of mental retardation and autistic behavior. Our results demonstrate abnormalities in both regional energy metabolism and in several behavioral characteristics in the male fmr1 KO mouse.
Materials and Methods
Animals.
All procedures were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and the approval of the Institute Animal Care and Use Committee. FVB/NJ-Fmr1tm1Cgr breeding pairs (heterozygous females and hemizygous males) were obtained from The Jackson Laboratory. Heterozygous female and WT male offspring were mated to produce two experimental groups: hemizygous males (n = 43) and WT males (n = 42). All mice were housed in a central facility and maintained under controlled conditions of normal humidity and temperature, with standard alternating 12-h periods of light and darkness. Rodent chow and water were provided ad libitum. A total of 85 mice between 16 and 20 weeks of age were studied.
Genotyping.
Genomic DNA was extracted from a section of tail taken from each animal (Puregene, Gentra Systems). Primers to screen for the presence or absence of the mutant allele were 5′-ATCTAGTCATGCTATGGATATCAGC-3′ and 5′- GTGGGCTCTATGGCTTCTGAGG-3′. The DNA, a PCR buffer, and TaqDNA polymerase (AmpliTaq Gold, Applied Biosystems) were combined and subjected to 35 cycles at 95, 62, and 72°C. After amplification, the products were separated by electrophoresis on a 1.5% agarose gel at 100 V for 1 h. The PCR product at ≈800 bp indicated the presence of the KO allele.
Neurologic Screen.
Groups of 10 fmr1 KO and 10 WT control littermates at 10–14 months of age were examined for home-cage behavior and were subjected to a neurologic screen for motor and sensory functions, as described (22). Physical characteristics of mice were noted, such as appearance of fur and whiskers, exophthalmos, and piloerection. The presence of behaviors in the home cage, such as wild running, sniffing, freezing, licking, rearing, jumping, defecation, urination, and movement throughout the cage, was recorded. Animals were tested for the eye blink, ear twitch, trunk curl, toe pinch, reaching, and righting reflexes. Animals were also subjected to the wire-suspension (22) and hind-leg resistance tests.
Locomotor Activity in an Open Field.
Locomotor activity was evaluated by placing mice in an open field consisting of a clear Plexiglas box (40 × 40 × 30 cm) with a black floor in standard room light. Activity was recorded at 2-min intervals for 30 min and quantified by a computer-operated tracking system of 16 photo beams per side (TruScan System, Coulbourn Instruments, Allentown, PA). Total distance moved, distance moved in the margins of the field (within 6.25 cm of walls), and number of entrances into the center zone (area >6.25 cm from walls) were measured. The margin distance/total distance ratio can be used as an index of anxiety-related responses (23).
Passive Avoidance.
Animals were trained in a passive avoidance apparatus (Small Animal Shocker, Coulbourn Instruments) with one lighted and one dark compartment separated by a guillotine door. On training day, each mouse was placed in the lighted compartment and given access to the dark compartment by raising the guillotine door after 5 sec. On entrance into the dark compartment, the guillotine door was closed, and an electric shock (0.2 mA for 1 sec) administered. The mouse was removed from the apparatus after 5 sec and returned to its home cage. Mice that did not enter the dark compartment within 60 sec were eliminated from the study. After 24 h, each animal was placed in the lighted compartment and the latency to enter the dark compartment was recorded up to a maximum of 300 sec.
Surgical Preparation of Animals.
Mice were prepared for metabolic studies by insertion under light halothane anesthesia of polyethylene catheters (PE-10) into one femoral artery and vein as described (24). Mice were permitted to move freely throughout the 18- to 20-h recovery period. Food and water were available ad libitum.
Physiological Variables.
Mean arterial blood pressure, hematocrit, and arterial plasma glucose concentrations were measured to evaluate each animal's physiological state (24). Rectal temperature and mean arterial blood pressure were monitored with a model BAT-12 thermometer (Sensortek, Clifton, NJ) and a Digi-Med Blood Pressure Analyzer (Micro-med, Louisville, KY), respectively.
Determination of CMRglc.
CMRglc was determined by the autoradiographic [14C]deoxyglucose (DG) method as previously described for the rat (25), except for the withdrawal of smaller and less frequent blood samples to avoid excessive blood loss. The experimental period was initiated by an i.v. pulse injection of 120 μCi/kg (1 Ci = 37 GBq) of 2-deoxy-d-[1-14C]glucose (specific activity, 50–55 μCi/mmol; Perkin–Elmer) contained in ≈40 μl of physiological saline. Timed arterial samples were collected during the following 45 min for determination of the time courses of the plasma glucose and [14C]DG concentrations. At the end of the experimental interval, mice were killed by an i.v. injection of a lethal dose of sodium pentobarbital, and brains were removed rapidly and frozen in isopentane (−40°C). Serial sections, 20 μm thick, were cut in a Leica 1850 cryostat (Leica, Deerfield, IL) at −18°C, thaw mounted on gelatin-coated slides, immediately dried in a stream of air, and exposed to EMC-1 film (Kodak) along with calibrated [14C]methylmethacrylate standards as described previously (24). Sections were then stained with thionin. Autoradiograms and stained sections were digitized with a pixel size of 11 μm by means of a 10-bit DVC-1310 digital camera (DVC, Austin, TX). Images were aligned and analyzed with an MCID Elite image processing system (Imaging Research, St. Catherine's, ON, Canada). Regions of interest were located and outlined on the Nissl-stained section by reference to a mouse brain atlas (26), and ODs were measured on the autoradiograms. Concentrations of 14C were determined from the OD vs. 14C concentration curve determined from the calibrated plastic standards, and CMRglc was calculated from the pixel-weighted average local tissue 14C concentration and the time courses of plasma [14C]DG and glucose concentrations by means of the operational equation of the method (25). We used the rate constants and the lumped constant determined in the normoglycemic, conscious rat (25). Two animals in the series were not analyzed because of problems with the autoradiography. Global CMRglc and brain volume were determined by analysis of autoradiograms of all sections of the entire brain digitized (42-μm pixel size) by means of a Multirad 850 Howtek Film Digitzer (Howtek, Hudson, NH). Weighted average CMRglc was determined as described above, and brain volume was determined from the total number of pixels and the calibrated pixel size.
Statistical Analyses.
Locomotor activities in an open field were analyzed by means of a two-way (genotype × epoch) ANOVA with repeated measures on epoch. Data sets were further analyzed by two-tailed Student's t tests. Physiological variables and CMRglc measurements were analyzed for statistically significant differences between the two genotypes by two-tailed Student's t tests. Corrections for multiple comparisons were made by means of a modified Hochberg procedure with the number of true null distributions estimated by the graphical P-plot method; family-wise error was set at α = 0.05 and 0.01 (27).
Results
Neurologic Screen.
A simple assessment of home-cage behavior and a screen for neurological deficits were performed before any behavioral studies were done. These assessments demonstrated no differences between WT and fmr1 KO mice in home-cage behavior, fighting patterns, level of interaction among animals, and physical appearance. Reflexes tested were found to be normal in both genotypes. Hind-leg resistance in a supine position was similar for both genotypes. Only wire-suspension performance was impaired in the fmr1 KO mice compared with WT controls. Mean (± SEM) latencies (min) to fall from a wire were 0.20 ± 0.06 (n = 5) and 0.54 ± 0.24 (n = 10) in fmr1 KO and WT mice, respectively. Means were statistically significantly different (P < 0.05).
Open-Field Activity.
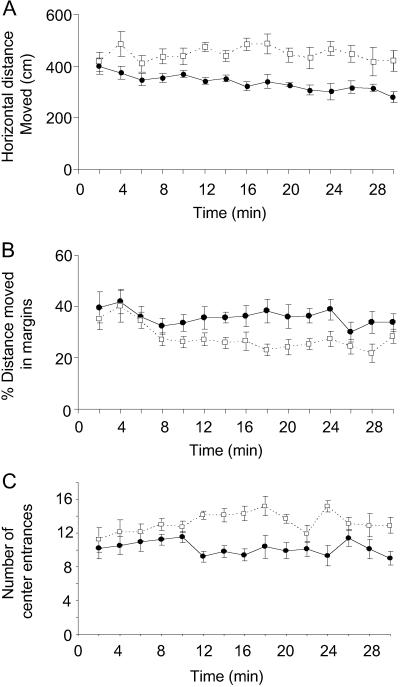
The distance moved in an open field during 2-min epochs (Fig. 1A) showed no statistically significant interaction between genotype and epoch (repeated measures ANOVA). Regardless of epoch, fmr1 KO mice moved a greater distance in the open field than WT, F(1, 14) = 110.4, P < 0.001. Regardless of genotype, the distance moved was not significantly different over the 15 epochs, F(1, 14) = 1.05, P = 0.40, indicating no significant signs of habituation during the 30-min session for either genotype. As an indicator of the level of anxiety, we also analyzed where in the field the mice moved. Normally rodents seek cover and avoid open spaces; these behaviors are thought to be associated with anxiety (23). The ratio of the distance moved in the margins to the total during each epoch showed an interaction between genotype and epoch approaching statistical significance, F(1, 14) = 1.621, P = 0.07. During the first 8 min in the field, the margin distance ratio decreased for both genotypes. Thereafter, movements of the WT mice were increasingly in the margins compared with the movements of the fmr1 KO mice (Fig. 1B). Post hoc analysis of each epoch showed statistically significant differences between the means of the two genotypes for the 14- and 22-min epochs (P < 0.01, Student's t tests). Analysis of the number of entrances into the center of the field during 2-min epochs (Fig. 1C) also showed an interaction between genotype and epoch approaching statistical significance, F(1, 14) = 1.472, P = 0.12. Post hoc analysis of each epoch showed statistically significant differences between the means of the two genotypes for the 12- to 16-, 20-, 24-, and 30-min epochs (P < 0.01, Student's t tests).
Fig 1.
Open-field activity in fmr1 KO mice (n = 10; open squares) and WT controls (n = 10; filled circles). Each point represents the mean ± SEM for each epoch. Data were analyzed by means of a two-way (genotype × epoch) ANOVA with repeated measures on epoch. (A) Total distance moved in the horizontal plane. (B) Distance moved in the margins of the field (% of total). (C) Number of entries into center of the field.
Passive Avoidance.
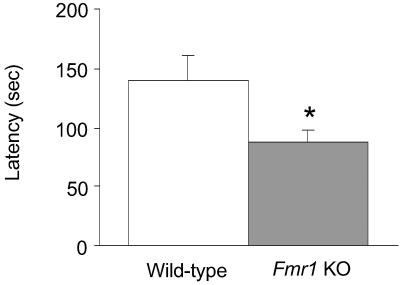
Twenty-four hours after a one-trial training session, the mean latency to enter the dark chamber was 38% shorter for the fmr1 KO mice compared with WT; the difference was statistically significant, P < 0.05 (Fig. 2).
Fig 2.
Passive avoidance task. Bars represent the mean ± SEM latency to enter the dark chamber for fmr1 KO mice (n = 10; shaded bar) and WT controls (n = 10; open bar) 24 h after a single training session in which mice received a shock (0.2 mA for 1 sec) when they entered the dark chamber. *, Statistically significantly different from WT; P < 0.05, Student's t test.
Physiological Measurements.
Physiological measurements (Table 1) were made on the mice in which CMRglc was measured. The two groups of mice were well matched with respect to the physiological variables measured, except for testis weight, which was 28% higher in the fmr1 KO mice.
Table 1.
Physiological variables
| WT (9) | fmr1 KO (11) | |
|---|---|---|
| Age, days | 124 ± 4 | 132 ± 3 |
| Body weight, g | 33 ± 2 | 34 ± 1 |
| Brain weight, g | 0.484 ± 0.005 | 0.482 ± 0.004 |
| Testis weight, g | 0.180 ± 0.003 | 0.232 ± 0.008 |
| Body temperature, °C | 37.6 ± 0.2 | 37.8 ± 0.1 |
| Hematocrit, % | 42 ± 1 | 40 ± 1 |
| Arterial plasma glucose concentration, mM | 7.8 ± 0.4 | 7.8 ± 0.3 |
| Mean arterial blood pressure, mmHg† | 97 ± 3 | 95 ± 1 |
Values are means ± SEM for the number of mice indicated in parentheses, except for brain weights, which were measured in a separate series of 13 WT and 9 fmr1 KO mice.
Statistically significantly different from WT, P < 0.05, Student's t test.
†1 mmHg = 133 Pa.
CMRglc.
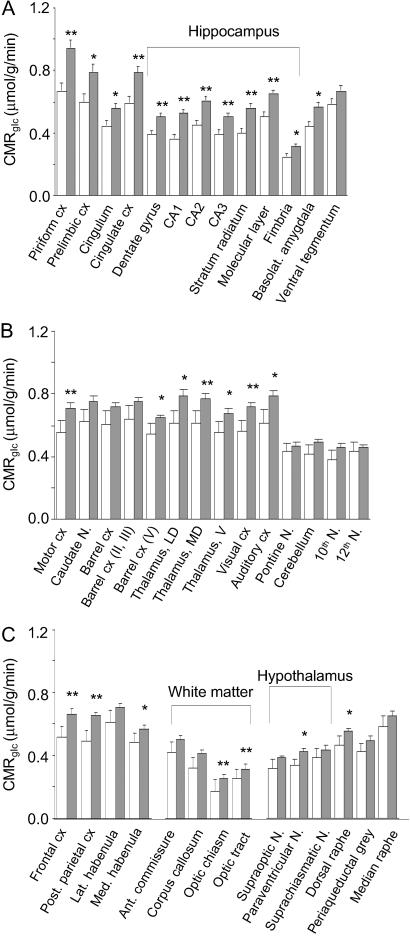
The global CMRglc (Table 2) in fmr1 KO mice was statistically significantly higher by 26% compared with WT, and global brain volume was unchanged. In all 38 structures examined, CMRglc was found to be higher by 12–46% in fmr1 KO mice compared with controls (Fig. 3). All of the regions in the limbic system examined except the ventral tegmental area were statistically significantly increased, and the largest effects were in the hippocampus (Fig. 4). Other cortical regions, including primary motor, visual, and posterior parietal cortex, were also statistically significantly affected. In the somatosensory barrel cortex, only layer V showed a statistically significant effect. Of the hypothalamic nuclei and regions of white matter examined, only the paraventricular nuclei and optic tract and chiasm were statistically significantly increased.
Table 2.
Weighted average CMRglc and volume of whole brain
| WT (8) | fmr1 KO (10) | |
|---|---|---|
| CMRglc, μmol/g/min | 0.439 ± 0.024 | 0.554 ± 0.003 |
| Volume, cm3 | 0.467 ± 0.012 | 0.470 ± 0.011 |
Values are ± SEM for the number of mice indicated in parentheses.
Statistically significantly different from WT, P < 0.01, Student's t test.
Fig 3.
CMRglc (μmol/g/min) in 38 brain regions of fmr1 KO mice (n = 10; shaded bars) and WT controls (n = 8; open bars). Statistically significantly different from controls, Student's t tests with correction for multiple comparisons; *, P < 0.05; **, P < 0.01.
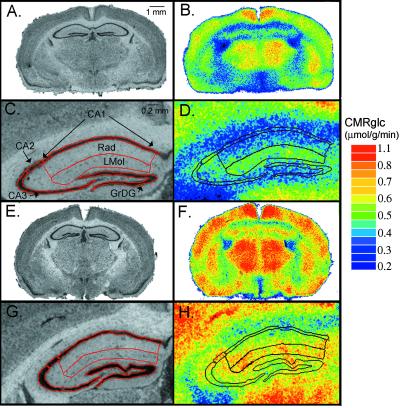
Fig 4.
Digitized [14C]DG autoradiograms (B, D, F, and H) and their corresponding Nissl-stained coronal sections (A, C, E, and G) at the level of the dorsal hippocampus from mice representative of the two genotypes studied. [14C]DG autoradiograms have been color-coded for CMRglc. A–D are from a WT mouse; C and D are higher-resolution images of the left hippocampus in A and B, respectively. E–H are from an fmr1 KO mouse; G and H are higher-resolution images of the left hippocampus in E and F, respectively. Nissl-stained sections have been coregistered with the corresponding autoradiograms. Outlines of regions of interest placed on the Nissl-stained sections were superimposed on autoradiograms for localization. GrDG, granule cell layer of dentate gyrus; Lmol, stratum lacunosum moleculare of hippocampus; Rad, stratum radiatum of hippocampus; CA1–3, pyramidal cell layer of CA1–3 sectors. The color bar (Right) provides the calibration scale for the range of values of CMRglc in μmol/g/min. (Bar in A is 1 mm and applies to B, E, and F. Bar in C is 0.2 mm and applies to D, G, and H.)
Discussion
The present study, to our knowledge, is the first study of regional brain functional activity in the mouse model of fragile X syndrome. Our results show that in the adult male fmr1 KO mouse, CMRglc is increased throughout the brain, particularly in the regions of the limbic system. These increases are accompanied by deficits in performance on a passive avoidance task, general hyperactivity, and a tendency for lower anxiety-like behavior in an open field compared with control littermates.
Behavioral Assessment.
The clinical manifestation of fragile X syndrome is mental retardation in which learning and memory may be profoundly impaired (28). One of our aims was to test the fmr1 KO mouse for its ability to perform a learning and memory task. Our observations were preceded by a thorough assessment of behavior in the home cage and a neurologic screen demonstrating that neither genotype had any gross abnormality that might interfere with the ability to perform on behavioral tests. Initially, we tried to use the Morris water maze, a spatial navigation task frequently used to evaluate learning and memory in rodents. In our hands, neither genotype learned this task well enough to meet established criteria, and we suspect that retinal degeneration in the FVB/NJ background may have interfered with their ability to interpret the spatial cues. We also tried to test the animals on a fear-conditioning task in which we measured freezing behavior in response to either a conditioned stimulus or context. In contrast to our work with other strains of mice, we found it very difficult to assess freezing behavior in the FVB/NJ strain. For this reason, we used the passive avoidance task as a test of the ability of the fmr1 KO mouse to learn and remember an association between an aversive experience (a foot shock) and environmental cues (a dark chamber). Impairment on passive avoidance tasks has been reported in rats with discrete hippocampal lesions in CA1 and CA3 (29). One caveat associated with this test is a potential difference in pain threshold between the two genotypes. Before beginning these studies, we tested a separate group of mice for their reactions to a 1-sec foot shock at 0.2 mA, and we noted vocalizations, jumping behavior, and excessive running in both genotypes as evidence that they had detected the stimulus.
Hyperactivity and autistic-like behavior are common features of fragile X syndrome (2). Our results in a 30-min open-field test demonstrate hyperactivity and suggest decreased anxiety-related behavior in the fmr1 KO mouse. These studies confirm and expand on previously reported results in the fmr1 KO on a C57BL/6 background (17, 30, 31), suggesting that the effect of the mutation on these behaviors is specific for the mutation and not influenced by genetic background. In agreement with a study on C57BL/6 mice (31), our results indicate that during the first 8 min of our 30-min test period, the two genotypes behave very similarly. Thereafter, the WT show decreasing levels of activity and an increased tendency to remain in the margins of the field, whereas the fmr1 KO mice remain at approximately the same level of activity with increasing exploration into the center of the field. As in the human disease, the fragile X mouse demonstrates hyperactivity, but the finding of reduced anxiety-related behaviors in the fragile X mouse contrasts with the human disease in which heightened levels of anxiety, albeit social anxiety, are reported (2). The issue of whether increased levels of exploration into the center of an open field is due to decreased anxiety remains one of interpretation. It may be that an aversion to open spaces is a learned response that the fmr1 KO mice have neither assimilated nor remembered.
Deficits in performance on learning and memory tasks in the fmr1 KO have been difficult to demonstrate (32). Effects of the mutation on performance in the Morris water maze are subtle (17) and even with the plus-shaped water maze variable (33, 34). The background strain appears to be one important consideration (33). Results of cued and contextual conditioning studies in the fmr1 KO mouse are also variable (30, 33–35). Our results with the passive avoidance test indicate some impairment in learning and memory in the fmr1 KO. To our knowledge, there is only one other study in which this test was used on the fmr1 KO mouse (17), and the results showed no effect of the mutation on the C57BL/6J background.
Regional Cerebral Energy Metabolism.
We applied the autoradiographic [14C]DG method to measure CMRglc as a measure of regional functional activity. The method was originally devised for use in the rat (25) but has been applied to many other species (36). There are several practical considerations in the adaptation to the mouse. First, the implantation of catheters in a mouse requires a longer surgical duration and a period of anesthesia between 1 and 2 h. In the original description of the DG method, 2 h were allowed for recovery from anesthesia. We have allowed a period of 18–20 h for recovery to be sure there are no lingering effects of anesthesia during the study. We have also used a button tether system to ensure that mice cannot access the catheters during this period and to allow them freedom of movement. Second, we scaled down the assays, reduced the number of samples, and returned dead-space blood to the animal so that the physiological state was not affected.
In our calculations of CMRglc we used the rate constants and lumped constant determined in the rat (25). The design of the method is such that it is relatively insensitive to the values of the rate constants (25), so inaccuracies in these values have negligible effects on the determined CMRglc. The CMRglc, however, is inversely proportional to the value of the lumped constant. Whereas there may be some difference between values of the lumped constant in mouse and rat, it is unlikely that there is a difference between fmr1 KO and WT mice. It has been shown that the lumped constant remains constant under all physiological conditions that have been studied and that changes occur only in pathophysiological states, such as severe hypoglycemia (37). Therefore, it is highly unlikely that the relative changes in CMRglc between fmr1 KO and WT mice reported in our study are due to any methodological issues. Our values for CMRglc in WT mice are in good agreement with those reported previously in Swiss albino mice (38–40) but are lower than values reported in CFW (41) and C57BL/6J mice (42, 43). Whether this disparity is a function of the strain studied or a difference in procedure remains to be determined.
The increase in CMRglc in the fmr1 KO mouse was unexpected. In view of the behavioral impairments found, we expected the level of functional activity in brain to be reduced. Reduced functional activity is generally associated with a decreased metabolic rate (36). Other studies of diseases with mental retardation have reported decreased or unchanged CMRglc in cretinism in adult rats (44) and in Down's syndrome in humans (45), respectively. New Zealand Black mice characterized by marked deficits in the performance of tasks requiring learning and memory had widespread decreases in CMRglc (41). In an [18F]fluorodeoxyglucose study of adult fragile X patients, global CMRglc was unchanged, but selective increases were reported in the right caudate nucleus, right calcarine cortex, and vermis (15). Such regionally selective increases in metabolism may be related to the hyperactivity and hyperreactivity seen in the disease.
Regional selectivity is not a striking feature in our study. CMRglc was higher throughout the brain in the fmr1 KO mice, but effects were greatest in magnitude and most generalized in the hippocampus. Behavioral and physiological manifestations of hippocampal abnormalities have not been uniformly observed in the fmr1 KO mouse. Impaired performance in the Morris water maze, a spatial memory task, has been difficult to demonstrate (19, 33, 34). Several groups have shown that long-term potentiation in the hippocampus is unaffected in the fragile X mouse (35, 46). Recently, however, an augmentation of long-term depression in the Schaffer collateral synapses in area CA1 has been reported in the fmr1 KO (47). This finding is of interest in light of our study in which increases in CMRglc in the CA1 pyramidal cell layer and stratum radiatum were the largest of any brain region examined.
Changes in CMRglc reflect changes in functional activity, and it is the neuropil-rich regions of the nervous system in which changes in functional activity require the greatest expenditure of energy (36). This is because most of the energy expenditure in brain is used for fueling the Na+, K+-ATPase, the enzyme largely responsible for maintaining ion gradients across neuronal membranes. Areas in which the surface-to-volume ratio is highest will require the highest maintenance. The increased density and altered morphology of dendritic spines in fmr1 KO mice (48) with consequent increased surface-to-volume ratios may be responsible for increased metabolic needs of the tissue.
The immature spines in fragile X may also produce an imbalance between excitatory and inhibitory inputs rendering cortical neurons in a hyperexcitable state. The long apical dendrites of cortical pyramidal cells receive excitatory afferents primarily as excitatory amino acid synapses on spines of dendritic branches (49). Inhibitory inputs are in the form of γ-aminobutyric acid (GABA) synapses on cell bodies and proximal axons (49). It is the balance between excitatory and inhibitory inputs that determines whether individual neurons will depolarize. Dendritic abnormalities in fragile X syndrome may be responsible for unbalanced excitatory inputs causing a hyperexcitability, increased sensitivity to sensory stimulation (3, 21), and increased CMRglc.
The finding of augmented long-term depression (LTD) (47) in the hippocampus of fmr1 KO mice may elucidate this process. It has been shown that activation of metabotropic glutamate receptors (mGluR) triggers LTD at Schaffer collateral synapses in CA1 of the hippocampus (50) and that postsynaptic dendritic protein synthesis is involved in the long-term nature of the depression. One of the proteins translated in response to mGluR activation is FMRP (51) a putative negative regulator of translation in the dendrite (11, 52). Without the control of FMRP, synapses may undergo enhanced turnover in response to stimulation. Huber et al. (47) have suggested that the absence of FMRP may interfere with the establishment and maintenance of strong synapses required for normal brain function. Numerous such unstabilized synapses in the fmr1 KO mouse may increase tissue energy requirements.
Conclusion
In keeping with the clinical symptoms characteristic of fragile X syndrome, our studies demonstrate that fmr1 KO mice are hyperactive and have some deficits in learning and/or memory. Contrary to expectations, our results show that the level of functional activity as measured by CMRglc is increased in the conscious and freely moving fmr1 KO mouse. Increased rates of energy metabolism in cortical structures correlate with findings of abnormal dendritic spine morphology and reflect an increased excitatory state. The increased excitatory state may be a consequence of an imbalance between excitatory amino acid synapses on dendritic spines and GABAergic synapses on cell bodies favoring the excitatory inputs. The imbalance may result from a failure to stabilize the synapses on dendritic spines due to the lack of FMRP and its regulatory function on translation in the dendrite.
Acknowledgments
We thank R. Cohen for helpful discussions at the outset of the study; J. Crawley for counsel on the behavioral aspects of this study; H. Klein for her work on the home-cage observations and neurologic screening of the animals; T. Burlin for help with image processing; and K. Schmidt for help with the multiple comparison statistical analyses.
Abbreviations
FMRP, fragile X mental retardation protein
CMRglc, cerebral metabolic rate for glucose
KO, knockout
DG, deoxyglucose
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Turner G., Webb, T., Wake, S. & Robinson, H. (1996) Am. J. Med. Genet. 64 196-197. [DOI] [PubMed] [Google Scholar]
- 2.Hagerman R. J. (2002) in Fragile X Syndrome: Diagnosis, Treatment, and Research, eds. Hagerman, R. J. & Hagerman, P. J. (John Hopkins Univ. Press, Baltimore), pp. 3–109.
- 3.Miller L. J., McIntosh, D. N., McGrath, J., Shyu, V., Lampe, M., Taylor, A. K., Tassone, F., Neitzel, K., Stackhouse, T. & Hagerman, R. J. (1999) Am. J. Med. Genet. 83 268-279. [PubMed] [Google Scholar]
- 4.Verkerk A. J., Pieretti, M., Sutcliffe, J. S., Fu, Y. H., Kuhl, D. P., Pizzuti, A., Reiner, O., Richards, S., Victoria, M. F. & Zhang, F. P. (1991) Cell 65 905-914. [DOI] [PubMed] [Google Scholar]
- 5.Pieretti M., Zhang, F. P., Fu, Y. H., Warren, S. T., Oostra, B. A., Caskey, C. T. & Nelson, D. L. (1991) Cell 66 817-822. [DOI] [PubMed] [Google Scholar]
- 6.Sutcliffe S., Nelson, D. L., Zhang, F., Pieretti, M., Caskey, C. T., Saxe, D. & Warren, S. T. (1993) Hum. Mol. Genet. 1 397-400. [DOI] [PubMed] [Google Scholar]
- 7.Feng Y., Gutekunst, C.-A., Eberhart, D. E., Yi, H., Warren, S. T. & Hersch, S. M. (1997) J. Neurosci. 17 1539-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khandjian E. W., Corbin, F., Woerly, S. & Rousseau, F. (1996) Nat. Genet. 12 91-93. [DOI] [PubMed] [Google Scholar]
- 9.Ashley C. T., Wilkinson, J. R., Reines, K. D. & Warren, S. T. (1993) Science 262 563-566. [DOI] [PubMed] [Google Scholar]
- 10.Oostra B. A. (2002) Trends Mol. Med. 8 102-103. [DOI] [PubMed] [Google Scholar]
- 11.Laggerbauer B., Ostareck, D., Keidel, E. M., Ostareck-Lederer, A. & Fischer, U. (2001) Hum. Mol. Genet. 10 329-338. [DOI] [PubMed] [Google Scholar]
- 12.Rudelli R. D., Brown, W. T., Wisniewski, K., Jenkins, E. C., Laure-Kamionowska, M., Connell, F. & Wisniewski, H. M. (1985) Acta Neuropathol. 67 289-295. [DOI] [PubMed] [Google Scholar]
- 13.Hinton V. J., Brown, W. T., Wisniewski, K. & Rudelli, R. D. (1991) Am. J. Med. Genet. 41 289-294. [DOI] [PubMed] [Google Scholar]
- 14.Marin-Padilla M. (1967) J. Comp. Neurol. 13 475-490. [DOI] [PubMed] [Google Scholar]
- 15.Schapiro M. B., Murphy, D. G. M., Hagerman, R. J., Azari, N. P., Alexander, G. E., Miezejeski, C. M., Hinton, V. J., Horwitz, B., Haxby, J. V., Kumar, A., et al. (1995) Am. J. Med. Genet. 60 480-493. [DOI] [PubMed] [Google Scholar]
- 16.Reiss A. L., Aylward, E., Freund, L. S., Joshi, P. K. & Brayan, R. N. (1991) Ann. Neurol. 29 26-32. [DOI] [PubMed] [Google Scholar]
- 17.Bakker C. E., Verheij, C., Willemsen, R., vander Helm, R., Oerlemans, F., Vermey, M., Bygrave, A., Hoogeveen, A. T., Oostra, B., Reyniers, E., et al. (1994) Cell 78 23-33.8033209 [Google Scholar]
- 18.Irwin S. A., Galvez, R. & Greenough, W. T. (2000) Cereb. Cortex 10 1038-1044. [DOI] [PubMed] [Google Scholar]
- 19.D'Hooge R., Nagels, G., Franck, F., Bakker, C. E., Reyniers, E., Storm, K., Kooy, R. F., Oostra, B. A., Willems, P. J. & De Deyn, P. P. (1997) Neuroscience 76 367-376. [DOI] [PubMed] [Google Scholar]
- 20.Musumeci S. A., Bosco, P., Calabrese, G., Bakker, C., De Sarro, G. B., Elia, M., Ferri, R. & Oostra, B. A. (2000) Epilepsia 41 19-23. [DOI] [PubMed] [Google Scholar]
- 21.Chen T. & Toth, M. (2001) Neuroscience 103 1043-1050. [DOI] [PubMed] [Google Scholar]
- 22.Paylor R., Nguyen, M., Crawley, J. N., Patrick, J., Beaudet, A. & Orr-Urtreger, A. (1998) Learn. Mem. 5 302-316. [PMC free article] [PubMed] [Google Scholar]
- 23.Crawley J. N. (1989) Curr. Opin. Psychiatry 2 773-776. [Google Scholar]
- 24.Smith C. B. & Kang, J. (2000) Proc. Natl. Acad. Sci. USA 97 11014-11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokoloff L., Reivich, M., Kennedy, C., Des Rosiers, M. H., Patlak, C. S., Pettigrew, K. D., Sakurada, O. & Shinohara, M. (1977) J. Neurochem. 28 897-916. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G. & Franklin, K. B. J., (2001) The Mouse Brain in Stereotaxic Coordinates (Academic, New York).
- 27.Turkheimer F. E., Smith, C. B. & Schmidt, K. (2001) NeuroImage 13 920-930. [DOI] [PubMed] [Google Scholar]
- 28.Bennetto L. & Pennington, B. F. (2002) in Fragile X Syndrome: Diagnosis, Treatment, and Research, eds. Hagerman, R. J. & Hagerman, P. J. (John Hopkins Univ. Press, Baltimore), pp. 206–248.
- 29.Stubley-Weatherly L., Harding, J. W. & Wright, J. W. (1996) Brain Res. 716 29-38. [DOI] [PubMed] [Google Scholar]
- 30.Peier A. M., McIlwain, K. L., Kenneson, A., Warren, S. T., Paylor, R. & Nelson, D. L. (2000) Hum. Mol. Genet. 9 1145-1159. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen D. M., Derber, W. J., McClellan, D. A. & Crnic, L. S. (2002) Brain Res. 927 8-17. [DOI] [PubMed] [Google Scholar]
- 32.Fisch G. S., Hao, H. K., Bakker, C. & Oostra, B. (1999) Am. J. Med. Genet. 84 277-282. [DOI] [PubMed] [Google Scholar]
- 33.Dobkin C., Rabe, A., Dumas, R., El Idrissi, A., Haubenstock, H. & Brown, W. T. (2000) Neuroscience 100 423-429. [DOI] [PubMed] [Google Scholar]
- 34.Van Dam D., D'Hooge, R., Hauben, E., Reyniers, E., Gantois, I., Bakker, C. E., Oostra, B. A., Kooy, R. F. & De Deyn, P. P. (2000) Behav. Brain Res. 117 127-136. [DOI] [PubMed] [Google Scholar]
- 35.Paradee W., Melikian, H. E., Rasmudden, D. L., Kenneson, A., Conn, P. J. & Warren, S. T. (1999) Neuroscience 94 185-192. [DOI] [PubMed] [Google Scholar]
- 36.Sokoloff L. (1996) in Comprehensive Human Physiology, eds. Greger, R. & Windhorst, U. (Springer, Berlin), Vol. 1, pp. 579–602. [Google Scholar]
- 37.Sokoloff L. (1989) in Regulatory Mechanisms of Neuron to Vessel Communication in the Brain, ed. Battaini, F. (Springer, Berlin), pp. 345–392.
- 38.Melzer P., Crane, A. M. & Smith, C. B. (1993) Eur. J. Neurosci. 5 1638-1652. [DOI] [PubMed] [Google Scholar]
- 39.Melzer P. & Smith, C. B. (1995) Cereb. Cortex 5 301-306. [DOI] [PubMed] [Google Scholar]
- 40.Melzer P. & Smith, C. B. (1997) Neuroscience 83 27-41. [DOI] [PubMed] [Google Scholar]
- 41.Wree A., Beck, T., Bielenberg, G. W., Schleicher, A. & Zilles, K. (1989) Histochemistry 92 343-348. [DOI] [PubMed] [Google Scholar]
- 42.Jay T. M., Jouvet, M. & Des Rosiers, M. H. (1985) Brain Res. 342 297-306. [DOI] [PubMed] [Google Scholar]
- 43.Itoh Y., Esaki, T., Cook, M., Qasba, P., Shimoji, K., Alroy, J., Brady, R. O., Sokoloff, L. & Moore, D. (2001) J. Neurochem. 79 1217-1224. [DOI] [PubMed] [Google Scholar]
- 44.Dow-Edwards D., Crane, A., Rosloff, B., Kennedy, C. & Sokoloff, L. (1986) Brain Res. 373 139-145. [DOI] [PubMed] [Google Scholar]
- 45.Shapiro M. B., Grady, C. T., Kumar, A., Herscovitch, P., Haxby, J. V., Moore, A. M., White, B., Freidland, R. P. & Rapoport, S. I. (1990) J. Cereb. Blood Flow Metab. 10 199-206. [DOI] [PubMed] [Google Scholar]
- 46.Godfraind J. M., Reyniers, E., De Boulle, K., D'Hooge, R., DeDeyn, P. P., Bakker, C. E., Oostra, B. A., Kooy, F. & Willems, P. J. (1996) Am. J. Med. Genet. 64 246-251. [DOI] [PubMed] [Google Scholar]
- 47.Huber K. M., Gallagher, S. M., Warren, S. T. & Bear, M. F. (2002) Proc. Natl. Acad. Sci. USA 99 7746-7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Comery T. A., Harris, J. B., Willems, P. J., Oostra, B. A., Irwin, S. A., Weiler, I. J. & Greenough, W. T. (1997) Proc. Natl. Acad. Sci. USA 94 5401-5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kandel E. R. & Schwartz, J. H. (1991) in Principles of Neuroscience, eds. Kandel, E. R., Schwartz, J. H. & Jessell, T. M. (Elsevier, New York), pp. 151–172.
- 50.Huber K. M., Kayser, M. S. & Bear, M. F. (2000) Science 288 1254-1256. [DOI] [PubMed] [Google Scholar]
- 51.Weiler I. J., Irwin, S. A., Klinstova, A. Y., Spencer, C. M., Brazelton, A. D., Miyashiro, K., Comery, T. A., Patel, B., Eberwine, J. & Greenough, W. T. (1997) Proc. Natl. Acad. Sci. USA 94 5395-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z., Zhang, Y., Ku, L., Wilkinson, K. D., Warren, S. T. & Feng, Y. (2001) Nucleic Acids Res. 29 2276-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]