Abstract
The thyrotropin (TSH) receptor (TSHR) is a member of the heterotrimeric G protein-coupled family of receptors whose main function is to regulate thyroid cell proliferation as well as thyroid hormone synthesis and release. In this study, we generated a TSHR knockout (TSHR-KO) mouse by homologous recombination for use as a model to study TSHR function. TSHR-KO mice presented with developmental and growth delays and were profoundly hypothyroid, with no detectable thyroid hormone and elevated TSH. Heterozygotes were apparently unaffected. Knockout mice died within 1 week of weaning unless fed a diet supplemented with thyroid powder. Mature mice were fertile on the thyroid-supplemented diet. Thyroid glands of TSHR-KO mice produced uniodinated thyroglobulin, but the ability to concentrate and organify iodide could be restored to TSHR-KO thyroids when cultured in the presence of the adenylate cyclase agonist forskolin. Consistent with this observation was the lack of detectable sodium-iodide symporter expression in TSHR-KO thyroid glands. Hence, by using the TSHR-KO mouse, we provided in vivo evidence, demonstrating that TSHR expression was required for expression of sodium-iodide symporter but was not required for thyroglobulin expression, suggesting that the thyroid hormone synthetic pathway of the mouse could be dissociated into TSHR-dependent and -independent steps.
The glycoprotein hormone receptors, a subgroup within the heterotrimeric G protein-coupled receptor family, is composed of the receptors for the glycoprotein hormones: thyrotropin (TSH), luteinizing hormone, and follicle-stimulating hormone. A large ectodomain and a small intracellular tail, relative to most other G protein-coupled receptors, characterize this subfamily of receptors. Sequence comparison of the TSH receptor (TSHR) (1–3) with the other glycoprotein hormone receptors revealed that the TSHR was distinct from the other subgroup members in several potentially important aspects. The TSHR has two unique stretches of amino acids, one of 8 aa close to the amino terminus of the receptor and one of 50 aa toward the carboxyl end of the ectodomain. Also unique to the TSHR was a posttranslational cleavage phenomenon first described during earlier protein studies (4), which involved removal of the unique 50-aa insert, leaving most of the ectodomain (A or α subunit) tethered to the membrane-bound portion of the receptor (B or β subunit) by disulfide bonds (5, 6).
Binding of TSH to the TSHR transmits the major trophic stimulus to the thyroid gland, as well as relaying the signals necessary for the production and release of thyroid hormones. TSH stimulation of the TSHR transcriptionally regulates the genes necessary for thyroid hormone synthesis, namely those of the sodium-iodide symporter (NIS) (7), thyroperoxidase (8), and thyroglobulin (Tg) (9). For these reasons, the TSHR can be regarded as the major regulator of thyroid function.
Inactivating and activating somatic mutations in the TSHR have been reported in several species and are commonly found in thyroid tumors (10). In addition, genomic mutations have been reported in humans and mice. Inactivating mutations lead to the human syndrome of resistance to TSH, as first described in 1995 (11). The mutation which gives rise to the hypothyroid (hyt) mouse (12, 13) was also traced to a point mutation in the transmebrane region of the TSHR (14).
In this study, we report the development and characterization of a TSHR knockout (TSHR-KO) mouse as a model system in which to examine the role of the TSHR in thyroid cell function. TSHR-KO mice were severely hypothyroid and presented with an abnormal thyroid histopathology. When placed on thyroid hormone replacement therapy from weaning, TSHR-KO mice were rendered euthyroid, resulting in fertile animals, providing an opportunity to examine residual thyroid cell function in the absence of the TSHR. By using the TSHR-KO mouse, we demonstrated in vivo that Tg transcription and translation are TSH-independent whereas Tg iodination is TSH-dependent.
Materials and Methods
Genomic DNA Cloning.
A BAC 129Sv mouse genomic DNA library was screened by homology by Genome Systems (St. Louis) to a 138-bp fragment of the murine TSHR (mTSHR) cDNA spanning nucleotides 68–190 (exon 1). Three positive clones were identified. A 9-kb EcoR1 fragment (clone 2E) containing exon 1, 5.5 kb of upstream DNA, and 3.5 kb of intron 1, was selected for use as the foundation of the targeting vector.
Assembly of Targeting Vector.
An XhoI site was introduced into position 1 of mTSHR exon 1 coding sequence with the QuikChange site-directed mutagenesis kit (Stratagene), creating 2E1-Xho. pSVL (Amersham Pharmacia) was cut with SalI and blunted with T4 DNA polymerase, which was then cut with EcoR1, removing all of the built-in SV40 sequences (ΔpSVL). An 8.2-kb StuI-EcoR1 fragment of 2E1-Xho was ligated into ΔpSVL, yielding pTV4. The 3.26-kb EGFP-neo cassette was removed from pEGFPN1 (CLONTECH) with XhoI and DraI, which was then ligated into pTV4 (XhoI and Pme1), to yield the targeting vector, pKO4 (Fig. 1A).
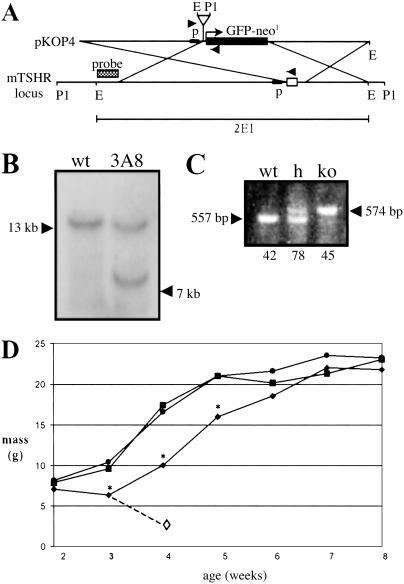
Fig 1.
(A) Targeting vector pKO4 used to generate the TSHR-KO mouse, as described in Materials and Methods. Arrowheads indicate placement of genotyping primers; p, TSHR promoter; open box, mTSHR exon 1; E, EcoR1; P1, Pst-1. (B) Southern blot of Pst-1-digested ES cell DNA. ES cell clone 3A8 had the proper restriction fragment length polymorphism when detected with the 5′ probe. (C) Representative genotyping assay showing WT allele (557 bp) and knockout (ko) allele (574 bp). Both alleles are present in heterozygotes. Numbers below the genotype indicate the number of pups of that genotype resulting from heterozygous matings. (D) Growth rates of mice weaned at 3 weeks and fed a diet containing 100 ppm thyroid powder. •, WT; ▪, h; ⧫, TSHR-KO; ◊, TSHR-KO weaned onto the unsupplemented diet die within 1 week. *, P < 0.01.
Embryonic Stem (ES) Cell Selection and Generation of Mutant Mice.
The TSHR mutation was generated in W9.5 ES cells derived by C. Stewart from a substrain of 129/Sv-+p+Tyr-cMgfSl-J/+ mice (The Jackson Laboratory, catalog no. JR0090), which, however, were wild-type for the Steel locus (15, 16). The 1:151 clone was identified as a homologous recombinant (Fig. 1B). The positive clone (3A8) was injected into C57BL/6J blastocysts to generate male chimeric offspring. Three chimeras of >90% ES cell origin were bred to C57BL/6J females to assess germ-line transmission of the mutation. The TSHR-KO line was derived from a single chimeric mouse.
Animals and Animal Husbandry.
All mice used were C57/Bl6J-129 hybrids raised from heterozygous matings and were 6–8 weeks of age at use. Mice were maintained in light/dark cycles of 12:12 h and were given water and fed ad libitum Pico Rodent Chow-20 (Purina). The supplemented diet consisted of Pico Rodent Chow-20 supplemented with either 250 ppm desiccated thyroid powder (T250) or 100 ppm desiccated thyroid powder (T100), ad libitum (Purina). However, the T100 diet was subject to minor fluctuations in potency because the thyroid hormone content of various preparations of the thyroid supplement were not standardized by the manufacturer. When indicated, mice were maintained on a supplemented diet for a minimum of 3 weeks before use. Mice were killed with CO2. All animal experiments were performed according to approved institutional protocols.
Tissue Preparation and Microscopy.
For histological analysis, tissues were fixed in formalin and paraffin-embedded by using standard protocols. For fluorescence microscopy, fresh tissues were frozen in aqueous media. Six-micrometer sections were cut and transferred to glass at −40°C (Cryo-Jane, Instrumedics, Hackensack, NJ), mounted in 50% glycerol, and GFP was determined as emission at 510–560 nm with excitation at 460–500 nm.
Western Blotting.
Thyroids were removed from the trachea and homogenized in 50 mM Tris⋅HCl, pH 7.5, with protease inhibitor mixture (GIBCO/BRL). Homogenates were cleared by centrifugation at 16 × g for 10 min at 4°C followed by centrifugation at 100,000 × g at 4°C for 1 h to purify total cellular membranes. Membranes were suspended in homogenization buffer with soluble and membrane fractions stored at −70°C until use. For TSHR and NIS, 40 μg of membranes were resolved on SDS-9% polyacrylamide gel, transferred to a poly(vinylidene difluoride) membrane, and detected, in turn, by specific antibody [anti-TSHR: A10 was a kind gift from P. Banga, King's College, London (17); anti-NIS: anti-rat NIS antibody (18) was kindly provided by N. Carrasco, Albert Einstein College of Medicine]. For Tg and GFP, 20 μg of soluble fraction was resolved on SDS-5% polyacrylamide gel and transferred to a poly(vinylidene difluoride) membrane for detection with antibody [iodine-specific anti-Tg antibody 42C3 (19), polyclonal sera raised against murine Tg, or anti-GFP peptide antibody (CLONTECH)]. Reactive bands were visualized with ECL-Plus (Perkin–Elmer).
Genotype Determination.
ES cell or mouse tail DNA was prepared by incubating at 56°C in PCR lysis buffer (50 mM Tris⋅HCl, pH 8.0/20 mM NaCl/1 mM EDTA/0.2% SDS/100 μg/ml proteinase K). Southern Blot: 10 μg of DNA was digested with the appropriate restriction endonuclease and resolved on 0.8% agarose gel. Membranes were washed three times with 0.1× SSC, 0.2% SDS at 65°C. PCR: Tissue sample was digested in 20 μl of PCR lysis buffer, diluted with 200 μl of H2O, and heat-denatured for 5 min. Precipitated material was cleared by centrifugation and 4 μl of the supernatant was used for PCR (50 μl final volume, 1.5 mM MgCl2, 150 ng of each primer (mTSHR7: CAGGGTGGAGACGCACACTC; proMOUSE-1: AGAGAGTCCCACAACAGTC; EGFPrev: TCGCCGTCCAGCTCGACCAG). Cycling parameters: 94°C, 45 s; 54°C, 45 s; 72°C, 50 s; 40 cycles).
Thyroid Hormone Measurement.
Blood was drawn postmortem by cardiac puncture and sent to the Mount Sinai Medical Center clinical endocrinology laboratory for total T3 and total T4 measurement. Alternatively, total blood T4 was measured directly from the tail (Neonatal T4 kit, Diagnostic Products Corporation, Los Angeles) according to the manufacturer's instructions.
Radioactive Iodide Uptake.
Mice were administered 0.1 mCi/10 g body weight Na125I (Perkin–Elmer) i.p. and were killed 2 h after treatment. Tissues were immediately dissected and fixed in formalin before quantification in a γ-counter. An Uptake Index (UI) was calculated based on the following formula: (cpm thyroid + trachea/mass thyroid + trachea)/(cpm liver/mass liver).
Thyroid organ culture and forskolin treatment was performed as reported (20, 21), with some modifications. Thyroids from three WT or TSHR-KO mice were dissected, removed from the trachea, minced under sterile conditions, separated into two approximately equal portions, and incubated in 2 ml of DMEM/Ham's F-12/MCDB131 (GIBCO/BRL), 2:1:1, supplemented with 227 μM ascorbic acid, 5 μg/ml human insulin (Novo-Nordisc, Copenhagen)/15 μM NaI, 0.1 mCi/ml 125I− (Perkin–Elmer). Samples were incubated for 2 days at 37°C in a humidified, 5% CO2 incubator with 10 μM forskolin or an equal volume of DMSO (vehicle control). After incubation, tissue pieces were collected, rinsed three times with cold PBS, and homogenized as for Western blotting. After centrifugation at 16 × g for 10 min at 4°C, total protein was measured by using Bradford reagent (Bio-Rad), and the amount of 125I− incorporated into trichloroacetic acid-insoluble 10-μg protein aliquots was quantified in a γ-counter.
Serum TSH Determination and TSH Stimulation Test.
Serum TSH was measured as described (22). Bovine TSH (Sigma, catalog no. T8931) was administered i.p. as described (23). TSH-stimulated T4 release was measured with the Neonatal T4 RIA kit (Diagnostic Products Corporation).
RNA Isolation and RT-PCR Analysis.
Pooled thyroid tissue from five mice was homogenized in Trizol (GIBCO) and processed as directed. Poly(A) RNA was prepared from total RNA by using Oligotex spin columns (Qiagen, Chatsworth, CA). mRNA was reverse transcribed by using Superscript II reverse transcriptase (GIBCO). For the initial TSHR-specific PCR, cDNA was amplified with primer set 1 (150 ng each mTSHR 5: CCCACTGTTGGTACAACCC and mTSHR 8: GAGCCAGCAGACTGACAAACC; cycling parameters 94°C, 45 s; 59°C, 45 s, 72°C, 1 min; 25 cycles). The product of the initial PCR was diluted 11-fold and used as template for the nested PCR using the TSHR-specific primer set 2 (150 ng each mTSHR4: TTCCAGCCGCTGCAGAGTTGC and mTSHR 2: GAGTGTGCGTCTCCACCCTG; cycling parameters 94°C, 45 s; 61°C, 45 s, 72°C, 30 s; 25 cycles). A similar approach was used for PCR of GFP: primer set 1 (GFP-4: TTACTTGTACAGCTCGTCCATGCC and GFP-5: TGGTGAGCAAGGGCGAGG; cycling parameters 94°C, 45 s; 61°C, 45 s, 72°C, 50 s; 25 cycles); primer set 2 (GFP-1: CTCGTGACCACCCTGACC and GFP-2 GGTCACGAACTCCAGCAGG; 94°C, 45 s; 59°C, 40 s, 72°C, 30 s; 25 cycles). Actin was amplified by using 150 ng each ACT1 (GACGAGGCCCAGAGCAAGAGAG) and ACT2 (ACGTACATGGCTGGGGTGTTG): cycling parameters 94°C, 45 s; 51°C, 40 s; 72°C, 30 s; 30 cycles.
Statistical Analysis.
Measurements were expressed as mean ± SD. Probabilities were calculated using Student's t test with significance set at P < 0.05.
Results
TSHR-KO Growth and Viability.
Pups of heterozygous matings appeared normal at birth and litters had a genotypic ratio consistent with single-gene Mendelian inheritance (Fig. 1C), indicating that the TSHR null mutation did not lead to fetal loss and that the TSHR was not necessary for embryonic growth or viability. By weaning at day 21, TSHR-KO pups were severely runted, and TSHR-KO weanlings wasted and died within 1 week whereas the heterozygotes and wild-type mice continued to thrive (Fig. 1D). The TSHR-KO mice could be rescued by extending the weaning time to 28 days or by weaning at day 21 onto a diet supplemented with desiccated thyroid extract. On thyroid supplements, TSHR-KOs gained weight and became indistinguishable from their littermates within 3 weeks (Fig. 1D). Mice rescued by prolonged weaning and maintained on normal diet survived but did not reproduce, whereas both male and female TSHR-KO mice were fertile when fed a diet supplemented with thyroid powder.
Thyroid Function in TSHR-KO Mice Thyroid Function in TSHR-KO Mice.
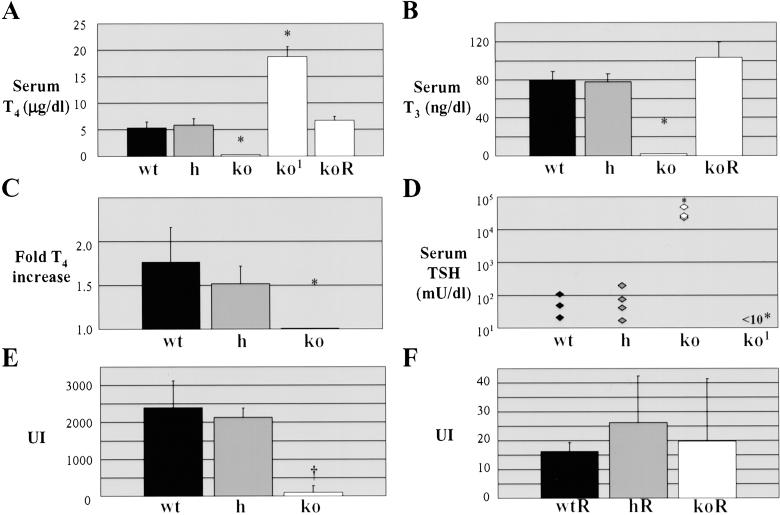
TSHR-KOs which were rescued by prolonged weaning and maintained on normal diet were severely hypothyroid, as judged by low serum T4 and T3 levels (Fig. 2 A and B) and elevated serum TSH [400-fold higher than wild type (Fig. 2D)], whereas heterozygous littermates had normal circulating thyroid hormone and TSH levels. When challenged with exogenous TSH, both wild-type and heterozygous mice responded by releasing thyroid hormone into the circulation, whereas TSHR-KO mice failed to respond (Fig. 2C). When TSHR-KO mice were fed normal rodent diet with a high dose thyroid supplement (T250 diet), the TSHR-KO mice became hyperthyroid (Fig. 2A) and their TSH levels were suppressed (Fig. 2D), indicating that the normal feedback mechanisms regulating thyroid hormone secretion remained intact. The hyperthyroidism was alleviated when the mice were switched to a diet containing a more modest thyroid supplement (T100 diet).
Fig 2.
Thyroid function of TSHR-KO mice. (A) Total T4. (B) Total T3. (C) TSH-induced increase in total T4. (D) Serum TSH. 1, TSHR-KO supplemented with 250 ppm thyroid powder; R, TSHR-KO supplemented with 100 ppm thyroid powder; *, P < 0.001. A–C represent mean ± SD, n = 5. Thyroidal radioiodide uptake index (UI) in mice on normal diet (E); and mice onT100 diet (F). (E and F) data represent two to three experiments of two to three animals each; †, P < 0.01.
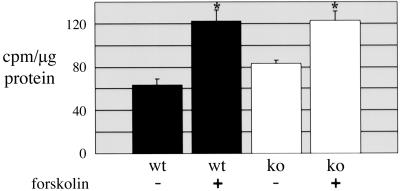
TSHR-KO mice failed to concentrate radioactive iodide (RAI) in their thyroid glands. Within 2 h, wild-type and heterozygous mice had intrathyroidal RAI levels 40-fold higher than knock-outs (Fig. 2E). As shown in Fig. 2F, even the low-dose T100 diet completely suppressed RAIU into wild-type and heterozygous thyroids, making those glands behave as TSHR-KO thyroids. Signals transmitted through the TSHR, primarily in the form of cAMP, drive thyroid hormone synthesis and secretion, including transcription of the genes necessary for iodide organification. When cAMP was supplied to cultured TSHR-KO thyroids by forskolin (an adenylate cyclase agonist), they gained the ability to take up and organify iodide, as measured by trichloroacetic acid-precipitable 125I-labeled protein (Fig. 3).
Fig 3.
Forskolin induced 125I− organification into thyroid protein. Three thyroids were minced, divided into four portions, and cultured with radioiodide tracer for 2 days, with or without forskolin. Tissue was then homogenized, and equal amounts of protein were trichloroacetic acid-precipitated and quantified. These data were representative of three independent experiments, each counted in triplicate; *, P < 0.002.
A GFP reporter gene was incorporated into the KO-targeting construct. However, high concentrations of GFP can be toxic to transfected cells (24), so the possibility existed that expression of the reporter gene may have damaged GFP-expressing cells, altering normal physiology. We observed that the “green mouse,” which ubiquitously expresses GFP under the control of the β-actin promoter (25), had no detectable thyroid abnormalities (unpublished observations). Because the TSHR promoter is much weaker than the actin promoter, we would expect less GFP to exist in the TSHR-KO cells than in the green mouse cells, greatly diminishing the concerns of GFP toxicity influencing the TSHR-KO phenotype.
Histology of the TSHR-KO Thyroid.
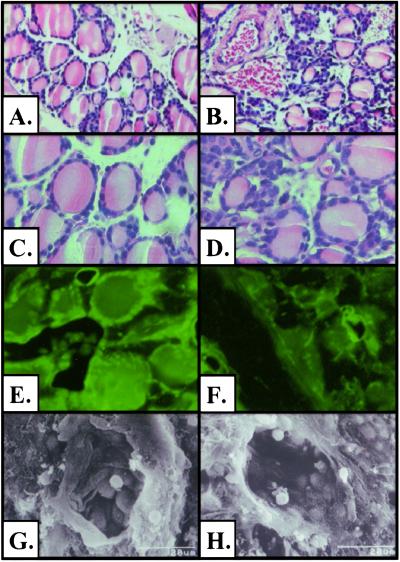
Past studies have determined that TSH was not essential for thyroid follicle formation (26). Therefore, it was reasonable to expect that the TSHR-KO mice would have thyroid glands. When normalized to body weight, the TSHR-KO thyroids from nonreplaced mice were ≈50% of the size of normal thyroids (body mass/thyroid mass × 105; WT, 16 ± 2.9; h: 14 ± 2.9; *ko, 9 ± 0.2; *, P < 0.005). TSHR-KO thyroids appeared to have fewer follicles and more non-follicle-associated cells within the gland than did wild type (Fig. 4 B and D). The GFP reporter gene was detected in thyrocytes of heterozygous and TSHR-KO thyroids by fluorescent microscopy (Fig. 4 E and F). Individual follicles were also examined by scanning electron microscopy. TSHR-KO follicles appeared mostly normal, showing epithelial cells covered with microvilli (Fig. 4 G and H).
Fig 4.
Histology of the TSHR-KO thyroid gland. (A–D) Hematoxylin/eosin-stained paraffin sections of WT (A and C) and TSHR-KO (B and D) thyroids. Fluorescent microscopy of h (E) and TSHR-KO (F) thyroids for GFP reporter gene expression. Scanning electron micrographs of individual WT (G) and TSHR-KO (H) thyroid follicles. (Magnification: A and B, ×100; C–F, ×400; G and H, ×1,500.)
Thyroid-Related Gene Expression.
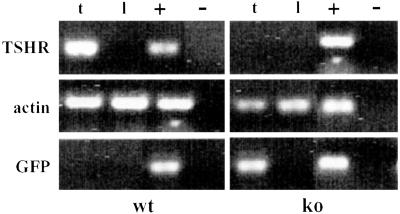
Inactivation of the TSHR gene in TSHR-KO mice was confirmed by using nested RT-PCR. Primer mTSHR2 was homologous to a portion of the mTSHR DNA sequence deleted during assembly of the targeting vector and, therefore, not present in any message produced from the knock-out thyroid epithelial cells. A TSHR-specific band was detected in cDNA prepared from wild-type thyroids but not in knock-out thyroids (Fig. 5). The converse experiment revealed that the TSHR reporter gene, GFP, was transcribed in knock-out thyroids but not in wild-type thyroids (Fig. 5). The reporter gene was also translated as demonstrated by immunoblot of thyroid cytosol for GFP. GFP was present in the thyroids of TSHR-KO mice and in heterozygotes, but not in wild type (Fig. 6B), verifying that GFP was expressed in thyroids under control of the TSHR promoter.
Fig 5.
RT-PCR analysis of TSHR-KO thyroids, demonstrating that TSHR-KOs expressed GFP (TSHR reporter gene) message in lieu of full-length TSHR message; t, thyroid, l, liver; +, plasmid control for PCR; −, no reverse transcriptase control for reverse transcription.
Fig 6.
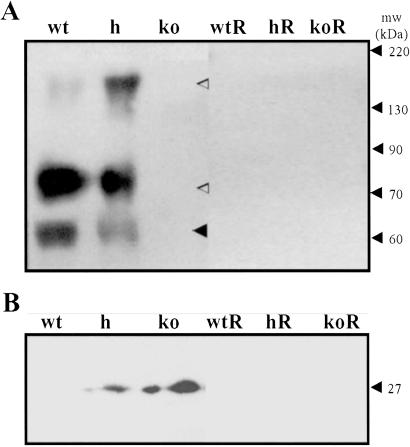
(A) Thyroid membranes immunoblotted proteins for TSHR and NIS; ◂, TSHR α subunit, ◃, NIS. (B) Thyroid cytosol immunoblotted for TSHR reporter gene GFP. R, mice on T100 diet.
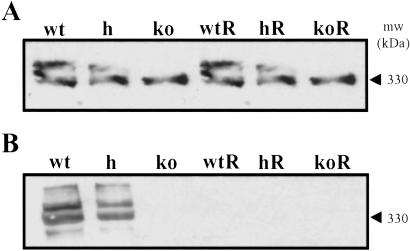
Thyroid homogenates taken from TSHR-KO mice fed a normal diet were immunoblotted for several of the proteins involved in thyroid hormone synthesis, namely Tg, NIS, and TSHR. As shown in Fig. 6A, thyroids from untreated TSHR-KO mice did not express detectable amounts of the 65-kDa TSHR α subunit, whereas heterozygotes expressed approximately half as much as wild-type. The TSHR-KOs also failed to express the 80- and 160-kDa NIS proteins, whereas the heterozygotes appeared to express both monomeric and dimeric forms of NIS, the relative abundances of which varied between preparations. Despite the absence of TSHR, the TSHR-KO mice continued to express Tg, which was detected with polyclonal serum raised against purified wild-type murine thyroglobulin (Fig. 7A). In contrast, Tg was undetectable by using an iodine-dependent monoclonal antibody to murine Tg (19) (Fig. 7B). These data suggested that TSHR-KO mice failed to scavenge iodide and iodinate Tg. After 3 weeks on the T100 diet, all mice, regardless of genotype, expressed Tg, but lost the ability to iodinate (Fig. 7). Replaced mice also failed to express measurable amounts of TSHR or NIS (Fig. 6). These data provided in vivo evidence that NIS expression was dependent upon TSHR-mediated signals, whereas Tg expression was not.
Fig 7.
(A) Thyroid cytosol immunoblotted for whole Tg using polyclonal anti-Tg serum. (B) Thyroid cytosol immunoblotted for iodinated Tg using the iodine-specific monoclonal antibody 42C3. R, mice on the T100 diet were thyroid-suppressed and did not iodinate Tg.
Discussion
In this study, we describe the generation and characterization of a TSHR-KO mouse. Several lines of evidence were provided that the TSHR was deleted from the TSHR-KO mice: TSHR mRNA was undetectable by using a sensitive and specific nested RT-PCR system; reporter gene (GFP) mRNA was transcribed and translated in lieu of TSHR mRNA in the knock-outs; and TSHR protein was undetectable by Western blot of TSHR-KO thyroid membranes. Despite being TSHR-deficient, the TSHR-KO produced thyroglobulin.
Several lines of hypothyroid mice are studied today, for example, the Snell Dwarf mouse (27), the cog mouse (28), and the hyt mouse (13). Of the preexisting models, the hyt mouse is the only model of hypothyroidism resulting from a TSHR mutation. The hyt mouse carries a point mutation in the 4th transmembrane domain of the TSHR, a change that rendered the TSHR-insensitive TSH stimulation but did not alter TSHR expression levels (14). The TSHR-KO mouse, however, did not express the TSHR and, therefore, had no residual signal transduction. Ligand-independent signals transmitted by the TSHR may also be physiologically important (29). The TSHR-KO mouse is a unique model for studying the affect of a true TSHR null mutation on normal physiology.
Being unable to respond to pituitary TSH, the TSHR-KO mice were hypothyroid. Pups had delayed eye opening and growth retardation that was reflected in both weight and length, features common to other hypothyroid mouse models (12, 30). If weaned at the usual time, the mice wasted and died, perhaps as a result of the small intestine defect seen in the thyroid hormone receptor α (TRα) knockout mouse (31). However, TSHR-KO mice survived when the preweaning period was extended to day 28, or if weaned at day 21 onto a thyroid powder-supplemented diet. Furthermore, TSHR-KOs weaned at day 28 could be maintained on a normal diet without thyroid hormone supplement, suggesting that the normal rodent chow provided sufficient amounts of thyroid hormone for survival. Hypothyroid TSHR-KOs seemed to be as curious and active as their littermates but did not reproduce. In contrast, thyroid replaced animals had normal gross developmental patterns and were fertile, despite the absence of the TSHR.
It has been shown previously that the TSHR elicited both protein kinase A and protein kinase C signaling cascades (32). In dog thyroid slices (33) and in the rat-derived FRTL-5 cell line (34), the predominant second messenger produced on TSHR stimulation was cAMP, whereas human thyrocytes exhibited both cAMP and Ca2+ responses (35). In the present study, we demonstrated that TSHR-KO thyroids responded to forskolin (an adenylate cyclase agonist) by organifying iodide, consistent with cAMP being sufficient to drive murine thyroid hormone synthesis by the protein kinase A pathway.
As seen in the hyt mouse, the TSHR-KOs were hypothyroid, and with hypothyroidism came a concomitant increase in serum TSH. The TSH produced by the TSHR-KO mice was biologically active, as evidenced by stimulating the generation of cAMP in CHO cells transfected with the hTSHR holoreceptor (data not shown). Although the thyroid glands of the knockouts did not function normally, the pituitary feedback mechanisms regulating thyroid hormone production and release remained intact, as TSHR-KOs made hyperthyroid had suppressed TSH levels.
In contrast to the hyt mouse, which retains the ability to concentrate iodide (36), radioiodide uptake into the glands of the TSHR-KO was not significant. Also, we were unable to detect NIS protein in the thyroids of TSHR-KO mice. In addition, wild-type and heterozygous mice on thyroid supplementation failed to express measurable amounts of NIS, indicating that their pituitary TSH was suppressed while on hormone replacement therapy, providing in vivo evidence that NIS transcription is TSH-dependent.
The ability of the TSHR-KOs to produce Tg in a noniodinated form was also consistent with the findings of several different studies. Developmentally, in mice, Tg transcription starts at E14.5 (37), before pituitary thyrotrophs produce functional TSH on E15.5 (38), making Tg transcription independent of TSH. In addition, we have previously shown that thyrocytes could be induced to form thyroid “organoids” in mice with suppressed TSH, giving rise to Tg-producing follicles (26). However, other studies suggested that TSH and, therefore the TSHR, was the major regulator of Tg transcription (9, 39). All of the afore-mentioned studies used either live animals (with presumably normal insulin and insulin-like growth factor levels) or were performed in cell culture with medium containing insulin. We now know that insulin/insulin-like growth factor-1 play an important role in Tg gene transcription (40, 41), making it clear that insulin/insulin-like growth factor-1 are potent stimulators of Tg production in the absence of TSH. Therefore, it was presumably insulin and insulin-like growth factor-1 that was driving Tg production in the TSHR-KO mice. Estimating the amount of Tg produced by the different genotypes was not performed because iodinated Tg has been shown to be more immunogenic than noniodinated Tg (42–44). By using the TSHR-KO mouse as a model, we were able to demonstrate that thyroid hormone synthesis in mice is divided into a TSH-independent phase consisting of Tg synthesis, and a TSH-dependent phase consisting of Tg iodination ending in hormone production and release.
By using the TSHR-KO mouse, we demonstrated that in vivo thyroid hormone production was TSH-dependent but Tg production was not. Additionally, thyroid hormone-replaced TSHR-KO mice seemed to develop normally, indicating that this model will allow the definition of the role of the TSHR in nonthyroidal tissues.
Acknowledgments
We thank Dr. Roy Weiss for the TSH measurements, Dr. Edward Diamond for the thyroid hormone measurements, and Dr. Rauf Latif for technical advice. We thank Dr. Naoko Arata for generously providing the anti-Tg serum. We thank Norman Katz for the electron microscopy and Yanan Li for the fluorescent microscopy. We thank Dr. Douglas Forrest and the ES Cell Core Facility at the Department of Human Genetics, Mount Sinai School of Medicine, and Dr. Kevin Kelley of the Mouse Genetics Shared Research Facility, Mount Sinai School of Medicine, for their help in preparing the knockout, and Drs. Douglas Forrest, Yaron Tomer, Reigh-yi Lin, and Roberto DiLauro for reading the manuscript. This work was supported in part by National Institutes of Health Grants DK52464, DK35764, and DK45011 (to T.F.D.); AG12951 and AR47700 (to H.C.B.); and CA88302 (to the Mouse Genetics Shared Research Facility); and grants to the Department of Veterans Affairs, and served as part of a Ph.D. thesis submitted to The Mount Sinai Graduate School of New York University.
Abbreviations
TSH, thyrotropin
TSHR, TSH receptor
TSHR-KO, TSHR knockout
Tg, thyroglobulin
NIS, sodium-iodide symporter
ES, embryonic stem
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Parmentier M., Libert, F., Maenhaut, C., Lefort, A., Gerard, C., Perret, J., Van Sande, J., Dumont, J. E. & Vassart, G. (1989) Science 246 1620-1622. [DOI] [PubMed] [Google Scholar]
- 2.Nagayama Y., Kaufman, K. D., Seto, P. & Rapoport, B. (1989) Biochem. Biophys. Res. Commun. 165 1184-1190. [DOI] [PubMed] [Google Scholar]
- 3.Misrahi M., Loosfelt, H., Atger, M., Sar, S., Guiochon-Mantel, A. & Milgrom, E. (1990) Biochem. Biophys. Res. Commun. 166 394-403. [DOI] [PubMed] [Google Scholar]
- 4.Buckland P. R. & Rees, S. B. (1984) FEBS Lett. 166 109-114. [DOI] [PubMed] [Google Scholar]
- 5.Chazenbalk G. D., Tanaka, K., Nagayama, Y., Kakinuma, A., Jaume, J. C., McLachlan, S. M. & Rapoport, B. (1997) Endocrinology 138 2893-2899. [DOI] [PubMed] [Google Scholar]
- 6.de Bernard S., Misrahi, M., Huet, J. C., Beau, I., Desroches, A., Loosfelt, H., Pichon, C., Pernollet, J. C. & Milgrom, E. (1999) J. Biol. Chem. 274 101-107. [DOI] [PubMed] [Google Scholar]
- 7.Riedel C., Levy, O. & Carrasco, N. (2001) J. Biol. Chem. 276 21458-21463. [DOI] [PubMed] [Google Scholar]
- 8.Gerard C. M., Lefort, A., Libert, F., Christophe, D., Dumont, J. E. & Vassart, G. (1988) Mol. Cell. Endocrinol. 60 239-242. [DOI] [PubMed] [Google Scholar]
- 9.Van Heuverswyn B., Streydio, C., Brocas, H., Refetoff, S., Dumont, J. & Vassart, G. (1984) Proc. Natl. Acad. Sci. USA 81 5941-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp P. (2001) Cell. Mol. Life Sci. 58 1301-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sunthornthepvarakui T., Gottschalk, M. E., Hayashi, Y. & Refetoff, S. (1995) N. Engl. J. Med. 332 155-160. [DOI] [PubMed] [Google Scholar]
- 12.Beamer W. J., Eicher, E. M., Maltais, L. J. & Southard, J. L. (1981) Science 212 61-63. [DOI] [PubMed] [Google Scholar]
- 13.Beamer W. G. & Cresswell, L. A. (1982) Anat. Rec. 202 387-393. [DOI] [PubMed] [Google Scholar]
- 14.Stein S. A., Oates, E. L., Hall, C. R., Grumbles, R. M., Fernandez, L. M., Taylor, N. A., Puett, D. & Jin, S. (1994) Mol. Endocrinol. 8 129-138. [DOI] [PubMed] [Google Scholar]
- 15.Lau M. M., Stewart, C. E., Liu, Z., Bhatt, H., Rotwein, P. & Stewart, C. L. (1994) Genes Dev. 8 2953-2963. [DOI] [PubMed] [Google Scholar]
- 16.Forrest D., Hanebuth, E., Smeyne, R. J., Everds, N., Stewart, C. L., Wehner, J. M. & Curran, T. (1996) EMBO J. 15 3006-3015. [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson L. B., Vlase, H., Graves, P., Nilsson, M., Molne, J., Huang, G. C., Morgenthaler, N. G., Davies, T. F., McGregor, A. M. & Banga, J. P. (1996) J. Mol. Endocrinol. 16 159-170. [DOI] [PubMed] [Google Scholar]
- 18.Levy O., Dai, G., Riedel, C., Ginter, C. S., Paul, E. M., Lebowitz, A. N. & Carrasco, N. (1997) Proc. Natl. Acad. Sci. USA 94 5568-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saboori A. M., Rose, N. R., Bresler, H. S., Vladut-Talor, M. & Burek, C. L. (1998) Clin. Exp. Immunol. 113 297-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Keymeulen A., Roger, P. P., Dumont, J. E. & Dremier, S. (2000) Biochem. Biophys. Res. Commun. 273 154-158. [DOI] [PubMed] [Google Scholar]
- 21.Lamas L., Santisteban, P., Turmo, C. & Seguido, A. M. (1986) Endocrinology 118 2131-2136. [DOI] [PubMed] [Google Scholar]
- 22.Pohlenz J., Maqueem, A., Cua, K., Weiss, R. E., Van Sande, J. & Refetoff, S. (1999) Thyroid 9 1265-1271. [DOI] [PubMed] [Google Scholar]
- 23.Yamada M., Saga, Y., Shibusawa, N., Hirato, J., Murakami, M., Iwasaki, T., Hashimoto, K., Satoh, T., Wakabayashi, K., Taketo, M. M., et al. (1997) Proc. Natl. Acad. Sci. USA 94 10862-10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanazono Y., Yu, J. M., Dunbar, C. E. & Emmons, R. V. (1997) Hum. Gene Ther. 8 1313-1319. [DOI] [PubMed] [Google Scholar]
- 25.Okabe M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. (1997) FEBS Lett. 407 313-319. [DOI] [PubMed] [Google Scholar]
- 26.Valentine M., Martin, A., Unger, P., Katz, N., Shultz, L. D. & Davies, T. F. (1994) Endocrinology 134 1225-1230. [DOI] [PubMed] [Google Scholar]
- 27.Cordier A. C., Denef, J. F. & Haumont, S. M. (1976) Am. J. Anat. 146 339-357. [DOI] [PubMed] [Google Scholar]
- 28.Taylor B. A. & Rowe, L. (1987) Proc. Natl. Acad. Sci. USA 84 1986-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M., Tong, K. P., Fremont, V., Chen, J., Narayan, P., Puett, D., Weintraub, B. D. & Szkudlinski, M. W. (2000) Endocrinology 141 3514-3517. [DOI] [PubMed] [Google Scholar]
- 30.Pierpaoli W., Baroni, C., Fabris, N. & Sorkin, E. (1969) Immunology 16 217-230. [PMC free article] [PubMed] [Google Scholar]
- 31.Fraichard A., Chassande, O., Plateroti, M., Roux, J. P., Trouillas, J., Dehay, C., Legrand, C., Gauthier, K., Kedinger, M., Malaval, L., et al. (1997) EMBO J. 16 4412-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vassart G. & Dumont, J. E. (1992) Endocr. Rev. 13 596-611. [DOI] [PubMed] [Google Scholar]
- 33.Mockel J., Laurent, E., Lejeune, C. & Dumont, J. E. (1991) Mol. Cell Endocrinol. 82 221-227. [DOI] [PubMed] [Google Scholar]
- 34.Bone E. A., Alling, D. W. & Grollman, E. F. (1986) Endocrinology 119 2193-2200. [DOI] [PubMed] [Google Scholar]
- 35.Laurent E., Mockel, J., Van Sande, J., Graff, I. & Dumont, J. E. (1987) Mol. Cell. Endocrinol. 52 273-278. [DOI] [PubMed] [Google Scholar]
- 36.Stein S. A., Shanklin, D. R., Krulich, L., Roth, M. G., Chubb, C. M. & Adams, P. M. (1989) Neuroendocrinology 49 509-519. [DOI] [PubMed] [Google Scholar]
- 37.Lazzaro D., Price, M., de Felice, M. & Di Lauro, R. (1991) Development (Cambridge, U.K.) 113 1093-1104. [DOI] [PubMed] [Google Scholar]
- 38.Lin S. C., Li, S., Drolet, D. W. & Rosenfeld, M. G. (1994) Development (Cambridge, U.K.) 120 515-522. [DOI] [PubMed] [Google Scholar]
- 39.Ossendorp F. A., Leer, L. M., Bruning, P. F., van den Brink, J. A., Sterk, A. & de Vijlder, J. J. (1989) Mol. Cell. Endocrinol. 66 199-205. [DOI] [PubMed] [Google Scholar]
- 40.Santisteban P., Kohn, L. D. & Di Lauro, R. (1987) J. Biol. Chem. 262 4048-4052. [PubMed] [Google Scholar]
- 41.Clement S., Refetoff, S., Robaye, B., Dumont, J. E. & Schurmans, S. (2001) Endocrinology 142 5131-5139. [DOI] [PubMed] [Google Scholar]
- 42.Mallet B., Lejeune, P. J., Ruf, J., Piechaczyk, M., Marriq, C. & Carayon, P. (1992) Mol. Cell. Endocrinol. 88 89-95. [DOI] [PubMed] [Google Scholar]
- 43.Ebner S. A., Lueprasitsakul, W., Alex, S., Fang, S. L., Appel, M. C. & Braverman, L. E. (1992) Autoimmunity 13 209-214. [DOI] [PubMed] [Google Scholar]
- 44.Champion B. R., Rayner, D. C., Byfield, P. G., Page, K. R., Chan, C. T. & Roitt, I. M. (1987) J. Immunol. 139 3665-3670. [PubMed] [Google Scholar]