Abstract
The kidney plays an important role in osmoregulation in freshwater teleosts, which are exposed to the danger of osmotic loss of Na+ and Cl−. However, ion-transport mechanisms in the kidney are poorly understood, and ion transporters of the fish nephron have not been identified thus far. From Mozambique tilapia, Oreochromis mossambicus, we have cloned a chloride channel, which is a homologue of the mammalian kidney-specific chloride channel, ClC-K. The cDNA of the channel, named OmClC-K, encodes a protein whose amino acid sequence has high homology to Xenopus and mammalian ClC-K (Xenopus ClC-K, 41.8%; rat ClC-K2, 40.9%; rat ClC-K1, 40.1%). The mRNA of OmClC-K was expressed exclusively in the kidney, and the expression level of mRNA was increased more in freshwater-adapted fish than seawater-adapted fish. The immunohistochemical study using a specific antibody showed that OmClC-K-positive cells were specifically located in the distal nephron segments. Immunoelectron microscopy further showed that immunoreaction of OmClC-K was recognizable on the structure of basolateral membrane infoldings in the distal tubule cells. The localization of OmClC-K and its induction in hypoosmotic media suggest that OmClC-K is involved in Cl− reabsorption in the distal tubule of freshwater-adapted tilapia.
The kidney plays an important role in osmoregulation in both freshwater and seawater teleosts, although its function is entirely different under the two different environmental conditions. Freshwater teleosts are exposed to the danger of osmotic water load and ion loss. Accordingly, the primary function of their kidneys is to excrete excess water, while reabsorbing most of the filtered solutes. The glomerular filtration rate of freshwater teleosts is higher than that of marine teleosts, and sometimes as much as 95% of the filtered water is excreted as copious dilute urine, which implies that the renal tubules of freshwater teleosts must generally have low water permeability. The nephron of freshwater teleosts is composed of a well-developed glomerulus, a ciliated neck segment, a proximal tubule, an intermediate segment, a distal tubule, and a collecting tubule, but it lacks the loop of Henle (1). The teleost kidney exhibits neither zonation, such as the cortex and medulla, nor a countercurrent system of tubular elements. Thus, teleosts are not able to excrete hyperosmotic urine.
Nishimura et al. (2) measured water and ion transport in nephron tubules isolated from freshwater-adapted rainbow trout, Oncorhynchus mykiss. They perfused nephron tubules and measured water and ion transport in the distal tubule. These results suggest that in freshwater-adapted rainbow trout, the distal tubule acts as a diluting segment, which is equivalent to the early distal segment of the frog kidney and the mammalian thick ascending limb of Henle's loop (TALH) (3, 4).
The extraction of NaCl from urine in the TALH is crucial to urinary concentration and dilution, which is accomplished by the active reabsorption of NaCl in the water-impermeable epithelia. Major advances have been made during the past few years in our understanding of NaCl transport mechanisms in the TALH. This understanding has been fueled by the molecular identification of Na-K-2Cl cotransporter (NKCC2) (5, 6), K+ secretory channel (ROMK) (7, 8), and basolateral Cl− channel (ClC-K2) (9–12) expressed in the TALH of mammalian kidney.
In teleost species, however, the ion-transport mechanisms in the kidney are less understood, and ion transporters of the fish nephron have not been identified thus far. In the present study, to investigate the molecular basis of ion transport in the teleost kidney, we have cloned a chloride channel from Mozambique tilapia, Oreochromis mossambicus. We named it OmClC-K, because it is a homologue of the mammalian kidney-specific chloride channels, ClC-K1 or -K2. To evaluate further the function of OmClC-K, immunohistochemical techniques were applied to the kidney of freshwater- and seawater-adapted tilapia. Our results indicate that OmClC-K is involved in the transepithelial Cl− reabsorption at the distal tubule of the kidney of hyperosmoregulating, freshwater tilapia.
Materials and Methods
Fish.
Mozambique tilapia (O. mossambicus), 15.8 ± 0.7 g of body weight, were obtained from our laboratory stock. Fish were maintained in aerated local tap water (freshwater: [Na+], 0.77 mM; [Cl−], 0.50 mM; [Ca2+], 0.51 mM; [Mg2+], 0.33 mM) and seawater ([Na+], 454 mM; [Cl−], 560 mM; [Ca2+], 12 mM; [Mg2+], 54 mM) at 25–26°C with a daily 12-h photoperiod. The water was continuously circulated through charcoal and sand filters and was partially replaced every day. Fish were fed artificial tilapia pellets (Tilapia 41M, Shikoku Kumiai Shiryo, Tokushima, Japan). To prepare seawater-adapted fish, freshwater fish were first acclimated to 50% seawater for 1 day and then to 100% seawater for at least 30 days. Animal care and protocols for animal experiments were performed according to the guidelines established by the Graduate School of Science, University of Tokyo.
RT-PCR.
Freshwater tilapia were anesthetized in 0.05% 2-phenoxyethanol. They were killed by decapitation, and the kidneys were immediately dissected out. Total RNA was extracted from the kidney by using ISOGEN Reagent (Nippon Gene, Tokyo). For RT-PCR, we made degenerate primers corresponding to the conserved sequences in the ClC-K family [rat ClC-K1 (GenBank accession no. D13927), K2 (D26111), Xenopus ClC-K (AJ011385) and partial sequence of zebra fish (Danio rerio) similar to mammalian ClC-K (BM776551)]: sense strand, 5′-CT(A/G)AA(C/T)GC(C/G)CAC(A/C)(A/G)(A/G)TGG-3′; antisense strand, 5′-GCCCA(C/G)TTT(G/T)CC(A/C/T)AG(A/G)AA-3′. These primers contained 5′ extensions of eight nucleotides with restriction sites for BamHI and EcoRI, respectively. One microgram of total RNA was reverse-transcribed by using SuperScript II reverse transcriptase (Invitrogen) at 42°C for 60 min and then heated at 94°C for 5 min. The synthesized cDNA was used for subsequent PCR with synthetic primers in the following profile: 94°C for 30 s, 55°C for 30 s, 72°C for 30 s, 30 cycles. The PCR products were cut with EcoRI and BamHI on both ends, ligated into EcoRI and BamHI-cut pSPORT1 (Invitrogen), and then sequenced.
Library Construction and Screening.
A freshwater tilapia kidney cDNA library in λTriplEx2 expression vector (CLONTECH) was constructed by the SMART cDNA Library Construction Kit (CLONTECH). A PCR product that has a 150-bp insert homologous to mammalian ClC chloride channel was screened from the library. The full-length clone (2.9 kbp) obtained from the cDNA library was subcloned into pTriplEx2 (CLONTECH), and nested deletion clones prepared with Erase-A-Base System (Promega) were sequenced.
RNase Protection Assay.
For detection of the expression of this clone, the PCR clone used for the library screening was linearized and used to prepare a radiolabeled antisense RNA probe. This probe (1 × 105 cpm per sample) was mixed with 20 μg of total RNA from the gills, kidney, urinary bladder, intestine, stomach, liver, spleen, skeletal muscle, and brain of freshwater- and seawater-adapted tilapia. After hybridization and subsequent RNase digestion (RPA II Kit, Ambion, Austin, TX), protected fragments were analyzed by electrophoresis in a 6% denaturing polyacrylamide gel.
Electrophysiological Analysis of OmClC-K in Xenopus Oocytes.
OmClC-K was cloned into the pSPUTK cloning vector (Stratagene) with the SP6 promoter and Xenopus β-globin 5′ UTR sequences. By using an in vitro capped RNA transcription kit (mMESSAGE mMACHINE SP6 kit, Ambion), capped cRNA was transcribed from the construct after linearization. For expression studies, oocytes were defolliculated manually after collagenase digestion. Two hours after defolliculation, stage V/VI oocytes were injected with either 20 nl of nuclease-free water or 20 nl of 1.0 ng/nl cRNA solution as prepared above with a 10-μl Digital microdispenser (Drummond Scientific, Broomall, PA). The injected oocytes were incubated in modified Barth's solution (88.0 mM NaCl/1.0 mM KCl/2.4 mM NaHCO3/5.0 mM Tris⋅HCl/0.82 mM MgSO4/0.33 mM Ca(NO3)2/0.41 mM CaCl2, pH 7.5). After 2–3 days at 16°C, they were mounted in a recording chamber and impaled with microelectrodes for measurement of current and voltage. Data acquisition and analysis were performed by using commercially available software (CLAMPEX PCLAMP8, Axon Instruments, Union City, CA). The oocytes were clamped at −30 mV holding potential, and 1-s voltage steps from −80 to +100 mV in 20-mV increments were applied. All recordings were performed at room temperature in ND96 buffer (96 mM NaCl/1.8 mM CaCl2/1 mM MgCl2/5 mM Hepes, pH 7.4).
Generation and Affinity Purification of Anti-OmClC-K Serum.
A specific antiserum was raised against a synthetic oligo peptide of OmClC-K in rabbits by Sawady Technology (Tokyo). The antigen was the C terminus of OmClC-K: Cys-Pro-Glu-Met-Lys-Arg-Ile-Leu-Glu-Asp-Leu-Ala-Lys-Glu-Ile. The amino acid sequence of the synthetic peptide had low homology with the corresponding sequence of other tilapia ClC chloride channels cloned in our previous study (13). The antigen was emulsified with complete Freund's adjuvant and immunization was performed in two New Zealand White rabbits. Boost injections were made into the rabbits with the peptide in incomplete Freund's adjuvant at days 14, 42, and 56 after the first injection. A specific antiserum from the rabbit with the highest titer was collected and the specific antibody was affinity-purified by using the antigen.
Western Blotting.
The specificity of the antibody against OmClC-K was confirmed by the molecular mass of the endogenous protein by using Western blot analysis. Membrane fractions were prepared from the kidney of freshwater- and seawater-adapted tilapia. The kidney was homogenized on ice in homogenization buffer [20 ml of 25 mM Tris (pH 7.4), 0.25 M sucrose containing Complete Protein Inhibitor Mixture, Roche Diagnostics]. The homogenate was first centrifuged at 8,000 × g for 15 min, and the supernatant was subjected to higher centrifugation at 400,000 × g for 1 h. The pellet was resuspended in the homogenization buffer. All procedures were performed at 4°C. Protein content of the sample was quantified by the BCA Protein Assay kit (Pierce). The samples (20 μg) were solubilized in a sample loading buffer (0.25 M Tris⋅HCl, pH 6.8/2% SDS/10% β-mercaptoethanol/30% glycerol/0.01% bromophenol) and heated at 70°C for 15 min. They were separated by SDS/PAGE using 7.5% polyacrylamide gels. As a molecular length marker, prestained SDS/PAGE standards (188–7.3 kDa, Bio-Rad) were electrophoresed in parallel. After electrophoresis, the protein was transferred from the gel to a poly(vinylidene difluoride) (PVDF) membrane (ATTO, Tokyo).
The membranes were preincubated in TBST [50 mM Tris-buffered saline (pH 7.6) containing 0.05% Triton X-100] and 2% skim milk at 4°C overnight and were then incubated with the primary antibody for 1 h at room temperature. The primary antibody was diluted at 1:500 with TBST. The specificity of the immunoreaction was also confirmed by incubating the membranes with preabsorbed antibody. After rinsing in TBST, the membranes were incubated with protein A gold conjugate (British Biocell International, Cardiff, U.K.) for 1 h at room temperature and then visualized by using silver-enhancing reagents (British Biocell International). The protein A was diluted at 1:500 with TBST.
Immunohistochemistry.
Tilapia (15–20 g) adapted to freshwater or seawater were killed by decapitation, and the kidneys were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB) for 20 h at 4°C. The kidney was then dehydrated in ethanol and embedded in Paraplast. Serial cross sections were cut at 4-μm thickness. Pairs of adjacent sections were mounted on separate slides for subsequent parallel examination and comparison; one section was stained with anti-Na+, K+-ATPase (14) and the other section was stained with an anti-OmClC-K serum. The sections were immunohistochemically stained with the Vectastain ABC kit (Vector Laboratories) as described by Uchida et al. (15). Some sections were also stained with hematoxylin and eosin to observe the general morphology of the kidney.
Electron Microscopic Observations.
Small pieces of the kidney (3 mm each) were fixed in 2% paraformaldehyde-2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 12 h at 4°C. The tissues were postfixed in 1% osmium tetroxide in the same buffer for 1 h. After dehydration in ethanol, the tissues were transferred to propylene oxide and embedded in Spurr's resin. Ultrathin sections were cut with a diamond knife and mounted on grids. The sections were stained with uranyl acetate and lead citrate, and examined a transmission electron microscope (Hitachi H-7100, Hitachi, Ibaraki, Japan).
Immunoelectron Microscopy.
The kidneys were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 12 h, and then immersed in 20% sucrose in PBS (0.1 M) for 1 h at 4°C. Then, the tissues were embedded in OCT compound (Sakura Finetek, Torrance, CA), and frozen in a freezer. For immunoelectron microscopy, sections (20 μm thick) of the kidney were cut with a cryostat (Leica CM1100, Leica Microsystems, Wetzlar, Germany), and mounted on gelatin-coated slides. The sections were stained by the ABC method. After staining, the sections were washed in 0.1 M PBS, postfixed with 1% osmium tetroxide, and dehydrated in ethanol. Then, the tissues were embedded in Spurr's resin. Ultrathin sections were cut with a diamond knife and examined as described above without poststaining.
Results
cDNA Cloning and Sequence Analysis.
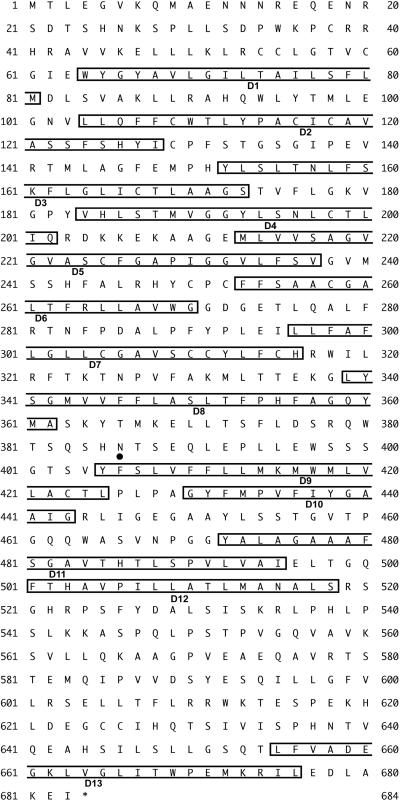
RT-PCR was performed with tilapia kidney RNA by using degenerate primers designed from consensus sequences of several ClC-K chloride channels. A single PCR product with the expected length (≈280 bp) was obtained. By using this PCR product as a probe, we screened the cDNA library from freshwater-adapted tilapia kidney and obtained a 2.9-kbp clone (GenBank accession no. AB075525). The ORF of this clone consisted of 2,052 nucleotides, resulting in a 684-aa protein with a molecular mass of ≈75 kDa (Fig. 1). The predicted amino acid sequence of this clone was highly homologous to rat ClC-K1 (41%), ClC-K2 (43%), and a Xenopus ClC-K (xClC-K) (45%) (Fig. 2A). Therefore, this clone was designated as OmClC-K (O. mossambicus ClC-K). The hydropathy analysis of the predicted protein (Fig. 2B) showed the presence of at least 13 hydrophobic regions, which may represent transmembrane domains, as in other members of the ClC family (16). The Asn-386 of OmClC-K located between hydrophobic regions D8 and D9 (Fig. 1) was a highly conserved glycosylation site among all ClC chloride channels identified to date. A consensus sequence for phosphorylation by cAMP-dependent protein kinase (Ser-546) also existed. To establish a possible evolutionary relationship among the ClC chloride channels, a phylogenetic tree was constructed by using the neighbor-joining method (Fig. 2A). A sequence comparison with other members of the ClC family showed that OmClC-K is closest to the ClC-K subset.
Fig 1.
Amino acid sequence of OmClC-K. Boxed amino acid sequences labeled D1 to D13 are similar to the hydrophobic domains previously characterized in other members of the ClC family. •, potential N-glycosylation site.
Fig 2.
(A) Similarity of the OmClC-K protein sequence to other members of the ClC family. A phylogenic tree was constructed by comparing the amino acid sequences of other members of ClC chloride channels by using the neighbor-joining method. The yeast (Saccharomyces cerevisiae) ClC chloride channel, scClC, was regarded as an outgroup. GenBank accession nos. are: ClC-0 (Torpedo mamorata), X56758; ClC-1 (rat), X62894; ClC-2 (rat), X64139; xClC-K (Xenopus laevis), AJ011385; ClC-K1 (rat), D13927; ClC-K2 (rat), D26111; ClC-6 (mouse), NM011929, ClC-7 (mouse), NM011930; ClC-4 (rat), Z36944; ClC-3 (rat), D17521; OmClC-3 (O. mossambicus), AF182215; ClC-5 (rat), Z56277; xClC-5 (X. laevis), Y09940; OmClC-5 (O. mossambicus), AF182216. (B) Hydropathy plot of OmClC-K by the Kyte–Doolittle method (window size, 12 residues). Upper lines labeled 1–13 are deduced transmembrane domains.
Tissue Distribution.
Tissue distribution of OmClC-K was examined by RNase protection assay with total RNA from freshwater- and seawater-adapted tilapia tissues. Protected bands migrated at 150 bp. As shown in Fig. 3, the expression was restricted to the kidney of freshwater-adapted tilapia. No detectable signal occurred in the other tissues of freshwater and seawater tilapia.
Fig 3.
Tissue distribution of OmClC-K in freshwater (A)- and seawater (B)-adapted tilapia by RNase protection assay. Probes were incubated with 20 μg of total RNA from yeast (control), and RNase protection analysis of 20 mg of total RNA isolation from gill, kidney, urinary bladder, intestine, stomach, liver, spleen, skeletal muscle, and brain of freshwater- and seawater-adapted tilapia. Arrows indicate the predicted size (150 nt) of protected fragments.
Expression of OmClC-K in Xenopus Oocytes.
After 2–3 days of incubation of Xenopus oocytes injected with the cRNA of OmClC-K, two-electrode voltage clamping was performed. However, we were unable to detect chloride current with OmClC-K. Injecting large amounts of cDNA, in contrast, yielded chloride current which activated slowly upon strong depolarization (>100 mV) (data not shown).
Renal Morphology of Tilapia, O. mossambicus.
The kidney of tilapia is composed of numerous nephrons arranged in an irregular pattern. The nephron largely consists of a renal corpuscle and proximal and distal tubule segments (Fig. 4A). The distal segment is formed by the cuboidal cells with scanty microvilli and basally located nuclei (Fig. 4B). The epithelial cells of the proximal tubule are tall columnar cells with a centrally located nucleus and dense brush border on the apical side (Fig. 4C).
Fig 4.
Structure of tilapia kidney. (A) Kidney section stained with hematoxylin and eosin. g, glomerulus; p, proximal tubule; d, distal tubule. (Scale bar = 50 μm.) Electron micrographs of epithelial cells in the distal tubule (B) and proximal tubule (C). (Bar = 1 μm.)
Western Blot Analysis.
Membrane fractions prepared from the kidney of freshwater- and seawater-adapted tilapia were analyzed by Western blot with the OmClC-K antibody (Fig. 5). The antibody recognized a major protein band with an approximate size of 90 kDa in kidney epithelia of freshwater-adapted tilapia. However, the immunoreactive band was not identified in seawater fish. Immunoreactivity disappeared after pretreatment of the antibody with the synthetic oligopeptide.
Fig 5.
Western blot analysis for OmClC-K protein expressed in tilapia kidney adapted to fresh water (FW) and seawater (SW). The membranes were incubated with an antibody specific for OmClC-K. Immunoreactivity of freshwater-adapted tilapia kidney disappeared after coincubation of the antibody with the synthetic oligopeptide (Abs).
Light Microscopic Immunohistochemistry.
The light microscopic immunocytochemistry showed that OmClC-K-immunoreactive cells were located in a restricted segment of the tubules in freshwater-adapted tilapia (Fig. 6A). Observations on the adjacent sections revealed that OmClC-K and Na+, K+-ATPase-immunoreactive cells were in the same nephron tubules (Fig. 6B). However, these immunoreactive cells were not observed in any renal tubules of seawater-adapted tilapia (Fig. 6 C and D).
Fig 6.
Adjacent sections of the kidney, stained with OmClC-K antibody (A and C) and Na+, K+-ATPase antibody (B and D), in freshwater (A and B)- and seawater (C and D)-adapted tilapia. OmClC-K and Na+, K+-ATPase immunoreactive cells were located in the same nephron tubules of freshwater-adapted tilapia, but no immunoreactive signal occurred in the nephrons of seawater-adapted tilapia. (Bar = 100 μm.)
Electron Microscopic Observations of OmClC-K-Immunoreactive Cells.
Ultrastructural observations revealed that OmClC-K-immunoreactive cells had a rich population of mitochondria and an extensive labyrinth of basolateral membrane infoldings in the cytoplasm (Fig. 7). Immunoreaction of OmClC-K was recognizable on the labyrinthine structure and rarely observed in other parts of the cells (Fig. 7). However, immunoreaction of OmClC-K was not detected in nephron tubules of seawater-adapted tilapia (data not shown).
Fig 7.
Immunoelectron micrographs of the distal tubule stained with anti-OmClC-K in the tilapia kidney adapted to freshwater. Immunostaining (arrows) is located mainly on the infolded basolateral membrane. m, mitochondria. (Bar = 1 μm.)
Discussion
The kidney, gills, and intestine are important organs for osmoregulation in teleosts (17). Morphology and physiology of the gill chloride cells have been studied extensively (18–20). Recently, various ion transporters have been identified in the chloride cells (21–24). However, the ion-transport mechanisms in the kidney and intestine are less understood. To our knowledge, no attempt has been made thus far to identify ion transporters in the kidney of teleost species. In this study, we have identified a kidney-specific chloride channel, OmClC-K, in the kidney of teleost fish. We have shown here that OmClC-K is expressed specifically in the kidney of freshwater-adapted tilapia. The sequence comparison and cellular localization demonstrated that the chloride channel that we had cloned was a tilapia homologue of the mammalian ClC-K chloride channel.
ClC-K chloride channels belonging to the ClC chloride channel family are predominantly expressed in the kidney. Since the initial cloning of the ClC family, the identification of ClC-0 from the electric ray, nine mammalian ClCs have been cloned. ClC-1 is established as the skeletal muscle chloride channel that controls the excitability of the muscle fiber (25). The ubiquitously expressed ClC-2 can be activated by cell swelling in oocytes and possibly plays a role in the regulation of cell volume (26). ClC-3 (27), -4 (28), and -5 (29, 30) constitute a subbranch of this gene family. ClC-3 was recently shown to be a volume-regulated chloride channel (31), and loss-of-function mutations of human ClC-5 were shown to result in X-linked recessive nephrolithiasis (32). These channels have been cloned not only from mammals, but also from lower vertebrates, bacteria, and some plants. We have also cloned OmClC-3 and OmClC-5 (homologue of mammalian ClC-3 and ClC-5) from tilapia gills (13). The expression of OmCLC-5 is restricted to the osmoregulatory organs such as the gill, kidney, and intestine. It is likely that OmCLC-5 acts as a chloride channel and is responsible for osmoregulation of tilapia. Therefore, the ClC chloride channels are critical contributors of chloride transport in tissues of various species.
The present study demonstrated that the expression of OmClC-K mRNA was restricted to the kidney of freshwater-adapted tilapia (Fig. 3), as well as the protein expression (Fig. 5). Therefore, OmClC-K has an essential role for freshwater adaptation in tilapia. Moreover, the expression of OmClC-K protein is restricted to a specific nephron segment of freshwater tilapia (Fig. 6). These segments of the tilapia nephron are readily distinguishable as distal segments according to the cell size, shape, and presence of brush border in the apical surface of the epithelial cells. In immunoelectron microscopy, immunoreactive nephrons comprised low columnar cells with scanty microvilli on the apical surface. The basolateral membrane infoldings were extensive and were visualized by the immunoreaction of OmClC-K. These features concur with the general characteristics of the distal tubule segment in teleost kidney (33, 34). These results indicate that OmClC-K protein is expressed in the basolateral membrane of the distal tubules.
Nishimura et al. (2) found by a perfusion technique that the distal tubule of the freshwater-adapted rainbow trout was nearly impermeable to water, whereas net chloride absorption was demonstrated. This finding suggests that the distal tubule acts as a diluting segment that helps to excrete excess water as dilute urine by extracting only NaCl from the forming urine, as shown by the mammalian TALH. Recently, Kobayashi et al. (12) demonstrated that ClC-K2, a mammalian homologue of OmClC-K, was expressed in the TALH, where it is localized in the basolateral membrane. The localization of ClC-K2 to this nephron segment strongly implies that ClC-K2 confers the basolateral Cl− conductance in the TALH, where the Cl− is taken up by Na-K-2Cl cotransporter at the apical membrane (5).
We demonstrated that immunoreaction of Na+, K+-ATPase was observed on the same tubules of freshwater-adapted tilapia that were immunoreactive to OmClC-K (Fig. 6). Thus, the ultimate driving force for Cl− reabsorption across the epithelium may be Na+ electrochemical gradient established by Na+, K+-ATPase located in the same cell. It is most probable that Cl− enters the cell across the apical membrane by way of Na-K-2Cl cotransport as in other Cl− transporting epithelia, although the apical Na-K-2Cl cotransporter in the distal tubule has not yet been identified in the teleost. According to Nishimura et al. (2), furosemide, an inhibitor of Na-K-2Cl cotransport, added to the lumen of the distal tubule of freshwater trout markedly decreased transepithelial ion transport, whereas no change was noted when it was added to the basolateral side. These results suggest that the Cl− reabsorption mechanism of the distal tubule in freshwater tilapia is the same as that of the mammalian TALH.
To confirm the Cl−-transporting function of OmClC-K, we attempted expression of OmClC-K in Xenopus oocytes, but no Cl− current could be recorded. Several possible reasons exist to explain why OmClC-K cannot be functionally expressed as chloride channel. First, the expression system (Xenopus oocytes) may not be appropriate. Second, OmClC-K may reside in intracellular organelles, which are known to possess chloride channel as well (35–37). For OmClC-K, however, immunoreaction has been shown in the basolateral plasma membrane (Fig. 7). Finally, a possible explanation for the nonfunctional expression might relate to the multimeric assembly of ClC chloride channels. Biochemical and functional analyses have shown that many ClCs display a dimeric quaternary structure (38–41), suggesting that dimerization is probably a general feature of ClC's architecture. Therefore, functional expression of OmClC-K might depend on its association with another ClC subunit of as yet unknown nature.
Recently, Estevez et al. (42) identified barttin that is mutated in a form of Bartter's syndrome and acts as an essential β-subunit for human ClC-Ka and ClC-Kb (human homologue of rat ClC-K1 and ClC-K2, respectively). Bartter's syndrome comprises several closely related disorders of transepithelial transport in TALH. The gene (43) encoding the integral membrane protein barttin is mutated in a form of Bartter's syndrome that is associated with congenital deafness and renal failure. When the barttin was coexpressed with ClC-Ka or ClC-Kb in Xenopus oocytes, large Cl− currents were observed. This result indicates that barttin is the functional subunit for ClC chloride channels. Although barttin has been identified only in mammals, tilapia could also have a teleostean homologue of mammalian barttin.
The kidney of freshwater-adapted teleosts regulates the body fluid and salt balance in the aquatic environment and excretes dilute urine. Therefore, the distal tubule of teleost kidney acts as a diluting segment that helps in excreting an excess of water as dilute urine. The primitive forms of the nephron exemplified by those of the hagfish, a marine cyclostome, possess only renal corpuscles and the first segment of the proximal tubule, and their internal osmolality depends on the environmental media (44). However, in the river lamprey, a freshwater cyclostome, urine was hypoosmotic (45). Therefore, the diluting segment evolved during an early stage of vertebrate phylogeny and was likely essential for freshwater invasion and hyperosmoregulation. On the other hand, the kidney of mammals adapted to terrestrial life actively reabsorbs water to avoid dehydration. However, the diluting segment (mainly in the TALH) takes an essential role for urine concentration. Reabsorption of NaCl increases the NaCl concentration of the interstitium surrounding the TALH and that will drive water absorption from the descending limb of all nephrons (46). Consequently, the same properties of the diluting segment may be important in terms of the renal-functional evolution of the kidney from aquatic to terrestrial animals. The availability of homologous transport mediators such as ClC-K channels will be helpful in improving our understanding of molecular evolution of kidney function in relation to environmental adaptation.
Acknowledgments
We thank Dr. Christopher A. Loretz of the State University of New York at Buffalo for critical reading of the manuscript. We also thank Ms. Sanae Hasegawa of Ocean Research Institute for excellent technical assistance. This study was supported in part by Grants-in-Aid for Creative Basic Research (12NP0201) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and for Scientific Research (A) (13304063) from Japan Society for the Promotion of Science. H.M. was supported by a Research Fellowship awarded by the Japan Society for the Promotion of Science for Young Scientists.
Abbreviations
TALH, thick ascending limb of Henle's loop
Data deposition: The sequence reported in this article has been deposited in the GenBank database (accession no. AB075525).
References
- 1.Hickman C. P. J. & Trump, B. F. (1969) in Fish Physiology, eds. Hoar, W. S. & Randall, D. J. (Academic, San Diego), Vol. 1, pp. 99–239. [Google Scholar]
- 2.Nishimura H., Imai, M. & Ogawa, M. (1983) Am. J. Physiol. 244 F247-F254. [DOI] [PubMed] [Google Scholar]
- 3.Burg M. B. & Green, N. (1973) Am. J. Physiol. 224 659-668. [DOI] [PubMed] [Google Scholar]
- 4.Rocha A. S. & Pang, P. K. T. (1973) J. Clin. Invest. 52 612-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gamba G., Miyanoshita, A., Lombardi, M., Lytton, J., Lee, W. S., Hediger, M. A. & Hebert, S. C. (1994) J. Biol. Chem. 269 17713-17722. [PubMed] [Google Scholar]
- 6.Payne J. A. & Forbush, B. (1994) Proc. Natl. Acad. Sci. USA 91 4544-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho K., Nichols, C. G., Lederer, W. J., Lytton, J., Vassilev, P. M., Kanazirska, M. V. & Hebert, S. C. (1993) Nature 362 31-38. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H., Tate, S. S. & Palmer, L. G. (1994) Am. J. Physiol. 266 C809-C824. [DOI] [PubMed] [Google Scholar]
- 9.Adachi S., Uchida, S., Ito, H., Hata, M., Hiroe, M., Marumo, F. & Sasaki, S. (1994) J. Biol. Chem. 269 17677-17683. [PubMed] [Google Scholar]
- 10.Takeuchi Y., Uchida, S., Marumo, F. & Sasaki, S. (1995) Kidney Int. 48 1497-1503. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa M., Uchida, S., Yamauchi, A., Miyai, A., Tanaka, Y., Sasaki, S. & Marumo, F. (1999) Am. J. Physiol. 276 F552-F558. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi K., Uchida, S., Mizutani, S., Sasaki, S. & Marumo, F. (2001) J. Am. Soc. Nephrol. 12 1327-1334. [DOI] [PubMed] [Google Scholar]
- 13.Miyazaki H., Uchida, S., Takei, Y., Hirano, T., Marumo, F. & Sasaki, S. (1999) Biochem. Biophys. Res. Commun. 255 175-181. [DOI] [PubMed] [Google Scholar]
- 14.Ura K., Soyano, K., Omoto, N., Adachi, S. & Yamauchi, K. (1996) Zoolog. Sci. 13 219-227. [DOI] [PubMed] [Google Scholar]
- 15.Uchida K., Kaneko, T., Yamauchi, K. & Hirano, T. (1996) J. Exp. Zool. 276 193-200. [Google Scholar]
- 16.Jentsch T. J., Gunther, W., Pusch, M. & Schwappach, B. (1995) J. Physiol. 482 19S-25S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans D. H. (1979) in Comparative Physiology of Osmoregulation in Animals, ed. Maloiy, G. M. (Academic, San Diego), Vol. 1, pp. 305–390. [Google Scholar]
- 18.Uchida K., Kaneko, T., Miyazaki, H., Hasegawa, S. & Hirano, T. (2000) Zool. Sci. 17 149-160. [Google Scholar]
- 19.Perry S. F. (1997) Annu. Rev. Physiol. 59 325-347. [DOI] [PubMed] [Google Scholar]
- 20.Zadunaisky J. A. (1997) in Ionic Regulation in Animals, ed. Flick, G. (Springer, Berlin), pp. 87–105.
- 21.Suzuki Y., Itakura, M., Kashiwagi, M., Nakamura, N., Matsuki, T., Sakuta, H., Naito, N., Takano, K., Fujita, T. & Hirose, S. (1999) J. Biol. Chem. 274 11376-11382. [DOI] [PubMed] [Google Scholar]
- 22.Singer T. D., Tucker, S. J., Marshall, W. S. & Higgins, C. F. (1998) Am. J. Physiol. 274 C715-C723. [DOI] [PubMed] [Google Scholar]
- 23.Wilson J. M., Laurent, P., Tufts, B. L., Benos, D. J., Donowitz, M., Vogl, A. W. & Randall, D. J. (2000) J. Exp. Biol. 203 2279-2296. [DOI] [PubMed] [Google Scholar]
- 24.Piermarini P. M. & Evans, D. H. (2001) J. Exp. Biol. 204 3251-3259. [DOI] [PubMed] [Google Scholar]
- 25.Steinmeyer K., Ortland, C. & Jentsch, T. J. (1991) Nature 354 301-304. [DOI] [PubMed] [Google Scholar]
- 26.Thiemann A., Grunder, S., Pusch, M. & Jentsch, T. J. (1992) Nature 356 57-60. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki M., Uchida, S., Monkawa, T., Miyawaki, A., Mikoshiba, K., Marumo, F. & Sasaki, S. (1994) Neuron 12 597-604. [DOI] [PubMed] [Google Scholar]
- 28.van Slegtenhorst M. A., Bassi, M. T., Borsani, G., Wapenaar, M. C., Ferrero, G. B., de Conciliis, L., Rugarli, E. I., Grillo, A., Franco, B., Zoghbi, H. Y., et al. (1994) Hum. Mol. Genet. 3 547-552. [DOI] [PubMed] [Google Scholar]
- 29.Fisher S. E., van Bakel, I., Lloyd, S. E., Pearce, S. H., Thakker, R. V. & Craig, I. W. (1995) Genomics 29 598-606. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto H., Kawasaki, M., Uchida, S., Sasaki, S. & Marumo, F. (1996) J. Biol. Chem. 271 10210-10216. [DOI] [PubMed] [Google Scholar]
- 31.Duan D., Winter, C., Cowley, S., Hume, J. R. & Horowitz, B. (1997) Nature 390 417-421. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd S. E., Gunther, W., Pearce, S. H., Thomson, A., Bianchi, M. L., Bosio, M., Craig, I. W., Fisher, S. E., Scheinman, S. J., Wrong, O., et al. (1997) Hum. Mol. Genet. 6 1233-1239. [DOI] [PubMed] [Google Scholar]
- 33.Hwang P. P. & Wu, S. M. (1987) Bull. Inst. Zool. Acad. Sin. (Taipei) 26 271-277. [Google Scholar]
- 34.Kamunde C. N. & Kisia, S. M. (1994) Acta Biol. Hung. 45 111-121. [PubMed] [Google Scholar]
- 35.Bae H. R. & Verkman, A. S. (1990) Nature 348 637-639. [DOI] [PubMed] [Google Scholar]
- 36.Mulberg A. E., Tulk, B. M. & Forgac, M. (1991) J. Biol. Chem. 266 20590-20593. [PubMed] [Google Scholar]
- 37.Reeves W. B. & Gurich, R. W. (1994) Am. J. Physiol. 266 C741-C750. [DOI] [PubMed] [Google Scholar]
- 38.Middleton R. E., Pheasant, D. J. & Miller, C. (1994) Biochemistry 33 13189-13198. [DOI] [PubMed] [Google Scholar]
- 39.Fahlke C., Knittle, T., Gurnett, C. A., Campbell, K. P. & George, A. L., Jr. (1997) J. Gen. Physiol. 109 93-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorenz C., Pusch, M. & Jentsch, T. J. (1996) Proc. Natl. Acad. Sci. USA 93 13362-13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jentsch T. J. (2002) Nature 17 287-294. [Google Scholar]
- 42.Estevez R., Boettger, T., Stein, V., Birkenhager, R., Otto, E., Hildebrandt, F. & Jentsch, T. J. (2001) Nature 414 558-561. [DOI] [PubMed] [Google Scholar]
- 43.Birkenhager R., Otto, E., Schurmann, M. J., Vollmer, M., Ruf, E. M., Maier-Lutz, I., Beekmann, F., Fekete, A., Omran, H., Feldmann, D., et al. (2001) Nat. Genet. 29 310-314. [DOI] [PubMed] [Google Scholar]
- 44.Hickman C. P. J. & Trump, B. F. (1969) in Fish Physiology, ed. Randall, D. J. (Academic, New York), Vol. 1, pp. 91–239. [Google Scholar]
- 45.Logan A. G., Morris, R. & Rankin, J. C. (1980) J. Exp. Biol. 88 239-247. [DOI] [PubMed] [Google Scholar]
- 46.Knepper M. A. & Rector, F. C. J. (1995) in The Kidney, ed. Rector, F. C. J. (Saunders, Philadelphia), pp. 532–570.