Abstract
Objectives
To assess whether bacterial vaginosis or chlamydial infection before 10 weeks' gestation is associated with miscarriage before 16 weeks.
Design
Prospective cohort study.
Setting
32 general practices and five family planning clinics in south London.
Participants
1216 pregnant women, mean age 31, presenting before 10 weeks' gestation.
Main outcome measure
Prevalence of miscarriage before 16 weeks' gestation.
Results
121 of 1214 women (10.0%, 95% confidence interval 8.3% to 11.7%) miscarried before 16 weeks. 174 of 1201 women (14.5%, 12.5% to 16.5%) had bacterial vaginosis. Compared with women who were negative for bacterial vaginosis those who were positive had a relative risk of miscarriage before 16 weeks' gestation of 1.2 (0.7 to 1.9). Bacterial vaginosis was, however, associated with miscarriage in the second trimester at 13-15 weeks (3.5, 1.2 to 10.3). Only 29 women (2.4%, 1.5% to 3.3%) had chlamydial infection, of whom one miscarried (0.32, 0.04 to 2.30).
Conclusion
Bacterial vaginosis is not strongly predictive of early miscarriage but may be a predictor after 13 weeks' gestation. The prevalence of Chlamydia was too low to assess the risk, but it is unlikely to be a major risk factor in pregnant women.
What is already known on this topic
Miscarriages are common and associated with considerable morbidity and costs
Bacterial vaginosis is associated with miscarriage after 16 weeks' gestation and preterm birth but the role of chlamydial infection is uncertain
What this study adds
Bacterial vaginosis is not a strong predictor of miscarriage before 16 weeks' gestation but may be associated with miscarriage at 13-15 weeks' gestation
The prevalence of chlamydial infection was too low for it to be a major risk factor for miscarriage in this population of healthy pregnant women
Non-invasive screening for bacterial vaginosis and chlamydial infection by using self administered vaginal swabs is feasible in pregnant women in the community
Introduction
Miscarriage is the most common adverse outcome of pregnancy. It causes psychological and physical morbidity and incurs considerable costs to the NHS. Bacterial vaginosis is associated with miscarriage after 16 weeks' gestation and with preterm birth but its role in early clinical pregnancy loss has never been properly investigated in healthy women in the community.1,2 The effect of chlamydial infection during pregnancy is also unclear.3 It is important to know whether these infections are associated with early miscarriage because treatment might be preventive. Equally if there is no evidence of an association or possible treatment benefit, the risks related to screening and treatment may be avoided.
In the United Kingdom most women who know or suspect they are pregnant either visit their doctor or attend a family planning clinic where pregnancy testing is free; they do not usually attend a hospital antenatal booking clinic until at least 10 weeks' gestation. General practices and family planning clinics are thus the ideal setting in which to recruit healthy women in early pregnancy.
It is now feasible to test non-invasively for bacterial vaginosis and chlamydial infection. Bacterial vaginosis can be detected by using Gram's method to stain a self administered vaginal smear.1 The test is simple, cheap, sensitive, and specific.4 Chlamydial infection can be detected by using ligase chain reaction assay on a first pass urine or self administered vaginal swab.5 None of these tests have previously been used in women in early pregnancy in the community, and there are no data on the prevalence of bacterial vaginosis or chlamydial infection in this group.6,7 We aimed to test the hypothesis that the risk of clinically recognised miscarriage before 16 weeks' gestation is increased in women with bacterial vaginosis or chlamydial infection detected before 10 weeks' gestation. We also aimed to determine if the risk of miscarriage related to infection depends on duration of gestation.
Methods
Recruitment
Practices
We invited 34 general practices and five family planning clinics in south London to take part in our study. All were on the St George's or Mayday Hospital courier systems to ensure regular transport of specimens to hospital laboratories. No practices or clinics declined to take part, but two general practitioners from singlehanded practices who agreed initially did not recruit any patients: one retired shortly after enrolment, the other was too busy.
Patients
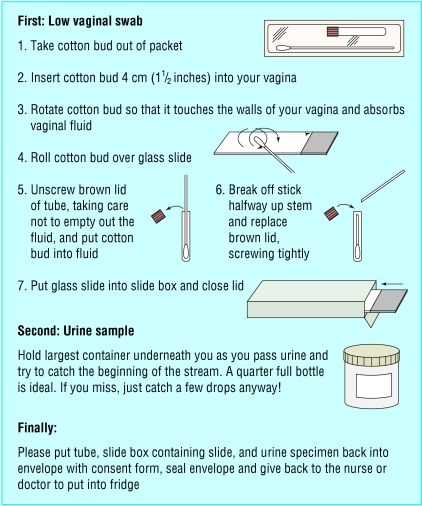
We gave the practices and clinics posters, laminated protocols, patient information sheets, and specimen packs and asked them to recruit consecutive pregnant women presenting before 10 weeks' gestation. We excluded women intending to have a termination. Women who gave informed consent were asked to provide a self administered vaginal swab, vaginal smear, and first pass urine sample immediately and to complete a confidential postal questionnaire at 16 weeks' gestation. The questionnaire asked about personal characteristics, medical history, and pregnancy outcome. To simplify recruitment, we had illustrated instructions printed on the specimen packs (fig 1) and displayed in the clinic lavatories. The specimen containers were prelabelled.
Figure 1.
Patient instructions for providing self administered vaginal swab and first pass urine
Analysis of specimens
Completed specimen packs were refrigerated and taken by courier to the local hospital laboratories. Vaginal smears were stained by Gram's method and examined for bacterial vaginosis by using Nugent's criteria.1 The slides were read by two observers (FS and PH) who were blinded to each other's results and to pregnancy outcome. Flora were graded normal (0-3, predominantly lactobacilli), intermediate (4-6, fewer lactobacilli mixed with other morphotypes), or bacterial vaginosis (7-10, few or absent lactobacilli with greatly increased numbers of Gardnerella vaginalis, other morphotypes, or both). When the observers disagreed, the slide was reviewed until consensus was reached.
Swabs and urine samples were stored at –70°C. They were tested for Chlamydia by ligase chain reaction assay (Abbott Diagnostics, Maidenhead). Positive results were confirmed by direct immunofluorescence (Syva MicroTrak, CA). Women were defined as Chlamydia positive if they had a confirmed positive result on either a swab or a urine specimen. Those doing the tests (BT, FS, and PH) were blind to the identity of the participants.
Pregnancy outcome
Gestation was calculated from the first day of the last menstrual period, if known, and modified when necessary after ultrasound examination. A miscarriage was defined as any report of clinically recognised miscarriage after a positive pregnancy test that occurred before 16 weeks' gestation. We chose 16 weeks as a cut-off point as our study was designed to investigate early miscarriage. At 16 weeks' gestation we informed the women's general practitioners or doctors at the family planning clinics of the results of the infection screen. Our study was approved by the St George's Healthcare and Mayday Hospital ethics committees.
Sample size calculations and statistical methods
We assumed that the prevalence of bacterial vaginosis was 15%, miscarriage 10%, and chlamydial infection 5%.1,8,9 The main relation under investigation was between bacterial vaginosis and miscarriage. A sample size of 1121 women would allow us to detect a relative risk of miscarriage of 2.1 for bacterial vaginosis and 3.0 for chlamydial infection, with 90% power and 5% significance.
We compared women who were positive for bacterial vaginosis with the combined group of those who were negative or intermediate for bacterial vaginosis. We used Cox regression to calculate the relative risk of miscarriage in women with bacterial vaginosis compared with those who were negative or intermediate for bacterial vaginosis. This allowed for variable gestation at recruitment or miscarriage. We included women who decided to have a termination of pregnancy after enrolment if the gestation at termination was known, censoring at that time. We excluded from the analysis women in whom the gestation at termination was not known. We adjusted for recognised risk factors for miscarriage: increasing age, history of miscarriage, and smoking during pregnancy.10–12 To test the assumption of constant relative risk across gestational age we fitted group by time interaction terms to the model.
Results
Between June 1998 and July 2000, 1216 pregnant women, mean age 31 (range 16-48), were recruited; 1126 were from general practices and 90 from family planning clinics. A further 38 women were excluded because ultrasound dating showed gestation was 10 weeks or more at enrolment (31 women), consent forms were not completed (4), all specimens were missing (2), and the woman was not pregnant (1). The median gestation at recruitment was 49 days (range 12-69). Ascertainment of pregnancy outcome at 16 weeks was 99.8% (1214 of 1216). Overall, 88% (1069) of women returned the questionnaire, 3% (39) were interviewed by telephone, and for the remaining 9% (106) pregnancy outcome was obtained from general practice or hospital records. Of the 1107 women who responded, 78% (867) described their ethnicity as white, 7% (75) as Afro-Caribbean, 4% (44) as black African, 6% (65) as of Indian subcontinent origin, and 5% (56) as other ethnic groups.
Prevalence of bacterial vaginosis and chlamydial infection before 10 weeks' gestation
We obtained slides of adequate quality for analysis from 1201 women; eight slides were inadequate and seven were missing. The prevalence of bacterial vaginosis was 14.5% (174 women, 95% confidence interval 12.5% to 16.5%). A further 4.5% (54) of women were intermediate for bacterial vaginosis. Bacterial vaginosis was more common in women under 25, those of Afro-Caribbean or black African ethnic group, those in social classes 3-5, single women, those who had previously used oral contraception or none, those who smoked during pregnancy, those with a history of termination, and those with concurrent chlamydial infection (table 1).
Table 1.
Characteristics of 1201 pregnant women according to bacterial vaginosis status at recruitment
| Characteristic
|
No (%)
|
Prevalence (proportion) of bacterial vaginosis among women
|
Relative risk (95% CI)
|
Age adjusted relative risk (95% CI)¶
|
|
|---|---|---|---|---|---|
| Women with characteristic
|
Women without characteristic
|
||||
| Age <25 (n=1201) | 150 (12.5) | 22.6 (34/150) | 13.3 (140/1051) | 1.7 (1.2 to 2.5)** | — |
| Afro-Caribbean or black African (n=1096) | 116 (11.1) | 33.6 (39/116) | 11.1 (109/980) | 3.0 (2.1 to 4.4)*** | 2.9 (2.0 to 4.2)*** |
| Social class 3 to 5† (n=1036) | 415 (40.0) | 16.5 (68/415) | 10.5 (65/621) | 1.6 (1.1 to 2.2)** | 1.5 (1.0 to 2.1)* |
| Single, widowed, or divorced (n=1095) | 94 (8.58) | 28.7 (27/94) | 11.6 (116/1001) | 2.5 (1.6 to 3.8)*** | 2.3 (1.5 to 3.7)*** |
| Previous oral contraception or none (n=1085) | 683 (62.9) | 14.8 (101/683) | 10.7 (43/402) | 1.4 (1.0 to 2.0) | 1.4 (1.0 to 2.0) |
| No previous pregnancies (n=1094) | 388 (35.5) | 11.1 (43/388) | 15.2 (107/706) | 0.7 (0.5 to 1.0) | 0.7 (0.5 to 1.0) |
| Smoked during this pregnancy‡ (n=786) | 117 (14.9) | 17.1 (20/117) | 10.8 (72/669) | 1.6 (1.0 to 2.6) | 1.4 (0.9 to 2.4) |
| Previous termination of pregnancy (n=1087) | 270 (24.8) | 21.9 (59/270) | 10.7 (87/817) | 2.1 (1.5 to 2.9)*** | 2.0 (1.5 to 2.8)*** |
| Previous miscarriage§ (if ever been pregnant n=700) | 225 (32.1) | 16.0 (36/225) | 14.9 (71/475) | 1.1 (0.7 to 1.6) | 1.1 (0.7 to 1.6) |
| Previous preterm birth at <37 weeks (if ever been pregnant n=700) | 39 (5.6) | 10.3 (4/39) | 15.2 (101/661) | 0.7 (0.3 to 1.8) | 0.7 (0.3 to 1.8) |
| Concurrent chlamydial infection (n=1199) | 29 (2.4) | 44.8 (13/29) | 13.8 (161/1170) | 3.3 (1.9 to 5.7)*** | 2.8 (1.6 to 5.0)*** |
P<0.05, **P<0.01, ***P<0.001.
For women who were unemployed or students, partner's social class was used when available.
Questions on smoking were omitted from questionnaires at start of study.
13 women had had ⩾3 miscarriages.
Adjusted for age <25 or ⩾25.
The overall prevalence of chlamydial infection was 2.4% (29 of 1214, 1.5% to 3.3%), but 8.5% (13 of 152, 4.1% to 12.9%) in women under 25 and 14.3% (6 of 42, 3.7% to 24.9%) in teenagers. In two women both specimens for Chlamydia testing were missing.
Miscarriage related to bacterial vaginosis or chlamydial infection
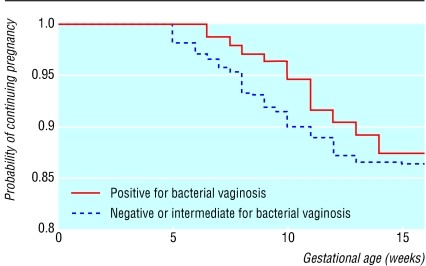
Overall, 121 women (10.0%, 8.3% to 11.7%) miscarried before 16 weeks' gestation. One woman had an ectopic pregnancy and was excluded from the analysis. Twenty two women decided after enrolment to have a termination of pregnancy; 10 of 174 women with bacterial vaginosis compared with 12 of 1025 without (relative risk 4.9, 2.2 to 11.2). The relative risk of miscarriage before 16 weeks in women who were positive for bacterial vaginosis compared with those who were negative or intermediate was 1.15 (0.70 to 1.87) (table 2). This relative risk did not change substantially when adjusted for risk factors for miscarriage—age over 37 (1.11, 0.68 to 1.81), history of miscarriage (1.34, 0.76 to 2.35), and smoking during pregnancy (1.04, 0.54 to 2.03)—or when adjusted for concurrent chlamydial infection (1.20, 0.74 to 1.97). Bacterial vaginosis was, however, associated with miscarriage in the second trimester at 13-15 weeks (3.45, 1.16 to 10.29). The interaction between gestational age at miscarriage and bacterial vaginosis status on the Cox regression was significant (χ22df=13.10; P<0.01). Figure 2 shows the survival of pregnancy by bacterial vaginosis status.
Table 2.
Outcome of pregnancy at 16 weeks' gestation related to bacterial vaginosis status at recruitment in 1189 women. Values are numbers (percentages) unless stated otherwise
| Bacterial vaginosis status
|
Relative risk (95% CI) of miscarriage if positive†
|
|||
|---|---|---|---|---|
| Positive (n=170)
|
Intermediate (n=54)
|
Negative (n=965)
|
||
| Any miscarriage | 19 (11.2) | 5 (9.3) | 97 (10.1) | 1.15 (0.70 to 1.87) |
| Gestation time of miscarriage: | ||||
| <10 weeks | 4 (2.4) | 2 (3.7) | 45 (4.7) | 0.53 (0.19 to 1.47) |
| 10-12 weeks | 10 (5.9) | 1 (1.9) | 45 (4.7) | 1.32 (0.67 to 2.62) |
| 13-15 weeks | 5 (2.9) | 2 (3.7) | 7 (0.7) | 3.45 (1.16 to 10.29)* |
| Still pregnant at 16 weeks | 144 (84.7) | 48 (88.9) | 863 (89.4) | |
| Termination of pregnancy at known date‡ | 7 (4.1) | 1 (1.9) | 5 (0.5) | |
P<0.05.
Calculated with Cox regression. Women positive for bacterial vaginosis compared with women negative or intermediate combined.
Date of termination unknown in further 9 women who decided to have termination after enrolment.
Figure 2.
Kaplan-Meier plot of survival of pregnancy by bacterial vaginosis status
Only one of 28 women with chlamydial infection miscarried (0.32, 0.04 to 2.30; when adjusted for bacterial vaginosis this was 0.30 (0.04 to 2.14); table 3). One woman with chlamydial infection was lost to follow up. Miscarriages before 16 weeks' gestation were more common in women over 37 and in those with a history of miscarriage.
Table 3.
Possible risk factors for miscarriage before 16 weeks' gestation
| Risk factor
|
Relative risk (95% CI) of miscarriage†
|
|---|---|
| Bacterial vaginosis positive (n=1189) | 1.15 (0.70 to 1.87) |
| Chlamydial infection (n=1202) | 0.32 (0.04 to 2.30) |
| Age >37 (n=1189) | 3.14 (1.98 to 4.98)*** |
| Previous miscarriage in women with previous pregnancy (n=697) | 1.76 (1.12 to 2.76)* |
| Smoked during this pregnancy (n=782) | 1.08 (0.60 to 1.96) |
P<0.05, ***P<0.001.
Calculated with Cox regression.
Discussion
Bacterial vaginosis is not a strong predictor of miscarriage before 16 weeks' gestation. However, the risk of miscarriage related to bacterial vaginosis status depends on length of gestation.
Strengths and weaknesses of study
Our study is unique as it was prospective and designed specifically to look at the relation between genital infection and early miscarriage in a community based cohort of healthy women. The community setting enabled us to recruit and screen women much earlier in pregnancy than studies based in hospitals. Our study is the largest of its kind to date, achieved despite difficulties of recruiting from inner city settings, and ascertainment at 16 weeks was over 99%. The women who completed questionnaires at 16 weeks' gestation were unaware of the results of their infection screen. In addition we showed that screening in primary care is feasible by self administered vaginal swabs even during pregnancy and by using only routine specimen storage and transport facilities. Finally, our finding that miscarriage was more common in women over 37 and in those with a history of miscarriage is similar to other studies.10,12
The main limitation of our study was that the low overall prevalence of chlamydial infection meant that we could not adequately evaluate any relation between Chlamydia and miscarriage. Our study was powered primarily to look at the influence of bacterial vaginosis rather than chlamydial infection on miscarriage. However, the low prevalence of chlamydial infection showed that it is unlikely to be a major risk factor for miscarriage in this population. (This may not apply to pregnant teenagers, in whom the prevalence of Chlamydia was 14%.) Our study is also the first to show that chlamydial infection in early pregnancy is associated with an almost threefold increase in the risk of bacterial vaginosis, independent of age.
One disadvantage of the community setting is that we could only assess clinically recognised miscarriage and had no information on preclinical miscarriages, ultrasound findings, or chromosomal analyses.13,14 We could not determine the timing of the biological events leading to a missed abortion. Secondly, although doctors and nurses were asked to recruit consecutively, we had no details on women not recruited. However, any recruitment bias would be unlikely to have a major influence on the relation between genital infection and miscarriage. In addition the ethnic distribution of participants was similar to that in Wandsworth in the 1991 census, and their mean age was comparable to the mean age (30) of women delivering at St George's Hospital in 1999. Finally, the omission of questions on smoking during pregnancy in the questionnaires at the start of the study meant we only had data on smoking for 74% (793 of 1069) of those who returned questionnaires. However other studies have not found smoking to be a strong risk factor for miscarriage.11,12,15
Comparison with other studies
Three hospital based studies have examined the relation between bacterial vaginosis and miscarriage in women who conceived naturally.1,2,16 These studies recruited women much later in pregnancy (9-24 weeks' gestation) than our study, and all were designed to look at preterm birth as well as miscarriage. In a prospective study of 783 women, Hay et al found a relative risk of 5.5 for miscarriage at 16-24 weeks' gestation.1 In a smaller study (228 women) Donders et al found a relative risk of 5.4 before 20 weeks' gestation.16 In a study of 1260 women mostly recruited during the second trimester, McGregor et al found a relative risk of 3.1 for miscarriage before 22 weeks' gestation.2
Two small hospital based studies have looked at bacterial vaginosis and miscarriage in the first trimester in women undergoing in vitro fertilisation, with conflicting results.13,17 Liversedge et al found no significant difference in miscarriage rates related to bacterial vaginosis status, but Ralph et al found bacterial vaginosis was associated with a twofold risk of miscarriage in the first trimester in 237 women who became pregnant after in vitro fertilisation.13,17 These women had high overall rates of both miscarriage (24%) and bacterial vaginosis (25%), and most miscarriages were preclinical, implying failure of implantation rather than loss of an established pregnancy. However when only clinically recognised miscarriages were included, as in our study, the relative risk of miscarriage before 13 weeks in women with bacterial vaginosis compared with those without bacterial vaginosis was 1.1, similar to our results.
Implications
Because bacterial vaginosis is not a strong risk factor for miscarriage before 16 weeks' gestation, it seems unlikely that screening and treatment of asymptomatic bacterial vaginosis would improve miscarriage rates, particularly in the first trimester. One reason may be because around 65-90% of clinically recognised early miscarriages are due to chromosomal abnormalities, and the occurrence of such abnormalities correlates strongly with maternal age.14 However, our results suggest that bacterial vaginosis is associated with miscarriage in the second trimester. The mechanism may be ascending spread of infection followed by an inflammatory response.16 Although in our cohort these late miscarriages comprised only 12% (14 of 121) of the total, they may be particularly traumatic. In one study 12% of women who had a miscarriage in the second trimester had a major depressive disorder in the following six months.18
Our study also shows that non-invasive screening for bacterial vaginosis and chlamydial infection using self administered vaginal swabs is feasible in pregnant women in the community. This might be important for prevention of adverse outcomes related to infection later in pregnancy and could involve collaboration between primary care and secondary care.3,19
Acknowledgments
We thank Brenda Thomas for doing the chlamydia assays, the patients, nurses, and doctors in the south London general practices and family planning clinics (Acorn Practice, 59 Addiscombe Road, Balham Health Centre, Balham Park Surgery, Barmouth Road Medical Centre, Battersea Rise Practice, Broad Green Health Centre, Bridge Lane Health Centre, Brigstock Medical Centre, Brocklebank Health Centre, Earlsfield Practice, Eversley Medical Centre, Falcon Road Medical Centre, Friends Road Medical Practice, Garratt Lane Surgery, Greyswood Practice, Lavender Hill Group Practice, Manor Health Centre, Mitcham Medical Centre, Norbury Health Centre, Parchmore Medical Centre, Parkside Group Practice, Queenstown Road Medical Centre, Southfields Group Practice, Thornton Heath Health Centre, Tod Practice, Tooting Health Clinic, Triangle Surgery, Trinity Road and Cavendish Road Partnership, Wandle Valley Group Practice, Winstanley Group Practice), Penny Oakeley, Azeem Majeed, and staff at the genitourinary clinics and microbiology laboratories at St George's and Mayday University Hospitals.
Footnotes
Funding: NHS London Regional Office Research and Development Programme.
Competing interests: PH has received payment for lectures and consultancy from Osmetech, which is developing a diagnostic test for bacterial vaginosis, 3M, which manufacturers 0.75% metronidazole vaginal gel, and Pharmacia and Upjohn, which manufacture 2% clindamycin vaginal cream. He has conducted clinical trials for which his unit has received reimbursement from Osmetech, 3M, Pharmacia, and Upjohn, and he has received financial support to attend conferences for these companies.
References
- 1.Hay P, Lamont R, Taylor-Robinson D, Morgan D, Ison C, Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ. 1994;308:295–298. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGregor J, French J, Parker R, Draper D, Patterson E, Jones W, et al. Prevention of premature birth by screening and treatment for common genital tract infections: results of a prospective controlled evaluation. Am J Obstet Gynecol. 1995;173:157–167. doi: 10.1016/0002-9378(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 3.Eschenbach DA. Chlamydia trachomatis in pregnancy. In: Bowie WR, Caldwell HD, Jones RP, Mardh P-A, Ridgway GL, Schachter J, editors. Chlamydial infections. Cambridge: Cambridge University Press; 1990. pp. 329–339. [Google Scholar]
- 4.Morgan DJ, Aboud CJ, McCaffrey MB, Bhide SA, Lamont RF, Taylor-Robinson D. Comparison of Gram-stained smears prepared from blind vaginal swabs with those obtained at speculum examination for the assessment of vaginal flora. Br J Obstet Gynaecol. 1996;103:1105–1108. doi: 10.1111/j.1471-0528.1996.tb09591.x. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson J, Carder C, Copas A, Robinson A, Ridgway G, Haines A. Home screening for chlamydial genital infection: is it acceptable to young men and women? Sex Transm Infect. 2000;76:25–27. doi: 10.1136/sti.76.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris M, Nicoll A, Simms I, Wilson J, Catchpole M. Bacterial vaginosis: a public health review. Br J Obstet Gynaecol. 2001;108:439–450. doi: 10.1111/j.1471-0528.2001.00124.x. [DOI] [PubMed] [Google Scholar]
- 7.CMO's Expert Advisory Group. London: Department of Health; 1998. Chlamydia trachomatis summary and conclusions of CMO's Expert Advisory Group.http://tap.ukwebhost.eds.com/doh/point.nsf/66b6f04bdca6defc0025693b0051ada0/a15b7b641170aa2f002566830051789c/$FILE/CHLAMYD.PDF (accessed 5 Oct 2002). [Google Scholar]
- 8.Everett C. Incidence and outcome of bleeding before the 20th week of pregnancy: prospective study from general practice. BMJ. 1997;315:32–34. doi: 10.1136/bmj.315.7099.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oakeshott P, Kerry S, Hay S, Hay P. Opportunistic screening for chlamydial infection at time of cervical smear testing in general practice: prevalence study. BMJ. 1998;316:351–352. doi: 10.1136/bmj.316.7128.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nybo Andersen A-M, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ. 2000;320:1708–1712. doi: 10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills JL. Cocaine, smoking and spontaneous abortion. N Engl J Med. 1999;340:380–381. doi: 10.1056/NEJM199902043400509. [DOI] [PubMed] [Google Scholar]
- 12.Regan L, Braude P, Trembath P. Influence of past reproductive performance on risk of spontaneous abortion. BMJ. 1989;299:541–545. doi: 10.1136/bmj.299.6698.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ralph SG, Rutherford AJ, Wilson JD. Influence of bacterial vaginosis on conception and miscarriage in the first trimester: cohort study. BMJ. 1999;319:220–223. doi: 10.1136/bmj.319.7204.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockwood C. Prediction of pregnancy loss. Lancet. 2000;355:1292–1293. doi: 10.1016/S0140-6736(00)02108-5. [DOI] [PubMed] [Google Scholar]
- 15. Harlap S, Shiono P. Alcohol, smoking and incidence of spontaneous abortions in the first and second trimester. Lancet 1980;173-6. [DOI] [PubMed]
- 16.Donders GG, Van Bulck B, Caudron J, Londers L, Vereecken A, Spitz B. Relationship of bacterial vaginosis and mycoplasmas to the risk of spontaneous abortion. Am J Obstet Gynecol. 2000;183:431–437. doi: 10.1067/mob.2000.105738. [DOI] [PubMed] [Google Scholar]
- 17.Liversedge N, Turner A, Horner PJ, Keay S, Jenkins J, Hull M. The influence of bacterial vaginosis on in-vitro fertilisation and embryo implantation during assisted reproduction treatment. Hum Reprod. 1999;14:2411–2415. doi: 10.1093/humrep/14.9.2411. [DOI] [PubMed] [Google Scholar]
- 18.Neugebauer R, Kline J, Shrout P, Skodol A, O'Connor P, Geller P, et al. Major depressive disorder in the 6 months after miscarriage. JAMA. 1997;277:383–388. [PubMed] [Google Scholar]
- 19.Carey JC, Klebanoff K, Hauth J, Hillier S, Thom E, Ernest J, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. N Engl J Med. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]