Abstract
DNA double-strand breaks (DSBs) are one of the most dangerous forms of DNA lesion that can result in genomic instability and cell death. Therefore cells have developed elaborate DSB-repair pathways to maintain the integrity of genomic DNA. There are two major pathways for the repair of DSBs in eukaryotes: homologous recombination and non-homologous end-joining (NHEJ). Until very recently, the NHEJ pathway had been thought to be restricted to the eukarya. However, an evolutionarily related NHEJ apparatus has now been identified and characterized in the prokarya. Here we review the recent discoveries concerning bacterial NHEJ and discuss the possible origins of this repair system. We also examine the insights gained from the recent cellular and biochemical studies of this DSB-repair process and discuss the possible cellular roles of an NHEJ pathway in the life-cycle of prokaryotes and phages.
Introduction
Non-homologous end-joining (NHEJ) was first discovered in the early 1980s as a rather mysterious mechanism involved in the integration of certain viruses into mammalian genomes [1–3]. Initially described as “illegitimate” or “indiscriminate” recombination, it soon became clear that this process could join together any DNA ends [2]. The term “non-homologous” came into vogue once it was realized that there was little or no homology between the DNA being incorporated and its site of integration, making this pathway distinct from the more familiar process of homologous recombination (HR) [4–7]. To illustrate the fact that the pathway is completely distinct from HR, the process has been called non-homologous “end-joining” since the mid-1990s [8–10].
It is now accepted that the NHEJ pathway acts to join breaks in genomic DNA, specifically double-stranded breaks (DSBs) [11–15]. This type of break may be introduced during normal physiological processes such as V(D)J joining of immunoglobulin genes, or may be due to errors in DNA metabolism (e.g., collapsed replication forks) or to exogenous damage (e.g., ionizing radiation). Thus, genomic DNA is frequently converted into DSBs, and efficient mechanisms are therefore required to deal with all types of breaks. Both NHEJ and HR can be utilized to repair DSBs, and eukaryotic cells use them to different extents. HR predominates in lower eukaryotes such as Saccharomyces cerevisiae [15]. In contrast, NHEJ appears to predominate in higher eukaryotes, probably as this pathway is active throughout the cell-cycle [12,14], whereas HR is limited to the late S and G2. NHEJ is perfectly suited to handling DSBs, as this pathway displays no sequence requirements for the recognition of DNA ends. This property is important when joining DSBs, as broken ends can have diverse chemical and physical characteristics.
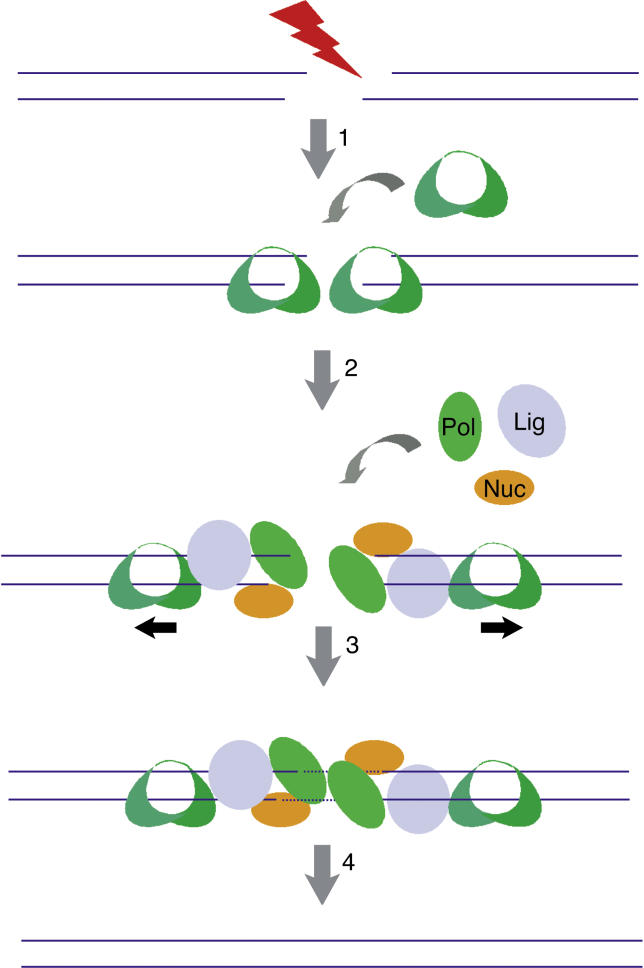
The molecular details of eukaryotic NHEJ and its various cellular functions have been summarized comprehensively elsewhere [12,13,15]. NHEJ uses a variety of proteins that process the ends and join them together (Figure 1). To summarize, once a DSB has been created, the first step in the re-joining process involves the binding of the Ku complex to the two DNA ends. The next proteins to be recruited to the complex are those that lead to resection of the ends of the DSB. In vertebrates, these include the nuclease Artemis and the polymerases pol μ and λ. The final set of steps in NHEJ lead to direct joining of the two ends [16,17] by a specific DNA ligase that restores the integrity of the DNA [18–21]. Currently, it is not clear how the repair complex disassembles from the ligated DNA. Whilst most of the proteins can leave the repair site by passive dissociation, the Ku dimer is likely to be left encircling the DNA [22]. It has been suggested that proteolytic cleavage may be the only way to remove the Ku dimer from DNA [22–24].
Figure 1. DSB Repair by the NHEJ Complex.
The Ku complex locates to the break site (step 1), where it may serve as an end-bridging and alignment factor. Following binding to the broken DNA ends, additional processing enzymes are recruited by Ku to the break site (step 2). Ku may translocate away from the ends, allowing access by other factors to the break termini. When DNA ends are non-complementary and/or are damaged, the DNA end-processing, gap-filling, and nucleolytic activities generate DNA termini, capable of being ligated, prior to ligation (step 3). Subsequently, the broken ends are joined by an NHEJ-specific DNA ligase (step 4) and the NHEJ complex dissociates.
Recently, homologues of several of the proteins involved in NHEJ have been identified in prokaryotes. Although the function of NHEJ in bacteria is still rather obscure, it now seems clear that this mechanism provides important physiological functions, at least in some bacteria. It has been hypothesized that such functions could include specialized forms of genome repair under conditions that lead to the accumulation of DSBs, or it may promote genetic diversity, particularly under difficult growth conditions where specific selection pressures may be in place. Here, we summarize current data and reflect on what these observations mean in terms of the evolution of the pathway and its function in different microorganisms.
NHEJ in Prokaryotes
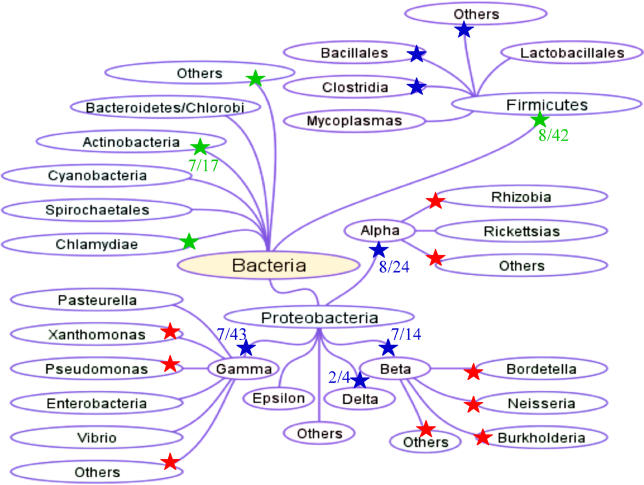
Discovery of Ku in prokaryotes and viruses. Historically speaking, all of the major DNA-repair pathways were first described in prokaryotes and, subsequently, equivalent eukaryotic counterparts were characterized. As discussed above, the NHEJ pathway was initially discovered in the eukarya and only recently has a homologous NHEJ apparatus been identified in prokaryotes [25–29]. The phylogenetic distribution of Ku genes in different bacterial species is summarized in Figure 2. These genes are most widely distributed in proteobacteria (α, β, γ, and δ families), firmicutes, and actinobacteria (Figure 2). However, it should be noted that Ku genes are not present in all bacteria and are conspicuously absent from many widely studied bacterial strains, such as Escherichia coli K12 (enterobacteria). The specific factors that link organisms possessing NHEJ proteins have yet to be identified. There is no obvious phylogenetic pattern between the bacteria that possess and those that do not possess this apparatus, leading to the possibility that NHEJ systems may most commonly be acquired by horizontal gene-transfer events. This important issue will be discussed later in the review.
Figure 2. Phylogenetic Distribution of the NHEJ Ku Protein in Bacteria.
A diagrammatic tree representation of major phyla, classes, and families of bacteria, as indicated by the relevant names is shown. Lines between the names illustrate well-established phylogenetic links within the various phyla. Stars in green indicate phyla that contain homologues of Ku. Numbers in green indicate the number of species with completed genome sequences that are within each phylum and contain one (or more) homologue(s) of Ku. Stars in blue indicate classes/orders that contain homologues of Ku. Numbers in blue indicate the number of species with completed genome sequences that are within each class that contain one (or more) homologue(s) of Ku. Stars in red indicate families that contain homologues of Ku. Note that there is no instance in which a Ku homologue is contained in all species within a phylum/class.
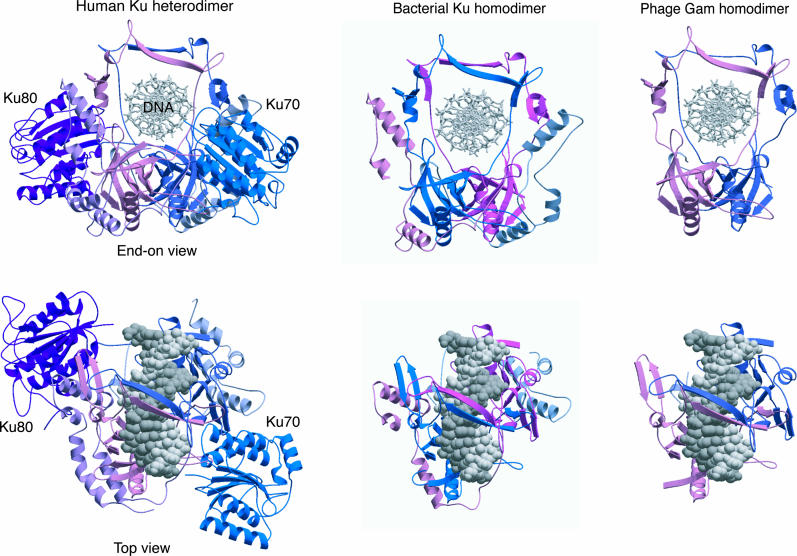
Ku is a major marker for the presence of the NHEJ pathway in many organisms, and the discovery of Ku homologues in prokaryotes led to the discovery of an NHEJ apparatus in many bacterial species [26,27]. The bacterial Ku proteins are approximately 30–40 kDa in size, in contrast to the much larger eukaryotic Ku complexes (70–80 kDa). The smaller bacterial Ku homologues represent a conserved “Ku domain” at the centre of the eukaryotic Ku complexes (amino acids 250–550 approximately; Figure 3) but lack other conserved domains present in the eukaryotic proteins [26,27]. The Ku domain of Ku70 and Ku80 is responsible for both heterodimerization and DNA binding, forming a ring-like structure through which the ends of the DNA break are threaded [22,30]. In contrast to the heterodimeric Ku complex of eukaryotes, the bacterial Ku complexes are predominantly homodimeric in structure, forming dimers that also bind preferentially to the ends of double-strand DNA [28] (Figure 3).
Figure 3. Structure of Eukaryotic, Prokaryotic, and Phage Ku Proteins.
The structure of the human Ku heterodimer bound to DNA [22] is depicted in two different orientations. Ku encircles the end of the DNA like a “nut on a bolt.” The predicted structures of the Ku from Mt and Gam homodimers are also shown. The smaller Ku homologues retain the regions required for dimerization and DNA binding but lack the N-terminal vWA domains, the C-terminal SAP (Ku70), and the DNA-PKcs binding (Ku80) domains present in the larger eukaryotic Ku proteins.
The Gam protein of bacteriophage Mu shares significant sequence and, probably, structural homology with both eukaryotic and prokaryotic Ku complexes [31]; (Figure 3). Gam orthologues are also present in a number of bacterial genomes, and these are believed to have derived from prophage insertions. In common with the prokaryotic Ku complexes, Gam is a homodimer that binds to DNA ends (Figure 3) and can protect linear Mu-phage DNA from nuclease digestion [31]. It has been proposed that Gam's role, following viral infection, is to bind to the ends of linear-phage DNA, preventing degradation by host exonucleases, and thus enhancing integration of the phage genome into the host's chromosome [31]. Interestingly, E. coli (strains expressing Gam, such as O157:H7) takes up linearized plasmid DNA much more efficiently than strains lacking this gene [32]. This strain, as well as other bacteria (e.g., Neisseria meningitidis, Haemophilus influenzae) possessing a phage-derived Gam, are naturally competent for DNA transformation. One of the major mechanisms for the evolution of bacteria involves the acquisition of genetic information by lateral gene transfer. Consequently, bacteria possessing Gam and other Ku homologues have the potential to acquire DNA directly from their surroundings and integrate this foreign genetic information into their genomes [31]. Therefore, it is possible that Ku may be instrumental in the process of prokaryotic evolution.
The similarity between the bacterial, phage, and eukaryotic Ku complexes suggests they have evolved from a common ancestral Ku, which functioned as a homodimeric complex that presumably bound to and protected the terminal ends of DNA (Figure 3). This primordial Ku most likely then evolved by merging with other functional domains to facilitate the recruitment of other factors, thus enhancing and diversifying the cellular role of Ku. Examples of probable domain acquisitions include the von Willebrant factor A (vWA) and SAP domains [26,27]. The vWA domain (Ku70/Ku80) enables Ku to recruit other proteins to sites of DNA damage, whilst the SAP motif (Ku70) binds to DNA and may prevent Ku from translocating away from the ends of DSBs [22]. The C-terminal end of Streptomyces coelicolor Ku protein shares significant homology with the SAP motif of Ku70 [26,27]. In effect, these apparent genetic acquisitions may have led to the evolution of an elementary NHEJ apparatus. Some bacterial genomes encode two distinct bacterial Ku genes within the same operon [26], possibly the result of an early gene-duplication event, giving rise to heterodimeric Ku complexes that are now the norm in eukaryotes.
Prokaryotic Ku and ligase form a two-component NHEJ repair complex. When the genome sequence for Mycobacterium tuberculosis H37Rv (Mt) was completed [33], it was immediately evident that this genome had the potential to encode four DNA ligases, referred to as Mt-LigA (NAD+-dependent ligase), Mt-LigB, Mt-LigC, and Mt-LigD (adenosine triphosphate [ATP]–dependent ligase). Although, at the time, this was thought to be rather unusual for a bacterial genome, many other prokaryotes are now known to harbour multiple DNA ligases [25,34]. Furthermore, bioinformatic analysis identified potential homologues of Ku in a similarly wide range of bacteria [26,27].
One striking observation, evident from the genetic order of the bacterial Ku genes, was that many of the Ku complexes are organized into operons containing ATP-dependent DNA ligases (homologues of Mt-LigD) [25–27]. It has now been established that this novel family of repair proteins are functional DNA ligases capable of catalyzing DSB rejoining in an ATP-dependent manner [28,29,35]. Eukaryotic Ku recruits DNA ligase IV to the termini of DSBs [16,17]. The circumstantial association of homologous genes in prokaryotic operons suggested that bacterial Ku complexes also recruit a repair ligase to DSBs [26,27]. Significantly, the end-joining activity of these ligases is specifically stimulated by the operon-associated Ku but not by eukaryotic Ku complexes, suggesting that together the Ku and ligase form a species-specific NHEJ complex [28]. Mutant strains of Bacillus subtilis bearing inactivating mutations in Ku (YkoU) and ligase (YkoV) homologues are sensitive to ionizing radiation in stationary phase, supporting the conclusion that Ku and ligase play a role in DSB repair in prokaryotes [28].
The majority of DSBs generated in vivo probably have ends that are non-complementary, and many breaks also have damaged termini. Therefore, the ends of these DSBs require processing by factors, including nucleases and DNA polymerases, to generate termini capable of being ligated (Figure 1). Eukaryotes produce many proteins that play a role in this processing [12,13,15]. In contrast, the prokaryotic NHEJ complexes appear to consist of only two repair factors (Ku and ligase) [25–27]. However, they have come up with a unique solution to compensate for the lack of additional processing factors. In addition to a ligase domain, many of the Ku-associated ligase genes encode domains with end-processing activities [25,27], including a polymerase that belongs to the Pol X family, members of which include human Pol μ, λ, and TdT, implicated in gap-filling during NHEJ. The Mt NHEJ repair ligase (Mt-LigD) possesses a remarkable variety of polymerase activities [29,36] including primase, terminal transferase, and gap-filling polymerase activities. A 3′ to 5′ single-strand DNA exonuclease activity also resides in the Mt-ligase polypeptide that is capable of removing 3′ overhangs [29,36]. Similar activities also reside in Pae-LigD, the homologous protein from Pseudomonas aeruginosa (37,38). Thus, many of the prokaryotic DNA-repair ligases are multi-functional DNA-repair enzymes that possess, with the exception of 5′-3′ exonuclease activity, all of the enzymatic activities required to process and join incompatible termini, irrespective of the ends. Indeed, DSB repair can be reconstituted in vitro simply by the addition of the Mt-Ku and ligase proteins.
Ku is not directly involved in the processing of breaks; however, it appears to be essential for recruiting ligase to the DSBs and may also modulate the order and extent of resection [29,36]. Mutations in yeast Ku and ligase genes can be complemented by ectopically expressing the bacterial NHEJ factors, confirming that reconstitution of NHEJ can also be established in vivo [28,29]. Together, these findings indicate that the prokaryotic NHEJ proteins constitute a fully functional two-component NHEJ repair complex.
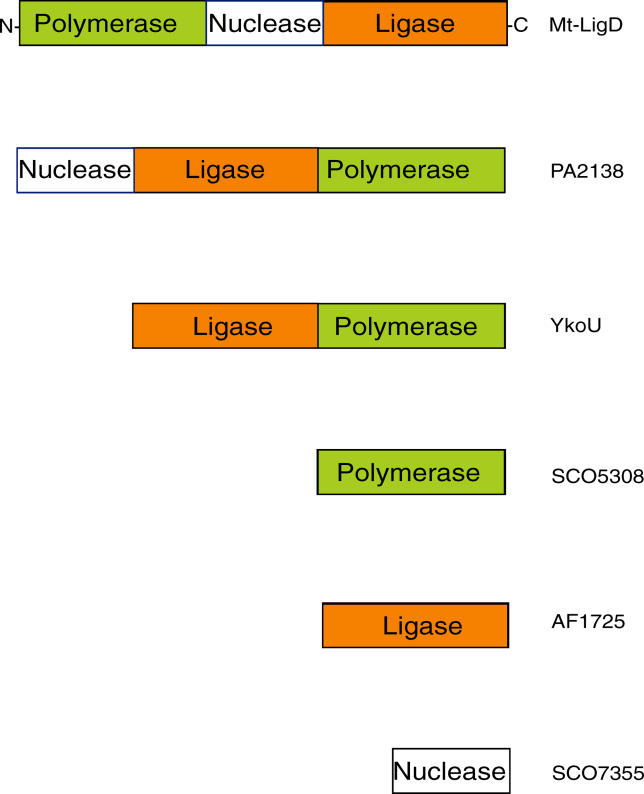
In the two best-studied examples of the end-joining ligases, Mt-LigD and Pae-LigD, the ancillary processing domains appear to be modular units [28,29,35–39]. Further analysis of genome sequences confirms this idea of distinct modules since homologous genes are organized in a variety of ways in different bacterial species (Figure 4). For example, in Mt, the polymerase and nuclease domains are distal and proximal amino-terminal extensions, respectively, of the ligase domain, and together they constitute a single polypeptide (Mt-LigD). The same activities can be found on a single polypeptide in P. aeruginosa, but the nuclease and polymerase domains are amino-terminal and carboxyl-terminal extensions, respectively, of the ligase domain. Furthermore, in B. subtilis, the polymerase domain resides at the C-terminal end of the ligase (YkoU), and the nuclease domain is absent [25,27]. Additional complexity is observed in other organisms, since the genome of S. coelicolor [26,27,40] encodes stand-alone genes for nuclease, polymerase, and ligase. Interestingly, Ku remains genetically associated with the separated genes for polymerase in S. coelicolor and ligase in Archaeoglobus fulgidus [26,27]. Thus, prokaryotes appear to have achieved a number of different solutions to providing a functional NHEJ pathway, although the manner in which these genes evolved is not yet clear. It also remains to be discovered whether or not the stand-alone ligase, nuclease, and polymerase proteins are capable of interacting in trans, reminiscent of eukaryotic NHEJ repair.
Figure 4. Domain Organization of Prokaryotic NHEJ Repair Enzymes.
Prokaryotic NHEJ DNA ligases exhibit a variety of arrangements of catalytic domains. Representative examples are presented showing the approximate location of the individual domains (amino acid): from Mt, Mt-LigD (polymerase, 1–280; nuclease, 281–460; ligase, 461–760); from P. aeruginosa, PA2138 (nuclease, 1–200; ligase, 201–550; polymerase, 551–841); and from B. subtilis, YkoU (ligase, 1–320; polymerase, 321–611). Putative “stand-alone” NHEJ proteins containing related DNA ligase, polymerase, and nuclease domains are also included—SCO refers to S. coelicolor and AF refers to A. fulgidus.
Cellular roles of the NHEJ pathway in prokaryotic organisms. Although it has been established that many bacterial species possess an NHEJ repair apparatus, the exact role and cell-cycle utilization of this pathway have yet to be elucidated. Many microorganisms that retain an NHEJ system spend much of their life-cycle in stationary phase, a stage of prolonged mitotic exit [41]. The transition from an actively dividing cell to a more quiescent cellular state is often a response to nutritional and environmental changes, and this transformation is accompanied by cellular adaptation, such as sporulation or microfilm formation. Prolonged cell-cycle exit has a number of important consequences for genome stability. During stationary phase, cells can be exposed for long periods of time to desiccation and other genotoxic agents that result in the accumulation of DSBs. Another consequence of stationary phase is that because there is a reduction in the average number of chromosomes per cell, it is expected that the ability of the cell to repair breaks via HR will be severely limited. Therefore it has been suggested that an NHEJ pathway may have evolved in bacteria to repair DSBs that arise at this particular stage of the cell-cycle [28,41].
Although deletion of the Ku and ligase in B. subtilis is not lethal, confirming that this pathway is non-essential for growth, the mutant strains do show mild sensitization to ionizing radiation in stationary phase [28]. Bacterial spores are highly resistant to irradiation and desiccation suggesting that they have an effective pathway(s) to repair resulting breaks or to prevent genome damage in the first place. As spores carry only one copy of their genome, it has been argued that an NHEJ pathway may be utilized to repair DSBs [42–44].
In some microorganisms, the onset of stationary phase results in the condensation of the chromosomal DNA into compact toroidal structures [42–45]. It has been argued that this morphology has a protective role and could also facilitate the repair of DSBs by limiting the diffusion of the termini of the breaks, thus enhancing the end-joining process, possibly by NHEJ [42–45]. A number of bacterial species that form these DNA structures, such as Deinococcus radiodurans, are capable of surviving very high levels of ionizing radiation (>15 kGy). This remarkable radio-resistance derives from the innate ability of these cells to repair hundreds of DSBs. Under conditions when chromosomal DNA is highly fragmented, it is likely to be more difficult to identify large regions of homologous DNA sequences, so HR is likely to be less effective. For example, the repair of DSB at the early stages following irradiation appears to be recA-independent [46], though ultimately, survival depends strongly on the recA HR protein.
Although Ku homologues have not been identified in D. radiodurans, arguing against the presence of NHEJ, a number of lines of evidence are emerging that may support the existence of an NHEJ-like pathway in this species. It has been reported that deletion of a unique radiation-inducible gene (PprA of D. radiodurans) leads to a significant sensitivity to ionizing radiation [47,48]. The PprA protein preferentially binds double-strand DNA carrying strand-breaks and stimulates DNA ligation, albeit in a non-specific way [48]. PprA could play a role similar to that of Ku although this remains to be established. It has also been reported that deletion of PolXDR, a member of the Pol X family, conferred sensitivity to irradiation, and this deletion strain also showed a significant delay in the restoration of genomic integrity following irradiation [49]. These reports suggest that D. radiodurans, and related bacteria, may possess a radiation-induced NHEJ apparatus that facilitates DSB repair, although the proteins that execute this repair process may be unique to these species.
Studies of mycobacteria have proved illuminating in respect of the in vivo function of the NHEJ proteins. Analysis of repaired junctions from individual NHEJ events confirmed that the DSBs are remodelled by the Mt end-joining complex [29,39]. Although LigD itself has the required activities to complete Ku-dependent NHEJ in mycobacteria, LigC may provide a back-up mechanism for LigD-independent repair of blunt-end DSBs [39]. Deletion strains of mtligC and mtligD are viable, so these gene products are not required for mycobacterial growth under laboratory conditions [35]. It is still possible that these genes influence the ability of the organism to infect their host cells. Indeed, a survey of deleted genes in 100 strains of clinically significant Mt did not find deletions in any of the ATP-dependent DNA ligases or in Mt-Ku [50]. In contrast, M. leprae has inactivated its ligase and Ku genes that could form an NHEJ apparatus, in accordance with the suggestions that this organism is downsizing its genome [51]. It has been speculated that the usefulness of NHEJ to mycobacteria is that it may allow repair of breaks induced by the genotoxic defence of human cells [39].
In addition, NHEJ may promote genetic diversity and, thus, some bacteria may have retained NHEJ since the genetic changes may provide growth advantages to bacteria under specific selection pressure, including the presence of antibiotics. NHEJ may represent a possible mechanism for generating such diversity via a process of stationary-phase mutagenesis that has been reported to occur in both prokaryotic and eukaryotic organisms [52–58]. Several molecular mechanisms have been proposed to explain the significant numbers of mutations that are produced during stationary phase and, indeed, various mechanisms may take place in different organisms or growth conditions [54–58]. There is evidence to implicate DNA damage in such types of mutation [52], but the roles of DNA-repair pathways in these processes have not been fully explored.
Current genomic-sequence data provide no obvious routes by which the prokaryotic NHEJ genes have been transferred between different organisms. Genomes of several rhizobia are now available [59] and provide perhaps the clearest indication that establishing evolutionary pathways for prokaryotic NHEJ will not be trivial. Homologues of Ku and the NHEJ ligase can be readily identified in Mesorhizobium loti and Sinorhizobium meliloti [25,26]. Using the Microbial Genome Database (http://mbgd.genome.ad.jp) [60], true homologues can also be identified in Bradyrhizobium japonicum, Rhodopseudomonas palustris, Agrobacterium tumefaciens, and Nitrobacter hamburgensis but are not present in species of Bartonella or brucella. This sporadic distribution of the genes suggests a complex phylogenetic relationship of NHEJ within the rhizobia (alpha-proteobacteria). A similar complex picture emerges when the other major phyla/classes of bacteria that contain NHEJ genes (β, γ, and δ proteobacteria, fimicutes, and actinobacteria) are considered in general (Figure 2). Although improvements in the number and depth of analysis of genome sequences might eventually alter these conclusions, the occurrence of these genes in bacteria is erratic. Given that the hosts are reasonably wide-ranging, it is not yet clear whether there are specific events or characteristics that promote acquisition and retention of the genes associated with NHEJ in prokaryotes. The most obvious explanation for this distribution is that the organisms acquired the NHEJ genes by horizontal gene transfer.
Perhaps the correlation of the possession of an NHEJ system is not determined by phylogeny but rather by the ecological environment of the bacterium. Indeed, it has been reported that among natural isolates of E. coli, the ability to engage in starvation/stress-induced mutagenesis is variable and is highly correlated with niche, and not with phylogeny [61]. Bjedov et al. showed that E. coli isolates from the gut of carnivores are more likely to be capable of stress-induced mutagenesis than isolates from the gut of herbivores [61]. This suggests that certain DNA metabolic activities, such as mutation and DNA repair, can evolve faster, depending on the niche, than the rest of the genome, which reflects phylogeny. Further studies are needed to determine if a similar environmental stress-driven evolutionary process accounts for the distribution of NHEJ genes in certain microorganisms and, if so, it may suggest what stresses (e.g., dessication, free-radical damage) promote this evolutionary process.
Conclusions
As discussed within this review, experimental evidence for the existence of functional NHEJ genes/proteins has been obtained from three different types of bacteria: bacillus, mycobacteria, and pseudomonas. However, open reading frames that encode homologous proteins exist in the genomes of a wide range of diverse bacteria, though notably not in many of the organisms that have been well studied in the laboratory, such as E. coli K12. Recent studies with the prokaryotic proteins have already unearthed some unexpected and exciting observations. The diversity within the prokaryotic systems appears to offer the potential for many more interesting findings, including the discovery of the evolutionary relationships between the prokaryotic systems and also the evolutionary relationship with the homologous eukaryotic NHEJ pathway. It is apparent that the bacterial NHEJ apparatus represents an elegantly simple experimental model system that can be exploited to delineate the molecular mechanisms that coordinate the processing and joining of DSBs by NHEJ and also provide insights into the role of this pathway in bacterial cell processes.
Acknowledgments
We thank Robbie Pitcher for comments on this manuscript. AJD is a Royal Society University Research Fellow, and AJD's work is supported by grants from BBSRC, CR-UK, AICR, and the Royal Society. RB's work has been supported by grants from AICR, Big C, and the Society for General Microbiology. Thanks are expressed to Professor Susan Rosenberg for her most helpful comments and suggestions.
Abbreviations
- ATP
adenosine triphosphate
- DSB
DNA double-strand break
- HR
homologous recombination
- Mt
Mycobacterium tuberculosis H37Rv
- NHEJ
non-homologous end-joining
- vWA
von Willebrant factor A
Supporting Information
Accession numbers The GenBank (http://www.ncbi.nlm.nih.gov/Genbank) accession numbers for the genes and gene products discussed in this paper are Gam from bacteriophage Mu (70843), Mt-LigA (15610151), Mt-LigB (15610199), Mt-LigC (15610867), and Mt-LigD (6226918), the nuclease Artemis (13872809), Pae-LigD (15597334), the polymerases pol μ (29165858) and λ (57209464), PolXDR (15805494), PprA (2257477), YkoU (2633694), and YkoV (2633695). The Swiss-Prot (http://www.ebi.ac.uk/swissprot) identification numbers for the S. coelicolor ligase, nuclease, and polymerase are SCO7345 (formerly SC4G10.24c), SCO7355 (formerly SC9H11.09c), and SCO5308 (formerly SC6G9.25), respectively, and for the Ku protein is SCO0601 (formerly SCF55.25c). The Swiss-Prot identification numbers for the Mt-LigD and and PolXDR proteins are Rv0938 and DR0467, respectively.
References
- Botchan M, Stringer J, Mitchison T, Sambrook J. Integration and excision of SV40 DNA from the chromosome of a transformed cell. Cell. 1980;20:143–152. doi: 10.1016/0092-8674(80)90242-1. [DOI] [PubMed] [Google Scholar]
- Wilson JH, Berget PB, Pipas JM. Somatic cells efficiently join unrelated DNA segments end-to-end. Mol Cell Biol. 1982;2:1258–1269. doi: 10.1128/mcb.2.10.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E, Keshet I. Indiscriminate recombination in simian virus 40-infected monkey cells. Proc Natl Acad Sci U S A. 1980;77:4861–4865. doi: 10.1073/pnas.77.8.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DB, Porter TN, Wilson JH. Mechanisms of nonhomologous recombination in mammalian cells. Mol Cell Biol. 1985;5:2599–2607. doi: 10.1128/mcb.5.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DB, Wilson JH. Relative rates of homologous and nonhomologous recombination in transfected DNA. Proc Natl Acad Sci U S A. 1985;82:3355–3359. doi: 10.1073/pnas.82.10.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thode S, Schafer A, Pfeiffer P, Vielmetter W. A novel pathway of DNA end-to-end joining. Cell. 1990;60:921–928. doi: 10.1016/0092-8674(90)90340-k. [DOI] [PubMed] [Google Scholar]
- Fishel R, Derbyshire MK, Moore SP, Young CS. Biochemical studies of homologous and non homologous recombination in human cells. Biochimie. 1991;73:257–267. doi: 10.1016/0300-9084(91)90211-i. [DOI] [PubMed] [Google Scholar]
- Goedecke W, Pfeiffer P, Vielmetter W. Nonhomologous DNA end joining in Schizosaccharomyces pombe efficiently eliminates DNA double-strand-breaks from haploid sequences. Nucleic Acids Res. 1994;22:2094–2101. doi: 10.1093/nar/22.11.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer P, Thode S, Hancke J, Vielmetter W. Mechanisms of overlap formation in nonhomologous DNA end joining. Mol Cell Biol. 1994;14:888–895. doi: 10.1128/mcb.14.2.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo PA. DNA breakage and repair. Adv Genet. 1998;38:185–218. doi: 10.1016/s0065-2660(08)60144-3. [DOI] [PubMed] [Google Scholar]
- Labhart P. Nonhomologous DNA end joining in cell-free systems. Eur J Biochem. 1999;265:849–861. doi: 10.1046/j.1432-1327.1999.00805.x. [DOI] [PubMed] [Google Scholar]
- Lieber MR, Ma Y, Pannicke U, Schwarz K. The mechanism of vertebrate nonhomologous DNA end joining and its role in V(D)J recombination. DNA Repair. 2004;3:817–826. doi: 10.1016/j.dnarep.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Weterings E, van Gent DC. The mechanism of non-homologous end-joining: A synopsis of synapsis. DNA Repair. 2004;3:1425–1435. doi: 10.1016/j.dnarep.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Critchlow SE, Jackson SP. DNA end-joining: From yeast to man. Trends Biochem Sci. 1998;23:394–398. doi: 10.1016/s0968-0004(98)01284-5. [DOI] [PubMed] [Google Scholar]
- Dudasova Z, Dudas A, Chovanec M. Non-homologous end-joining factors of Saccharomyces cerevisiae . FEMS Microbiol Rev. 2004;28:581–601. doi: 10.1016/j.femsre.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Chen L, Trujillo K, Sung P, Tomkinson AE. Interactions of the DNA ligase IV-XRCC4 complex with DNA ends and the DNA-dependent protein kinase. J Biol Chem. 2000;275:26196–26205. doi: 10.1074/jbc.M000491200. [DOI] [PubMed] [Google Scholar]
- Nick McElhinny SA, Snowden CM, McCarville J, Ramsden DA. Ku recruits the XRCC4-ligase IV complex to DNA ends. Mol Cell Biol. 2000;20:2996–3003. doi: 10.1128/mcb.20.9.2996-3003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grawunder U, Wilm M, Wu X, Kulesza P, Wilson TE, et al. Activity of DNA ligase IV stimulated by complex formation with XRCC4 protein in mammalian cells. Nature. 1997;388:492–495. doi: 10.1038/41358. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Grawunder U, Lieber MR. Yeast DNA ligase IV mediates non-homologous DNA end-joining. Nature. 1997;388:495–498. doi: 10.1038/41365. [DOI] [PubMed] [Google Scholar]
- Schär P, Herrmann G, Daly G, Lindahl T. A newly identified DNA ligase of Saccharomyces cerevisiae involved in RAD52-independent repair of DNA double-strand breaks. Genes Dev. 1997;11:1912–1924. doi: 10.1101/gad.11.15.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo SH, Jackson SP. Identification of Saccharomyces cerevisiae DNA ligase IV: Involvement in DNA double-strand break repair. EMBO J. 1997;16:4788–4795. doi: 10.1093/emboj/16.15.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- Jones JM, Gellert M, Yang W. A Ku bridge over broken DNA. Structure (Camb) 2001;9:881–884. doi: 10.1016/s0969-2126(01)00658-x. [DOI] [PubMed] [Google Scholar]
- Downs JA, Jackson SP. A means to a DNA end: The many roles of Ku. Nat Rev Mol Cell Biol. 2004;5:367–378. doi: 10.1038/nrm1367. [DOI] [PubMed] [Google Scholar]
- Weller GR, Doherty AJ. A family of DNA repair ligases in bacteria? FEBS Lett. 2001;505:340–342. doi: 10.1016/s0014-5793(01)02831-9. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Jackson SP, Weller GR. Identification of bacterial homologues of the Ku DNA repair proteins. FEBS Lett. 2001;500:186–188. doi: 10.1016/s0014-5793(01)02589-3. [DOI] [PubMed] [Google Scholar]
- Aravind L, Koonin EV. Prokaryotic homologs of the eukaryotic DNA-end-binding protein ku, novel domains in the ku protein and prediction of a prokaryotic double-strand break repair system. Genome Res. 2001;11:1365–1374. doi: 10.1101/gr.181001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller GR, Kysela B, Roy R, Tonkin L, Scanlan E, et al. Identification of a DNA non-homologous end-joining complex in bacteria. Science. 2002;297:1686–1689. doi: 10.1126/science.1074584. [DOI] [PubMed] [Google Scholar]
- Della M, Palmbos PL, Tseng HM, Tonkin LM, Daley JM, et al. Mycobacterial Ku and ligase proteins constitute a two-component NHEJ repair machine. Science. 2004;306:683–685. doi: 10.1126/science.1099824. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Jackson SP. DNA repair: How Ku makes ends meet. Curr Biol. 2001;11:R920–R924. doi: 10.1016/s0960-9822(01)00555-3. [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Weller GR, Doherty AJ, Jackson SP. The Gam protein of bacteriophage Mu is an orthologue of eukaryotic Ku. EMBO Rep. 2003;4:47–52. doi: 10.1038/sj.embor.embor709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akroyd J, Symonds N. Localization of the gam gene of bacteriophage mu and characterisation of the gene product. Gene. 1986;49:273–282. doi: 10.1016/0378-1119(86)90288-x. [DOI] [PubMed] [Google Scholar]
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- Wilkinson A, Day J, Bowater R. Bacterial DNA ligases. Mol Microbiol. 2001;40:1241–1248. doi: 10.1046/j.1365-2958.2001.02479.x. [DOI] [PubMed] [Google Scholar]
- Gong C, Martins A, Bongiorno P, Glickman M, Shuman S. Biochemical and genetic analysis of the four DNA ligases of mycobacteria. J Biol Chem. 2004;279:20594–20606. doi: 10.1074/jbc.M401841200. [DOI] [PubMed] [Google Scholar]
- Pitcher RS, Tonkin LM, Green AJ, Doherty AJ. Domain structure of a NHEJ DNA repair ligase from Mycobacterium tuberculosis . J Mol Biol. 2005;351:531–544. doi: 10.1016/j.jmb.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Zhu H, Shuman S. A primer-dependent polymerase function of Pseudomonas aeruginosa ATP-dependent DNA ligase (LigD) J Biol Chem. 2005;280:418–427. doi: 10.1074/jbc.M410110200. [DOI] [PubMed] [Google Scholar]
- Zhu H, Shuman S. Novel 3′ ribonuclease and 3′ phosphatase activities of the bacterial NHEJ protein, DNA ligase D. J Biol Chem. 2005;280:33707–33715. doi: 10.1074/jbc.M504002200. [DOI] [PubMed] [Google Scholar]
- Gong C, Bongiorno P, Martins A, Stephanou NC, Zhu H, et al. Mechanism of nonhomologous end-joining in mycobacteria: A low-fidelity repair system driven by Ku, ligase D and ligase C. Nat Struct Mol Biol. 2005;12:304–312. doi: 10.1038/nsmb915. [DOI] [PubMed] [Google Scholar]
- Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, et al. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2) Nature. 2002;417:141–147. doi: 10.1038/417141a. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Topper LM, Palmbos PL. Non-homologous end-joining: Bacteria join the chromosome break dance. Trends Biochem Sci. 2003;2:62–66. doi: 10.1016/S0968-0004(03)00005-7. [DOI] [PubMed] [Google Scholar]
- Frenkiel-Krispin D, Sack R, Englander J, Shimoni E, Eisenstein M, et al. Structure of the DNA-SspC complex: Implications for DNA packaging, protection, and repair in bacterial spores. J Bacteriol. 2004;186:3525–3530. doi: 10.1128/JB.186.11.3525-3530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander J, Klein E, Brumfeld V, Sharma AK, Doherty AJ et al. DNA toroids: Framework for DNA repair in Deinococcus radiodurans and in germinating bacterial spores. J Bacteriol. 2004;186:5973–5977. doi: 10.1128/JB.186.18.5973-5977.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin-Zaidman S, Englander J, Shimoni E, Sharma AK, Minton KW, et al. Ring-like structure of the Deinococcus radiodurans genome: A key to radioresistance? Science. 2003;299:254–256. doi: 10.1126/science.1077865. [DOI] [PubMed] [Google Scholar]
- Minsky A. Information content and complexity in the high-order organization of DNA. Annu Rev Biophys Biomol Struct. 2004;33:317–342. doi: 10.1146/annurev.biophys.33.110502.133328. [DOI] [PubMed] [Google Scholar]
- Daly MJ, Minton KW. An alternative pathway of recombination of chromosomal fragments precedes recA-dependent recombination in the radioresistant bacterium Deinococcus radiodurans . J Bacteriol. 1996;178:4461–4471. doi: 10.1128/jb.178.15.4461-4471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Earl AM, Howell HA, Park MJ, Eisen JA, et al. Analysis of Deinococcus radiodurans's transcriptional response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics. 2004;168:21–33. doi: 10.1534/genetics.104.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumi I, Satoh K, Cui S, Funayama T, Kitayama S, et al. PprA: A novel protein from Deinococcus radiodurans that stimulates DNA ligation. Mol Microbiol. 2004;54:278–285. doi: 10.1111/j.1365-2958.2004.04272.x. [DOI] [PubMed] [Google Scholar]
- Lecointe F, Shevelev IV, Bailone A, Sommer S, Hubscher U. Involvement of an X family DNA polymerase in double-stranded break repair in the radioresistant organism Deinococcus radiodurans . Mol Microbiol. 2004;53:1721–1730. doi: 10.1111/j.1365-2958.2004.04233.x. [DOI] [PubMed] [Google Scholar]
- Tsolaki AG, Hirsh AE, DeRiemer K, Enciso JA, Wong MZ, et al. Functional and evolutionary genomics of Mycobacterium tuberculosis: Insights from genomic deletions in 100 strains. Proc Natl Acad Sci U S A. 2004;101:4865–4870. doi: 10.1073/pnas.0305634101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, et al. Massive gene decay in the leprosy bacillus. Nature. 2001;409:1007–1011. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- Bridges BA. The role of DNA damage in stationary phase (‘adaptive') mutation. Mutat Res. 1998;408:1–9. doi: 10.1016/s0921-8777(98)00008-1. [DOI] [PubMed] [Google Scholar]
- Sung HM, Yasbin RE. Adaptive, or stationary-phase, mutagenesis, a component of bacterial differentiation in Bacillus subtilis . J Bacteriol 2002. 2002;184:5641–5653. doi: 10.1128/JB.184.20.5641-5653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich E, Novotny R, Kneidinger B, Holzmann V, Wintersberger U. Non-homologous end joining as an important mutagenic process in cell cycle-arrested cells. EMBO J. 2003;22:2274–2283. doi: 10.1093/emboj/cdg203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich E, Eisler H. Non-homologous end joining dependency of gamma-irradiation-induced adaptive frameshift mutation formation in cell cycle-arrested yeast cells. Mutat Res. 2004;556:201–208. doi: 10.1016/j.mrfmmm.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Rosenberg SM. Evolving responsively: Adaptive mutation. Nat Rev Genet. 2001;2:504–515. doi: 10.1038/35080556. [DOI] [PubMed] [Google Scholar]
- Hersh MN, Ponder RG, Hastings PJ, Rosenberg SM. Adaptive mutation and amplification in Escherichia coli: Two pathways of genome adaptation under stress. Res Microbiol. 2004;155:352–359. doi: 10.1016/j.resmic.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Matic I, Taddei F, Radman M. Survival versus maintenance of genetic stability: A conflict of priorities during stress. Res Microbiol. 2004;155:337–341. doi: 10.1016/j.resmic.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Puhler A, Arlat M, Becker A, Gottfert M, Morrissey JP, et al. What can bacterial genome research teach us about bacteria-plant interactions? Curr Opin Plant Biol. 2004;7:137–147. doi: 10.1016/j.pbi.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Uchiyama I. MBGD: Microbial genome database for comparative analysis. Nucleic Acids Res. 2003;31:58–62. doi: 10.1093/nar/gkg109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjedov I, Tenaillon O, Gerard B, Souza V, Denamur E, et al. Stress-induced mutagenesis in bacteria. Science. 2003;300:1404–1409. doi: 10.1126/science.1082240. [DOI] [PubMed] [Google Scholar]