Abstract
CD34+ bone marrow–derived progenitor cells contribute to tissue repair by differentiating into endothelial cells, vascular smooth muscle cells, hematopoietic cells, and possibly other cell types. However, the mechanisms by which circulating progenitor cells home to remodeling tissues remain unclear. Here we show that integrin α4β1 (VLA-4) promotes the homing of circulating progenitor cells to the α4β1 ligands VCAM and cellular fibronectin, which are expressed on actively remodeling neovasculature. Progenitor cells, which express integrin α4β1, homed to sites of active tumor neovascularization but not to normal nonimmune tissues. Antagonists of integrin α4β1, but not other integrins, blocked the adhesion of these cells to endothelia in vitro and in vivo as well as their homing to neovasculature and outgrowth into differentiated cell types. These studies describe an adhesion event that facilitates the homing of progenitor cells to the neovasculature.
Introduction
Bone marrow–derived, CD34+ progenitor cells have been shown to promote the repair of damaged tissues, offering promise for the treatment of hereditary and acquired human diseases (1–13). These cells differentiate into endothelia, hematopoietic cells, and possibly neurons, fibroblasts, and muscle (1–13). CD34+ and AC133+ progenitor cells may participate in neovascularization by differentiating into endothelial cells (1–6). Neovascularization stimulates healing of injured tissues (1–7, 14) but also promotes tumor growth and inflammatory disease (14–17). Circulating bone marrow–derived progenitor cells home to sites of neovascularization (1–7, 14–17), where they can give rise to approximately 15% of the tumor neovasculature (16). These cells may thus participate in tissue regeneration or pathogenesis (8–13). Although some studies indicate progenitor cells differentiate into a variety of cell types (1–13), others suggest they may fuse with differentiated hepatocytes or muscle cells, creating the appearance of differentiation (18, 19). Nevertheless, the evidence that these cells promote tissue repair is strong, although the molecular mechanism(s) that promote the homing and recruitment of bone marrow–derived progenitor cells to remodeling tissues remain unclear.
Integrins and their ligands promote endothelial cell migration and survival during angiogenesis (20). However, our studies demonstrate that the fibronectin receptor α4β1 plays 2 unique roles during angiogenesis. We recently found that this integrin mediates intercellular adhesion and survival of endothelial cells and pericytes during blood vessel formation in vivo and that this integrin is required for angiogenesis (21). However, integrin α4β1 is best known as a lymphocyte integrin that mediates adhesion of circulating lymphocytes to VCAM expressed on activated endothelia in inflamed tissues, thereby promoting extravasation of lymphocytes into inflamed tissue (22, 23). In the studies presented here, we found that integrin α4β1 promotes the homing of circulating bone marrow–derived progenitor cells to the α4β1 ligands, VCAM, and cellular fibronectin, which are expressed on neovasculature. By regulating the homing of these cells, this integrin also promotes their participation in angiogenesis and tumor growth.
Results
CD34+ cells home to the tumor periphery.
Bone marrow–derived progenitor cells have been shown to contribute to tumor neovasculature and other tissue repair processes by differentiating into endothelial cells, hematopoietic cells, and other cell types (1–7). To understand how progenitor cells as well as other circulating cells home to remodeling tissues, such as the tumor microenvironment, we initially employed real time–intravital microscopy to study the movement of circulating human cells transplanted into mice with breast carcinomas (Figures 1 and 2). CD34+ progenitor cells were isolated by magnetic bead affinity selection from human PBMCs; the purified CD34+ comprised approximately 0.1% of the total PBMC population and was 98% pure (Figure 3A). CD34+-positive cells were labeled with a red fluorescent cell tracking dye, 5-and-6-4-chloromethylbenzoylamino-tetramethylrhodamine (CMTMR). One million fluorescent CD34+ cells per mouse were injected into the tail veins of nude mice implanted with murine N202 breast carcinoma spheroids on mammary fat-pads under dorsal skinfold chambers (Figure 1A). Intravital microscopy enabled us to track cell homing within tumors and adjacent normal tissue. Tumors (Figure 1B) and associated blood vessels (Figure 1C) were visible in the transparent chambers, permitting analysis of real-time cell movement within the vasculature. Within a few minutes after intravascular injection, human bone marrow–derived CD34+ cells were observed circulating within the tumor vasculature. Approximately 10 minutes after injection, fluorescent cells were observed first rolling, then arresting in blood vessels at the tumor periphery (Figure 1D), but not at the tumor center (Figure 1D), neighboring breast fat-pad, or uninvolved skin (not shown). Cell homing was not dependent on the density of blood vessels in the tumor tissue, as extensive vascularization was observed in the center and periphery of the tumor (Figure 1D). Within 15 minutes after injection, no further fluorescent cells were observed arresting in blood vessels. The arrested cells remained in the tumor periphery during the 30-minute initial observation period. From 5- to 10-fold more cells arrested in the tumor periphery than in the tumor center or in the neighboring fat-pad (Figure 1E).
Figure 1.
Human CD34+ progenitor cells home to peripheral tumor vasculature. (A) Nude mice with GFP-N202 breast carcinomas under dorsal skinfold transparent chambers are injected with labeled progenitor cells. (B) Real-time video microscopy: GFP tumors in transparent chambers. Magnification, ×10. (C) Tumor vasculature is visible in the transparent chambers. Magnification, ×100. (D) Two images each of peripheral and central tumor vascular beds at 15 minutes after injection in animals injected with CMTMR-labeled CD34+ cells (arrowheads). Magnification, ×200. Blood vessels are observed as dark channels (arrows). (E) Average number of CMTMR CD34+ cells in the tumor center, tumor periphery, and fat-pad per ×200 field ± SEM. *P < 0.0019. (F) Five micron cryosections of the same tumors from animals injected with CD34+ cells were fixed in acetone, then incubated with rat anti-mouse CD31 and goat anti-rat FITC antibodies and photographed. Magnification, ×200. Arrowheads indicate CD34+ cells. Arrows indicate CD31+ blood vessels. Scale bar: 50 μm. (G) Average number of CD34+ cells per ×200 microscopic field in 5 μm cryosections. **P < 0.015. (H) Cryosections from F observed at ×630 magnification indicate CD34+ cells (arrows) are found adhering to the blood vessel wall or within tissues near blood vessels (arrowheads).
Figure 2.
Human CD34– mononuclear cells also home to peripheral tumor vasculature. (A) Intravital microscopy of CMTMR-labeled CD34– mononuclear cells homing to tumor vessels. Shown are peripheral and central tumor vascular beds as well as neighboring fat-pad at 20 minutes after injection in animals injected with CMTMR-labeled CD34– mononuclear cells (arrowheads). Magnification, ×200. Tumor center and periphery at 72 hours after injection of cells are also shown. (B) Average number of CMTMR CD34– cells in the tumor center, tumor periphery, and fat-pad per ×200 field ± SEM after 15 minutes. *P < 0.0009. (C) Postmortem tumor section shows CD34– cells (red; arrows) at the GFP+ (green) tumor periphery (left). Magnification, ×400. Also shown are CD31+ blood vessels (red) at the GFP+ (green) tumor periphery (right). Magnification, ×200.
Figure 3.
Integrin α4β1 mediates adhesion of human CD34+ cells to endothelia. (A) FACs profiles for CD34, CD133, and α4β1, α5β1, αvβ3, αvβ5, and β2 integrins on CD34+ cells and HUVECs. FACS profile in outline represents fluorescence of isotype control antibody. Percentage of positive cells is indicated by number in right-hand corner of each profile. hCD34+, human CD34+. (B and C) CD34+ cell adhesion to EC monolayers in the presence of medium, anti-α4β1, α5β1, αvβ3, αvβ5, or β2, or rsVCAM. (B) Average number of CD34+ cells adhering to EC per ×200 microscopic field ± SEM. *P < 0.002 (anti-α4β1) and P < 0.005 (rsVCAM). (C) Red fluorescent CD34+ cells adhering to ECs as in B. cIgG indicates control, isotype-matched immunoglobulin. (D) FACs profile of VCAM expression on cultured HUVECs. (E) Average number of cells adhering to recombinant CS-1 fibronectin fragments in the presence of culture medium or medium with antibody antagonists of integrin α4β1 (HP2/1) or integrin α5β1 (JBS5) per ×200 microscopic field ± SEM.
Postmortem analysis of thin sections of tumors isolated 60 minutes after cell injection confirmed that CD34+ cells had selectively arrested in the tumor periphery rather than tumor center (Figure 1, F and G; Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI24751DS1). Importantly, CD34+ cells were observed adhering to vessel walls within the lumens of CD31+ blood vessels, within vessel walls, and near the vessels within the tumor parenchyma (Figure 1H). These studies indicate that bone marrow–derived progenitor cells home selectively to the growing peripheral tumor vasculature and transmigrate into tumor tissue, suggesting that specific cell attachment and migration mechanisms may play a role in this homing response.
To determine whether CD34– circulating cells (such as monocytes) also arrest within the peripheral vasculature of tumors, CD34– mononuclear cells isolated from peripheral blood were labeled with a red fluorescent cell tracking dye and were injected into circulation of mice implanted with GFP+N202 breast carcinoma spheroids. Fluorescent cells were observed first rolling, then arresting in blood vessels at the tumor periphery (Figure 2A) but not at the tumor center (Figure 2A), neighboring breast fat-pad (Figure 2A), or uninvolved skin (not shown). Within 15–20 minutes after injection, no further fluorescent cells were observed arresting in blood vessels. The arrested cells remained in the tumor periphery during the 30-minute initial observation period and for up to 3 days later (Figure 2A). A significantly greater number of cells arrested in the tumor periphery than elsewhere (Figure 2B). Postmortem analysis of tissues confirmed that the human CD34– cells were present only at the GFP+ tumor periphery, just beyond the edge of the GFP+ tumor (Figure 2C), even though CD31+ blood vessels were present both within the tumor and at the periphery of the tumor (Figure 2C). These studies show that CD34– cells have the capacity to home to the tumor peripheral vasculature as well.
Integrin α4β1 is expressed by CD34+ progenitor cells.
To determine how circulating progenitor cells and other cells arrest in the peripheral tumor vasculature, we examined the roles of cell adhesion molecules in this process. Circulating cells such as lymphocytes utilize selectins to initiate contact with the blood vessel (rolling) and integrins α4 or β2 to arrest on the endothelium and extravasate from the circulation (22, 23). Hematopoietic precursor cells also use integrin α4 to adhere to bone marrow endothelia and to home back to the bone marrow from the circulation (24–26). To evaluate a role for specific adhesion proteins in progenitor cell homing, we examined the expression of integrins on circulating CD34+ cells. CD34+ cells were isolated to 98% purity from human PBMCs by 3 rounds of anti-CD34 magnetic bead affinity chromatography. CD34+ cells expressed significant levels of integrins α4 (100%), α5β1 (99%), αvβ3 (99%), and β2 (68%) but little integrin αvβ5 (Figure 3A). The integrin α4 subunit can combine with either the β1 or the β7 subunits, although the β7 subunit has been detected only in small subsets of bone marrow–derived cells. We observed that the CD34+ cell population expresses high levels of the integrin β1 subunit but did not express the integrin β7 subunit (Supplemental Figure 2). Thus, the integrin α4β1 (VLA-4) is the primary α4 integrin on CD34+ progenitor cells. Like CD34+ cells, CD34– mononuclear cells also express high levels of the same integrins (Supplemental Figure 3). While endothelial cells express high levels of integrins α5β1, αvβ3, and αvβ5, they express low levels of integrin α4β1, no β2 integrins, and no CD133. Thus, as CD34+ cells express CD133 and high levels of α4β1 and β2 integrin, but no αvβ5, these cells are clearly not of endothelial origin (Figure 3A).
Integrin α4β1 mediates progenitor cell adhesion to endothelia in vitro.
We next determined whether integrins on CD34+ cells mediate adhesion to the vascular endothelia by plating fluorescently labeled cells on confluent EC monolayers in vitro. Human CD34+ cells bound strongly to ECs (Figure 3, B and C). The adhesion of CD34+ cells to endothelia was blocked by antibodies to integrin α4 (Figure 3, B and C) and β1 (Supplemental Figure 2) as well as by recombinant soluble VCAM (rsVCAM), another competitive inhibitor of integrin α4 function, but not by antibodies to other integrins (Figure 3, B and C). Together, these studies demonstrate that integrin α4β1 selectively mediates the attachment of CD34+ progenitor cells to endothelia in vitro. As integrin α4β1 is a receptor for connecting segment-1 (CS-1) fibronectin, an alternatively spliced form of cellular fibronectin (27), and for VCAM, an immunoglobulin superfamily molecule expressed on endothelia in inflamed tissues (22), these studies indicate that α4-VCAM or α4-fibronectin interactions can promote progenitor cell adhesion to endothelia. Indeed, proliferating endothelial cells express the α4β1 ligands cellular fibronectin (27) and VCAM (Figure 3D). CD34+ cells also adhere to the CS-1 domain of cellular fibronectin in an integrin α4β1–dependent manner (Figure 3E). By comparison, CD34– mononuclear cells also express high levels of integrin α4β1 and adhere to endothelia and CS-1 fibronectin in an α4β1-dependent manner (Supplemental Figure 3).
Integrinα4β1 mediates progenitor cell adhesion to endothelia in vivo.
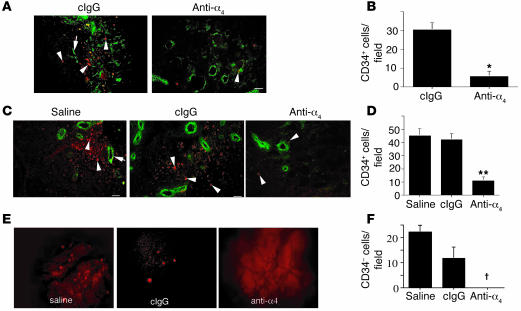
To determine if integrin α4β1 mediates CD34+ progenitor cell or CD34– mononuclear cell attachment to endothelia in vivo, fluorescently labeled cells were injected into nude mice bearing N202 tumors in the presence of integrin antagonists. Postmortem analysis of these tumors isolated within 60 minutes of cell injection confirmed that an anti-α4β1 antibody inhibited CD34+ cell arrest within tumors (Figure 4, A and B). Similarly, fluorescently labeled CD34+ cells were injected into nude mice with Lewis lung carcinoma (LLC) tumors. Postmortem analysis of these tumors isolated within 60 minutes of CD34+ cell injection showed that progenitor cells had arrested in the periphery of LLC tumors (Figure 4C). Few human cells were observed in tumor centers (data not shown). Anti-α4β1 antibodies, but not antagonists of other integrins, including integrins αvβ3 (Figure 4, C and D) and αvβ5 (not shown), substantially reduced progenitor cell accumulation in tumors. Antibody antagonists of human α4β1 also inhibited the homing of CD34– cells to N202 tumor vessels. Intravital microscopy imaging over 30 minutes demonstrated that no CD34– cells arrested in tumor vessels in the presence of anti-integrin α4β1 antibodies (Figure 4, E and F). While CD34+ progenitor cells and CD34– cells homed significantly to tumors, neither cell type homed significantly to adjacent normal tissues, to the lung (Supplemental Figure 4), or to the bone marrow (not shown). In contrast, i.v.-injected Chinese hamster ovary tumor cells expressing integrin α4β1 were entrapped solely in the lung and not in tumors, suggesting that circulating bone marrow–derived cells specifically home to the tumor vasculature. These studies indicate that integrin α4β1 regulates the homing of human bone marrow–derived progenitor cells and other mononuclear cells to the neovasculature in vivo. These results discount nonspecific leakage of cells from tumor vessels as a means to explain these findings because progenitor cells do not lodge in central tumor vessels, and integrin α4β1-expressing tumor cells do not lodge in tumors.
Figure 4.
Integrin α4β1 regulates CD34+ and CD34– cell homing to tumor vasculature. (A) Cryosections of N202 breast carcinomas from mice injected with CD34+ cells (red, arrowheads) and with either saline or anti-human α4β1 antibodies. Blood vessels were identified by CD31 staining (green, arrows) and CD34+ cells by CMTMR labeling (red). (B) Average number of CD34+ cells ± SEM per ×200 field. *P < 0.0007. (C) Cryosections of LLC lung carcinomas from mice injected with CD34+ cells (red, arrowheads) and with saline, anti-human α4β1 antibodies, or isotype-matched control anti-integrin antibody (cIgG, anti-αvβ3). Blood vessels were identified by CD31 staining (green, arrows). (D) Average number of CD34+ cells ± SEM per ×200 field. **P < 0.0006. (E) Video microscopy of tumors in animals injected with CMTMR-labeled CD34– human cells in the presence of saline, anti-α4β1, or isotype-matched control anti-integrin antibody (anti-αvβ3). (F) Average number of CD34– cells ± SEM per ×200 field. †P < 0.011. Scale bars: 50 μm.
Human CD34+ progenitor cells have been previously shown to differentiate into endothelial cells in vivo and to thereby participate in blood vessel formation in vivo (1–3). We also found that a subset of CD34+ progenitor cells injected into animals bearing subcutaneous tumors differentiated into endothelial cells that helped to form blood vessel structures (Supplemental Figure 5). Importantly, antagonists of human integrin α4β1 but not antagonists of integrin αvβ3 inhibited CD34+ progenitor cell differentiation into endothelial cells (Supplemental Figure 5). These human CD34+ cells in CD31+ blood vessels expressed the proliferating cell antigen Ki67, indicating their proliferative status (Supplemental Figure 5). These studies suggest that integrin α4β1 regulates CD34+ progenitor cell roles in blood vessel formation by promoting progenitor cell homing to tissues.
Integrin α4β1 promotes progenitor cell homing and subsequent blood vessel formation.
To determine whether α4β1 promotes murine progenitor cell homing and subsequent participation in neovascularization in vivo, we isolated murine bone marrow–derived progenitor cells from enhanced GFP (ACTB-EGFP [EGFP expressed under control of the chicken β-actin promoter]) mice. These cells have been previously shown to participate in blood vessel formation in vivo by differentiating into endothelial cells (3, 28). We compared the integrin expression and adhesion profiles of mouse bone marrow–derived lineage-positive (Lin+) and lineage-negative (Lin–) progenitor cell populations and Lin–Sca1+ stem cell–enriched populations (29). Like human bone marrow–derived cells, murine Lin+, Lin–, and Lin–Sca1+ cells expressed high levels of integrins α4β1, α5β1, αv, and β2 (Figure 5A). Each of these cell populations adhered to cultured ECs exclusively in an α4β1-dependent manner, as only anti-integrin α4β1 antibodies and rsVCAM, but not other integrin inhibitors, were able to suppress cell adhesion to endothelia (Figure 5, B–E).
Figure 5.
Integrin expression and function in murine bone marrow–derived progenitor cells. (A) FACs profiles for integrins α4β1, α5β1, αv, and β2 on murine bone marrow–derived Lin+, Lin– progenitor cells and Lin–Sca1+ stem cells. The small numbers in the corners of each FACS profile indicate the percentage of positive cells. (B–E) Adhesion of murine bone marrow–derived Lin+ and Lin– progenitor cells and Lin–Sca1+ stem cells to endothelial cell monolayers in the presence of medium, and function blocking antibodies against integrins α4β1, α5β1, αv, β2, and rsVCAM. (B) Micrographs showing EGFP Lin– green fluorescent cells adhering to ECs in the presence of medium, anti-α4β1, anti-α5β1, anti-αv, or anti-β2 (BD Biosciences — Pharmingen). (C) Average number of Lin– cells adhering per ×200 microscopic field ± SEM. *P < 0.016. (D) Average number of Lin–Sca1+ cells adhering per ×200 microscopic field ± SEM. **P < 0.015. (E) Average number of Lin+ cells adhering per ×200 microscopic field ± SEM. †P < 0.0065.
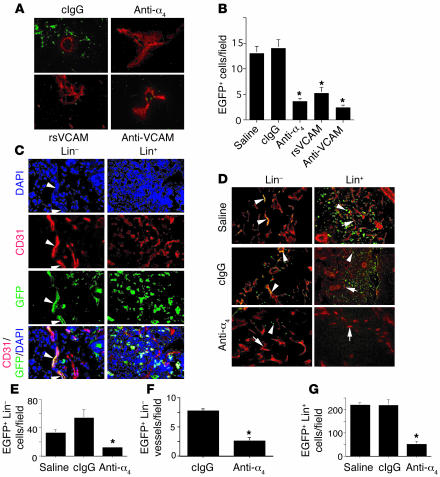
To evaluate the ability of murine progenitor cells to home to tumor blood vessels, we injected EGFP+Lin– progenitor cells into animals with LLC tumors in the presence of saline, anti-α4β1, anti-VCAM, rsVCAM, or control IgG for 12 hours. We found that Lin– cells homed to tumors and that this homing was inhibited by anti-α4β1, rsVCAM, and anti-VCAM (Figure 6, A and B). These results indicate that Lin– progenitor cells home to tumor neovasculature in an integrin α4β1- and VCAM-dependent manner.
Figure 6.
Integrin α4β1 mediates homing of Lin– murine progenitor cells to tumor neovasculature. (A) High-power images of anti-CD31 (red) immunostained tumor sections from animals 12 hours after they were injected with EGFP+Lin– cells (green) in the presence of saline, isotype-matched normal rat IgG, rat anti-mouse α4, rat anti-mouse VCAM, or rsVCAM. Magnification, ×400. (B) Average number of EGFP+Lin– cells per field ± SEM from A. *P < 0.0001. (C) High-power images of peripheral tumor sections from animals 5 days after they were injected with either EGFP+Lin– or Lin+ cells that were immunostained with DAPI (blue) and anti-CD31 (red). Merged images show Lin–EGFP+ vessels in yellow. Arrowheads indicate EGFP+ cells. Magnification, ×400. (D) Cryosections of LLC tumors from mice injected with EGFP+Lin– or EGFP+Lin+ cells (arrowheads) and treated for 5 days with saline, isotype-matched, control anti-integrin antibodies (cIgG), or anti-α4β1 antibodies. Magnification, ×200. Anti-CD31 staining (red, arrows) identifies blood vessels. EGFP+ blood vessels are yellow. (E) Average number of EGFP+Lin– cells per field ± SEM. (F) Average number of EGFP+Lin– blood vessels ± SEM in antibody-treated groups. (G) Average number of EGFP+ cells ± SEM.
To evaluate the ability of murine progenitor cells to participate in blood vessel formation, we injected EGFP+Lin– progenitor cells and EGFP+Lin+ cells into animals with LLC tumors. After 5 days in vivo, EGFP+Lin– cells had homed to tumors, and some cells had formed EGFP+ blood vessels, primarily at the tumor periphery (Figure 6C and Supplemental Figure 6). Importantly, EGFP+Lin+ bone marrow–derived cells similarly injected homed to tumors but did not contribute to vessel formation although these cells homed to tumors and were found within the tumor tissue (Figure 6C). We next injected murine EGFP+Lin– progenitor cells into animals with LLC tumors in the presence or absence of anti-α4β1 or isotype-matched anti-integrin β2 antibodies. In contrast to control-treated animals, few EGFP+Lin– cells (Figure 6, D and E, and Supplemental Figure 6) or EGFP+ blood vessels (Figure 6, D and F) were observed in tumors of anti-α4β1–treated mice.
We also injected murine EGFP+Lin+ cells into animals with LLC tumors in the presence or absence of anti-α4β1 or isotype-matched anti-integrin antibodies. Numerous EGFP+Lin+ cells were found in the tumor periphery of control-treated mice, but few EGFP+Lin+ cells were observed in tumors of anti-α4β1–treated mice (Figure 6, D and G). Our studies show that integrin α4β1 promotes homing of circulating progenitor cells and other bone marrow–derived mononuclear cells to tumor vessels. These studies also indicate that inhibition of progenitor cell homing to the tumor vasculature prevents their participation in neovascularization by differentiation into endothelial cells. Our studies thus indicate that integrin α4β1 mediates progenitor cell attachment to the tumor endothelium, extravasation, and subsequent participation in blood vessel formation.
Integrin α4β1 ligands are expressed primarily at the tumor periphery.
To determine whether ligands for the integrin α4β1 are expressed by the tumor neovasculature, we examined tissues undergoing neovascularization for expression of the α4β1 ligands VCAM and cellular fibronectin. Both VCAM and fibronectin are expressed in breast tumor endothelium, which is identified by anti-CD31 immunostaining (Figure 7). In fact, both ligands are expressed at much greater levels in the tumor periphery than in the tumor center (Figure 7). These ligands are rarely expressed by normal endothelia, although fibronectin was occasionally observed around large vessels in normal tissues (Figure 7). These results demonstrate that the α4β1 ligands VCAM and fibronectin are expressed in the tumor periphery where they appear to promote the adhesion of α4β1+ progenitor cells.
Figure 7.
Integrin α4β1 ligands are preferentially expressed at the tumor periphery. Cryosections of murine breast carcinomas or normal tissue immunostained for CD31 (red) and VCAM or fibronectin (green). Arrowheads indicate blood vessels. Yellow indicates EC expression of VCAM or fibronectin. Scale bar: 50 μm.
Integrin α4β1 but notβ2 integrins promote intrinsic bone marrow–derived progenitor cell homing.
Our studies indicate that integrin α4β1 mediates the homing of bone marrow–derived progenitors to the peripheral neovasculature. To ascertain whether integrin α4β1 plays a role in the natural homing of progenitor cells from bone marrow to neovasculature, mice were transplanted with bone marrow from Tie2LacZ mice. In Tie2LacZ mice, endothelial cells express β-galactosidase under the control of the promoter of the endothelial protein Tie2 (Supplemental Figures 7 and 8). Large vessels as well as small, single-celled vessels stain positively for β-galactosidase expression by both colorimetric (Supplemental Figure 7) and fluorescent immunostaining (Supplemental Figure 8) methods. In mice transplanted with Tie2LacZ bone marrow, bone marrow–derived endothelial cells expressed β-galactosidase under the control of the promoter of the endothelial protein Tie2 (1, 2).
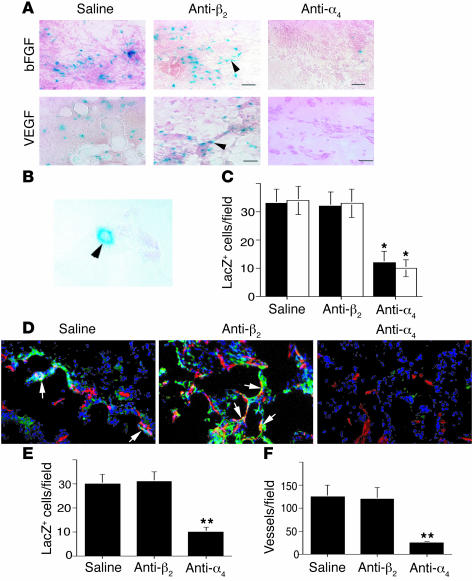
Angiogenesis was stimulated in the Tie2LacZ bone marrow–transplanted mice by implantation of growth factor–reduced Matrigel saturated with VEGF or bFGF. These growth factors induced an angiogenic response as well as the homing of β-galactosidase–positive (βGal+) cells (Figure 8, A and B). Many of these cells formed small vessels composed of single cells, which could be detected by microscopy at high magnification (Figure 8B). Treatment of mice with anti-α4β1 but not anti-integrin β2 function-blocking antibodies blocked the incorporation of βGal+ cells into the Matrigel (Figure 8, A–C). Immunological detection of β-galactosidase and CD31 expression demonstrated that many βGal+ cells incorporated into CD31+ blood vessels (Figure 8D and Supplemental Figure 9). Importantly, antagonists of α4β1 but not of β2 integrin blocked this incorporation into the vasculature (Figure 8, D–E) and suppressed angiogenesis (Figure 8F).
Figure 8.
Integrin α4β1 promotes stem cell homing from the bone marrow. (A) Cryosections of bFGF- or VEGF-saturated Matrigel from mice transplanted with Tie2LacZ bone marrow and treated with saline, anti-α4β1, or control isotype-matched anti-integrin β2 antibodies (cIgG) were stained to detect β-galactosidase (arrowheads). Magnification, ×200. Scale bars: 50 μm. (B) Micrograph of a β-galactosidase–positive single-celled vessel (arrowhead). Magnification, ×600. (C) Average numbers of LacZ+ cells per ×200 field ± SEM in VEGF (black bars) and FGF (white bars) saturated Matrigel plugs. *P < 0.035. (D) Cryosections immunostained for β-galactosidase (green) and CD31 (red). LacZ+/CD31+ vessels are yellow (arrows). (E) Average number of LacZ+/CD31+ cells per ×200 field ± SEM. **P < 0.03. (F) Average number of vessels per ×200 field ± SEM.
Our studies indicate that integrin α4β1, but not other integrins, potentiates progenitor/stem cell trafficking to tumors and remodeling tissues undergoing neovascularization. This integrin promotes progenitor cell attachment to the neovascular endothelium, endothelial transmigration, and new blood vessel formation in remodeling tissues.
Discussion
Our studies demonstrate that integrin α4β1-VCAM/fibronectin interactions promote the homing of progenitor cells and other circulating mononuclear cells to vascular endothelia during neovascularization and tissue remodeling. Our work identifies a key mechanism regulating progenitor cell homing to target tissues. This is a significant advance in our understanding of how progenitor and/or stem cells find their way to a specific tissue microenvironment. Once localized in a tissue, progenitor cells may promote tissue repair by differentiating into a number of cell types, including endothelial cells, smooth muscle cells, muscle cells, and neuronal cells (1–13). The use of CD34+ bone marrow–derived progenitor cells is thus under consideration for the therapy of diseased or damaged tissues. Development of methods to enhance homing of progenitors to target tissues could facilitate tissue repair. Our studies suggest that manipulation of the expression and/or function of α4β1 and its ligands may improve therapeutic bone marrow transplantation or be used to inhibit progenitor/stem cell contributions to pathological processes such as tumor angiogenesis.
Integrin α4β1-VCAM interactions promote several key heterotypic cell adhesion events in vivo. During normal embryonic development, this integrin promotes fusion of the chorion with the allantois and fusion of endocardium with myocardium (30, 31). Integrin α4β1 and fibronectin or VCAM interactions are also critical for T cell and natural killer cell trafficking during inflammation (22, 23) and cancer (32), for adhesion of immune cell precursors to bone marrow endothelia, and for circulating cell homing to the bone marrow (24, 25). More recently, we showed that this integrin also promotes intercellular adhesion of endothelial cells and pericytes during angiogenesis, thereby promoting subsequent blood vessel formation (21). Although previous studies showed that integrin α4β1 antibodies mobilize progenitor cell release from bone marrow and block progenitor cell return to bone marrow (24, 25), our studies now show that these antibodies also block, rather than promote cell homing to neovascular tissue. The studies presented here indicate yet another role for this integrin-ligand pair, the homing of progenitor cells and other circulating bone marrow–derived cells to proliferating or activated endothelia.
In our studies of progenitor cell homing to tumors, we observed that cells traveled throughout all the vessels of the tumor but only attached within the tumor vessels at the periphery of the tumor. We identified biochemical differences, expression of VCAM and fibronectin, between the peripheral and central vasculature. These differences were not attributable to central tumor necrosis, as the tumors observed were small with no evidence of necrosis. Our studies include dynamic analyses of homing as the in vivo studies successively analyzed the movement of adoptively transferred cells within a time frame of minutes (intravital microscopy), hours (N202 and LLC tumors with CD34+ cells and Lin– cells), and days (CD34+ and Lin– cell transfer). Use of intravital microscopy in studies of CD34+ cells could not distinguish cells adhering to the vascular wall from cells just beneath the endothelium. However, immunohistochemistry of thin cryosections shows that cells can be found both within vessels, adhering to the vessel wall, and inside tissues. Thus, our studies show that cells first attach to the vessel wall, then migrate into tissues. Longer term, 5-day studies show that Lin– cells form blood vessels while others remain as single cells within the tissues. In contrast, Lin+ cells never make vessels but do home to tumors and extravasate into tissues.
Our studies with human CD34+ cells and selective anti-human integrin antibodies demonstrated that human integrin α4β1 on CD34+ cells promotes homing of progenitor cells to murine neovessels. Our studies with murine Lin– cells and anti-murine α4β1 antibodies reproduced these findings. The in vitro and in vivo human and murine studies combined clearly demonstrate that integrin α4β1 on circulating progenitor cells promotes homing to neovessels. In our studies, only antagonists of integrin α4 and β1 integrins blocked cell adhesion to proliferating endothelia in vitro or in vivo. Our studies utilizing antagonists of integrin αvβ3, αvβ5, α5β1, and β2 integrins demonstrated that these other integrins are unlikely to play major roles in homing of circulating cells to tumors. Recent studies have suggested that β2 integrins, rather than integrin α4β1, promote endothelial progenitor cell homing to neovessels (33). In these studies, a population of adherent PBMCs cultured in endothelial growth medium were characterized as endothelial progenitor cells and served as the basis for adoptive transfer studies. We and others have found that this population of cells are derived from monocytes, as they express myelocytic markers, including CD14, CD68, and αMβ2, and fail to express CD34 or CD133, 2 key markers of progenitor cells (34). Our studies suggest that integrin β2 does not contribute to progenitor cell homing to tumors. However, as both of these integrins have been shown to promote lymphocyte trafficking, it remains possible that both receptors modulate progenitor cell trafficking in distinct clinical settings.
Our studies showed that bone marrow–derived progenitor cells home to tumor vasculature and can differentiate into endothelia, thereby participating in angiogenesis. We showed that only Lin– and not Lin+ cells formed blood vessels. Several previous studies have shown that murine Lin– and human CD34+ bone marrow–derived progenitor cell populations contain endothelial progenitor cells (1–4, 15, 16, 28). Although some controversy has arisen over the existence of bone marrow–derived endothelial precursors, recent studies have identified a common bone marrow–derived precursor for endothelial cell and hematopoietic cell lineages. For example, a common Lin–Sca1+ stem cell (the hemangioblast) for endothelial cell and hematopoietic cell lineages was isolated from adult murine bone marrow using single cell transplantation analysis (28), demonstrating the clear presence of common endothelia/hematopoietical progenitor cells among bone marrow–derived cell populations. Additionally, other studies have shown that endothelial and hematopoietic cells arise during embryogenesis from a common precursor cell, the hemangioblast (35, 36). Although recent studies demonstrated in a variety of animal models that only about 15–20% of tumor vessels arise from the bone marrow (16), other studies indicated that in the absence of sprouting angiogenesis, bone marrow–derived angiogenesis alone could promote tumor growth (15). Importantly, recently published work demonstrated that sex-mismatched human bone marrow transplant recipients who later develop cancer exhibit bone marrow–derived endothelial cells within tumor vessels (37). Furthermore, adoptively transferred CD34+ progenitor cells promoted angiogenesis and improved heart function in animal studies (1–3). Our studies thus indicate that integrin α4β1 mediates progenitor cell attachment to the tumor endothelium, extravasation, and, therefore, subsequent participation in blood vessel formation. As Lin–Sca1+ stem cells give rise to endothelial and hematopoietic cell lineages (28) and adhere to endothelia in an α4β1-dependent manner, our studies suggest that these bone marrow–derived stem cells also home to the tumor vasculature in an α4β1-dependent manner.
We found that both VCAM and cellular fibronectin are expressed at the tumor periphery, the location to which CD34+ cells home. Fibronectin is typically localized on the abluminal side of the blood vessel while VCAM is localized on the luminal side. It is possible that the fibronectin is accessible to circulating cells, as tumor neovasculature is characteristically leaky. Tumor-derived VEGF inhibits VE-cadherin–mediated intercellular adhesion of endothelial cells and allows circulating cells, including platelets, to contact the basement membrane of VEGF-stimulated vessels (38). It is also possible that circulating cells recognize VCAM on the luminal side of endothelia and use cellular fibronectin for migration beneath endothelia. As VCAM is also expressed on the endothelia of vessels in inflamed or ischemic tissues, it is likely that integrin α4β1 promotes the homing of circulating progenitor cells and other bone marrow–derived mononuclear circulating cells not only to tumor tissues but also to inflamed and ischemic tissues.
Antagonists of the bone marrow–derived component of angiogenesis could be useful in combination with inhibitors of sprouting angiogenesis to most thoroughly suppress tumor angiogenesis. Additionally, our studies suggest that manipulation of the expression and function of α4β1 and its ligands may improve therapeutic bone marrow transplantation or be used to inhibit progenitor/stem cell contributions to pathological processes such as tumor angiogenesis.
Methods
Progenitor cell isolation
PBMCs (3.7 × 109) were purified by gradient centrifugation from human blood obtained from the San Diego Blood Bank. From these, 3 × 106 CD34+ cells at 98% purity (as determined by FACS analysis for CD34 expression) were isolated by positive selection by 3 rounds of immune selection for human CD34 (Miltenyi Biotech).
All animal studies were approved by the Institutional Animal Care and Use Committees of UCSD, The Scripps Research Institute, and Sidney Kimmel Cancer Center. EGFP+ bone marrow–derived mononuclear cells (3 × 108) were purified by gradient centrifugation from the femurs and tibias of 6 ACTB-EGFP mice. Lin– cells (4.8 × 106) were isolated from bone marrow–derived mononuclear cells by 2 rounds of negative selection for a panel of lineage markers (CD5, B220, CD11b, Gr-1, 7-4, and Ter119; Miltenyi Biotech). The selected population was 90% negative for lineage markers and 80% positive for c-kit expression. Lin+ cells were 100% positive for lineage markers. Lin–Sca 1+ cells (70% Sca1+) (0.4 × 106) were isolated from Lin– cells by 3 rounds of immune selection for the stem cell marker Sca1+ (Miltenyi Biotech).
Intravital microscopy
Human cells were labeled with CMTMR (Invitrogen Corp.) for 15 minutes on ice and washed. PBMCs (2 × 106) or CD34+ cells (1 × 106) were injected i.v. in a volume of 100 μl into mice with N202 syngeneic GFP+ or negative tumors. N202 syngeneic breast tumor cells were cultured as tumor spheroids and were transplanted onto transplanted mammary fat-pad under transparent dorsal skinfold chambers. Animals were sedated while in vivo fluorescence microscopy was performed. Experiments were performed twice with 2 mice per treatment group. In some studies, cells were injected together with anti-integrin antibodies (final concentration of 50 μg/ml HP2/1 anti-human α4β1, P1F6 anti-αvβ5, or LM609, anti-αvβ3, or saline). Fluorescence microscopy was performed using a Mikron Instrument Microscope (Mikron) equipped with epi-illuminator and video-triggered stroboscopic illumination from a xenon arc (MV-7600; EG&G). A silicon intensified target camera (SIT68; Dage-MTI) was attached to the microscope. A Hamamatsu image processor (Argus 20) with firmware version 2.50 (Hamamatsu Photonic System) was used for image enhancement and to capture images to a computer. Zeiss Achroplan 20×/0.5 W, Zeiss long-distance 10/0.22, and Leitz PL1/0.04 objectives were used to capture images.
FACS analysis.
FACS analysis was performed at the UCSD Cancer Center Shared Resource. PE-conjugated mouse anti-human α4β1 (9F10) and PE-α5β1 (IIA1) were from BD. PE-conjugated anti-β2 (CLB-LFA 1/1) was from eBioscience, and FITC-conjugated anti-CD34 (AC136) and PE-conjugated anti-CD133 (AC133/1) were from Miltenyi Biotec. Mouse anti-human αvβ3 (FITC-conjugated LM609) and αvβ5 (PE-conjugated P1F6) were from Chemicon International. PE-conjugated rat anti-mouse α5β1 (5H10-27), αv (RMV-7), and β2 (Μ18/2) were from BD. PE-conjugated anti-mouse α4β1 (R1-2), c-kit (2B8), and Sca-1 (E13-161.7) were from eBioscience, and lineage markers were from Miltenyi Biotec. Expression of VCAM on ECs was determined with P8B1 (Chemicon International). Expression of β1 integrin was determined with mouse anti-human β1 P4C10 (a gift of David Cheresh, Moores UCSD Cancer Center), and expression of β7 integrin was determined with rat anti-human β7 FIB504 (BD Biosciences — Pharmingen) followed by incubation in Alexa 488–conjugated secondary antibodies (Invitrogen Corp.). Each FACS analysis was performed 2–3 times with identical results.
Immunohistochemistry.
Five micron cryosections were fixed in acetone and incubated in 5% BSA in PBS, then in primary antibody (5–10 μg/ml) for 2 hours at room temperature. Slides were well washed, incubated in Alexa 488 (green), Alexa 568 (red), or Alexa 350 (blue) conjugated secondary antibody for 1 hour at room temperature, washed and, in some cases, stained with DAPI. Primary antibodies were as follows: anti-fibronectin (TV-1; Chemicon International), rat anti-mouse VCAM (M/K-2 from Chemicon International), anti-pan species VCAM (H-276, sc-8304 from Santa Cruz Biotechnology Inc.), rat anti-mouse CD31 (MEC 13.3 from BD Biosciences — Pharmingen), and mouse anti-human Ki67 (Chemicon International). Each experiment was performed 3 times. In all immunohistochemistry studies, 8–10 fields per tissue sample and 3–6 tissues were quantified.
Adhesion assays.
Adhesion assays were performed for 30 minutes on 48-well plates coated with 5 μg/ml of recombinant H120 CS-1 fibronectin (from Martin J. Humphries, University of Birmingham, Birmingham, United Kingdom), plasma fibronectin, or vitronectin as described (39). Assays were performed in the presence of adhesion medium or 25 μg/ml function-blocking anti-α4β1 (HP2/1; a gift from Biogen), anti-α5β1 (JBS5; Chemicon International), anti-αvβ5 (P1F6; from David Cheresh) or anti-αvβ3 (LM609; from David Cheresh), or rsVCAM (R&D Systems). Each experiment was performed 3 times with identical results, with 4 replicates per treatment group. Statistical analysis was performed by 1-tailed Student’s t test.
Additionally, 25,000 CMTMR-labeled human cells per well were plated on HUVEC monolayers in 96-well culture plates for 30 minutes at 37°C in the presence of 25 μg/ml (2.5 μg/25,000 cells) HP2/1, JBS5, LM609, P1F6, P4C10, function-blocking anti-β2 antibodies (BD Biosciences — Pharmingen), and human rsVCAM (R&D Systems). A total of 25,000 EGFP+ bone marrow mononuclear cells, Lin+, Lin–, and Lin–Sca1+ cells/well were similarly incubated on HUVEC monolayers in the presence of 25 μg/ml (2.5 μg/25,000 cells) anti-α4β1 (PS/2; a gift from Biogen), anti-α5β1 (5H10-27; BD Biosciences — Pharmingen), anti-αv (RMV-7; BD Biosciences — Pharmingen), or anti-β2 (M18/2; BD Biosciences — Pharmingen). All assays were performed in HEPES-buffered HBSS containing 3% BSA, 1 mM CaCl2, 1 mm MgCl2, and 0.1 mm MnCl2. Each experiment was performed 2–3 times with identical results, with 4 replicates per treatment group. Representative fields were photographed at ×200, and the number of cells adhering per field was quantified in 5 fields per treatment condition. Statistical analysis was performed by Student’s t test.
Tumor studies.
CMTMR-labeled PBMCs (2 × 106) or CD34+ cells (1 × 106) were incubated in medium, 50 μg/ml of low-endotoxin anti-αvβ3 (LM609), or anti-human α4β1 (either HP2/1; Biogen or 9F10; BD) on ice for 30 minutes before injecting into nude mice bearing N202 breast carcinomas. In additional studies, 0.5 × 106 CD34+ progenitor cells were similarly incubated before injecting into nude mice bearing 0.5 cm LLC tumors (n = 6). After 1 hour, animals were sacrificed. In some studies, 0.5 × 106 GFP+ Chinese hamster ovary cells stably transfected to express integrin α4β1 were injected i.v. into nude mice bearing 0.5-cm LLC tumors (n = 3). Alternatively, 1 × 106 EGFP+Lin– or EGFP+Lin+ cells were injected into nude mice bearing 0.5-cm LLC tumors. In 1 study, animals (n = 4) were treated upon injection of EGFP+Lin– cells for 12 hours with 100 μl saline, 200 μg low-endotoxin IgG2b (BD Biosciences — Pharmingen), 200 μg low-endotoxin anti-α4β1 (PS/2; Biogen), low-endotoxin anti-VCAM (MK-1), or 200 μg rsVCAM. In other studies, animals (n = 6) were treated for 5 days with 100 μl saline, 200 μg low-endotoxin anti-αMβ2 (BD Biosciences — Pharmingen), or 200 μg low-endotoxin anti-α4β1 (PS/2; Biogen) every other day. Nude mice bearing 0.5-cm HT29 colon carcinoma tumors were injected with 1 × 106 CMTMR-labeled CD34+ cells and treated for 5 days with 100 μl saline, 200 μg anti-human α4β1 (HP2/1), or 200 μg anti-human αvβ5 (P1F6) (n = 10). Prior to sacrifice, animals were injected i.v. with FITC–Bandiera simplicifolia lectin. Tumors plus surrounding connective tissue were excised and cryopreserved. Microvascular density was determined by counting CMTMR+CD31+ vessels per ×200 microscopic field in 10 randomly selected fields per tumor in 10-μm thick cryosections. The mean vessel density in all tumors per treatment group was determined. Studies were performed twice with identical results. Statistical significance was determined using Student’s t test.
Bone marrow transplantation
Bone marrow from FVB/NJ mice was transplanted into irradiated FVB/N mice as previously described (1). After 12 weeks, mice were injected with 400 μl growth factor–reduced Matrigel supplemented with 400 ng/ml of bFGF or VEGF and treated on days 1 and 3 by i.v. injection of saline, 200 μg/mouse rat anti-mouse α4β1 (low-endotoxin PS/2), or rat-anti-mouse β2 integrin (low-endotoxin M1/70; BD). Plugs were excised after 5 days (n = 8). Cryosections were incubated in X-gal or immunostained with rabbit anti–β-galactosidase and rat anti-murine CD31 (MEC13.3; BD). Experiments were performed twice with identical results. Positive vessels were quantified at ×200 in 10 randomly selected microscopic fields per plug. Statistical analysis was performed by 1-tailed Student’s t test.
Acknowledgments
This work was supported by grants to J. Varner from the NIH, the UCSD Cancer Center, the Charlotte Geyer Foundation, and the American Heart Association. We would like to thank Margaret Hogan for assisting with confocal microscopy and Jeanine Kleeman, Yuhong Zhu, and Robbie Cheresh for excellent technical assistance.
Footnotes
Nonstandard abbreviations used: ACTB-EGFP, EGFP expressed under control of the chicken β-actin promoter; CMTMR, 5-and-6-4-chloromethylbenzoylamino-tetramethylrhodamine; CS-1, conecting segment-1; EGFP, enhanced GFP; Lin, lineage; LLC, Lewis lung carcinoma; rsVCAM, recombinant soluble VCAM.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Kawamoto A, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 3.Ott I, et al. Endothelial-like cells expanded from CD34+ blood cells improve left ventricular function after experimental myocardial infarction. FASEB J. 2005;19:992–994. doi: 10.1096/fj.04-3219fje. [DOI] [PubMed] [Google Scholar]
- 4.Otani A, et al. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat. Med. 2002;8:1004–1010. doi: 10.1038/nm744. [DOI] [PubMed] [Google Scholar]
- 5.Hattori K, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J. Exp. Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 7.Taguchi A, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J. Clin. Invest. 2004;114:330–338. doi:10.1172/JCI200420622. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Religa P, et al. Smooth-muscle progenitor cells of bone marrow origin contribute to the development of neointimal thickenings in rat aortic allografts and injured rat carotid arteries. Transplantation. 2002;74:1310–1315. doi: 10.1097/00007890-200211150-00019. [DOI] [PubMed] [Google Scholar]
- 9.Priller J, et al. Neogenesis of cerebellar Purkinje neurons from gene-marked bone marrow cells in vivo. J. Cell Biol. 2001;155:733–738. doi: 10.1083/jcb.200105103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 11.Torrente Y, et al. Human circulating AC133+ stem cells restore dystrophin expression and meliorate function in dystrophic skeletal muscle. J. Clin. Invest. 2004;114:182–195. doi:10.1172/JCI200420325. doi: 10.1172/JCI20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow–derived progenitor cells in pulmonary fibrosis. J. Clin. Invest. 2004;113:243–252. doi:10.1172/JCI200418847. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips RJ, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J. Clin. Invest. 2004;114:438–446. doi:10.1172/JCI200420997. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 15.Lyden D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 16.Ruzinova MB, et al. Effect of angiogenesis inhibition by Id loss and the contribution of bone-marrow-derived endothelial cells in spontaneous murine tumors. Cancer Cell. 2003;4:277–289. doi: 10.1016/s1535-6108(03)00240-x. [DOI] [PubMed] [Google Scholar]
- 17.Jain RK, Duda DG. Role of bone marrow-derived cells in tumor angiogenesis and treatment. Cancer Cell. 2003;3:515–516. doi: 10.1016/s1535-6108(03)00138-7. [DOI] [PubMed] [Google Scholar]
- 18.Nygren JM, et al. Bone-marrow derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion but not transdifferentiation. Nat. Med. 2004;5:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 19.Willenbring H, et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat. Med. 2004;7:744–748. doi: 10.1038/nm1062. [DOI] [PubMed] [Google Scholar]
- 20.Jin H, Varner J. Integrins: roles in cancer development and as treatment targets. Br J. Cancer. 2004;90:561–565. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garmy-Susini B, et al. Integrin α4β1−VCAM-1–mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J. Clin. Invest. 2005;115:1542–1551. doi:10.1172/JCI23445. doi: 10.1172/JCI23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan JL, Hynes RO. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990;60:53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- 23.Elices MJ, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 24.Simmons PJ, et al. Vascular cell adhesion molecule-1 expressed by bone marrow stromal cells mediates the binding of hematopoietic progenitor cells. Blood. 1992;80:388–395. [PubMed] [Google Scholar]
- 25.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001;98:2403–2411. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 26.Craddock CF, Nakamoto B, Andrews RG, Priestley GV, Papayannopoulou T. Antibodies to VLA4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood. 1997;90:4779–4788. [PubMed] [Google Scholar]
- 27.Elices MJ, et al. Expression and functional significance of alternatively spliced CS1 fibronectin in rheumatoid arthritis microvasculature. J. Clin. Invest. 1994;93:405–416. doi: 10.1172/JCI116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant MB, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat. Med. 2004;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 29.Terskikh AV, Miyamoto T, Chang C, Diatchenko L, Weissman IL. Gene expression analysis of purified hematopoietic stem cells and committed precursors. Blood. 2003;102:94–101. doi: 10.1182/blood-2002-08-2509. [DOI] [PubMed] [Google Scholar]
- 30.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 31.Kwee L, et al. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- 32.Melder RJ, et al. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat. Med. 1996;2:992–997. doi: 10.1038/nm0996-992. [DOI] [PubMed] [Google Scholar]
- 33.Chavakis E, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J. Exp. Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmeisser A, et al. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;49:671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 35.Patterson LJ, Gering M, Patient R. Scl is required for dorsal aorta as well as blood formation in zebrafish embryos. Blood. 2005;105:3502–3511. doi: 10.1182/blood-2004-09-3547. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Peters BA, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat. Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 38.Weis S, et al. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J. Cell Biol. 2004;167:223–229. doi: 10.1172/JCI20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am. J. Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]