It is a remarkable fact that antioxidants are nontoxic compounds that reduce the incidence of cancer. Antioxidant nutrients such as vitamin E, β-carotene, lycopene, and selenium are regularly found to reduce the risk of lung, prostate, stomach, or total cancers, as well as oral precancers, in epidemiologic studies (1). Foods containing these nutrients are similarly effective, as are nondietary antioxidants such as green tea phenols and various Oriental herbal medicines (2). In general, the level of risk reduction is on the order of 0.6, which might encouragingly be extrapolated to (0.6)n for those of us with n organs at risk. The risk reduction from an antioxidant can be 3-fold in the elderly, in smokers, and in subpopulations deficient in a second antioxidant (1). This result suggests that functional redundancy of antioxidant systems is hiding the importance of their biological roles until two actors have been removed, as often happens with gene knockouts. These nutrients, as well as nonnutrient antioxidants such as N-acetyl cysteine, also slow the appearance of tumors in mice (2–4). Why do antioxidants have these effects?
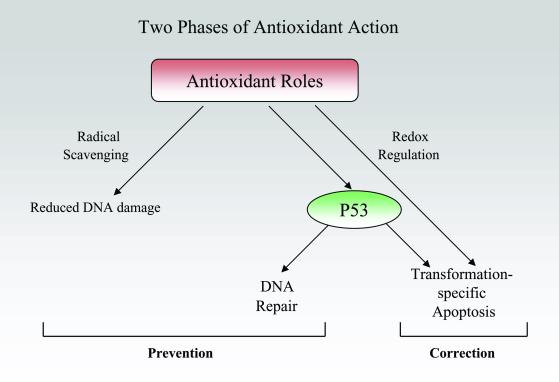
Biochemically, attention has traditionally centered on the obvious suspect, the ability of these compounds to scavenge free radicals, especially reactive oxygen species. Lipid-soluble antioxidants such as vitamin E act as chain-breakers to stop the propagation of sequential free radical reactions, as can water-soluble antioxidants such as vitamin C. Eradicating radicals will reduce damage to DNA and membranes (Fig. 1). But antioxidants have other molecular consequences, including inhibiting generation of reactive oxygen species, inhibiting metabolic activation of carcinogens, and altering the intracellular redox potential (5). The latter occurs with those water-soluble antioxidants having a high reducing potential, such as vitamin C and N-acetyl cysteine, which change the cell's redox state. Redox state, in turn, regulates the activity of many transcription factors (6). In this issue of PNAS, Seo et al. (7) present a novel mode of action for selenium: reduction of cysteines in the P53 tumor suppressor protein, leading to an increase in the efficiency of DNA excision repair.
Fig 1.
Chemopreventive antioxidants are usually considered in terms of radical scavenging, but Seo et al. (7) present evidence that selenium up-regulates DNA excision repair via P53. These preventive roles are complemented by other recent studies, indicating a corrective activity of antioxidants: inducing apoptosis selectively in transformed cells.
Selenium compounds have been intensively studied as cancer-preventive agents in mouse models and recently tested in phase II-III clinical trials for prostate cancer prevention (8). The form of selenium that is the focus of the Seo et al. study, selenomethionine (SeMet), is a relatively nontoxic compound and is especially interesting in terms of its antioxidant properties. SeMet is the form reported to be the major component of dietary selenium, and undergoes an intramolecular transsulfuration reaction to form selenocysteine. Two proteins containing this residue, glutathione peroxidase and thioredoxin reductase, are known for their antioxidant properties and maintain the redox balance in cells (9). The specific activity of both enzymes is highly sensitive to the concentration of Se in the cellular milieu. Thioredoxin reductase is also essential for converting ribonucleotides to the deoxyribonucleotides needed for DNA synthesis, and for regulating several transcription factors (9).
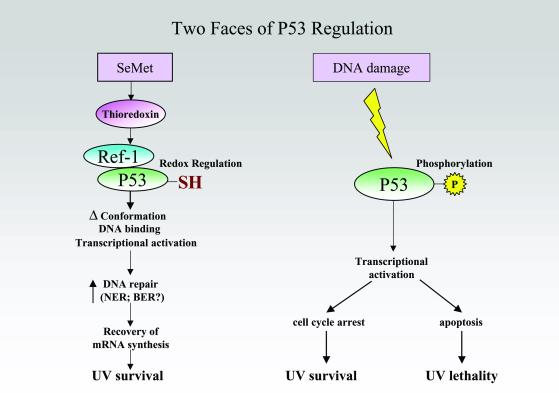
Ref-1 is a substrate for reduction by the thioredoxin reductase system (Fig. 2). Seo et al. (7) convincingly demonstrate that SeMet can activate P53, and that this activation is dependent on Ref-1; P53 was not activated when either a dominant-negative Ref-1 or a mutant P53 was used. They measured the extent of P53 reduction using an assay that is perhaps remarkable in its ability to “freeze” the protein's redox state (10). By lysing cells in a buffer containing N-ethylmaleimide (NEM) to bind free sulfhydryls, and then reducing the remaining disulfides by using dithiolthreitol followed by treatment with 3-maleimido-propionyl-biocytin (MBP), the original disulfide groups (now converted to the reduced form) were detected using a streptavidin–horseradish peroxidase conjugate. The reverse experiment was also carried out by using maleimide-activated alkaline phosphatase to directly label reduced sulfhydryls. The reaction was monitored by using a modified gel-shift assay. Using this technique, they also showed that at least one cysteine and possibly two, 275 and 277, in the C terminus of P53 were reduced by SeMet, shifting the 20-kDa peptide to a 70-kDa protein as a result of reacting with maleimide-activated alkaline phosphatase. In contrast to this clear-cut change in P53's sulfhydryl state, no phosphorylation was seen at any of the sites typically observed after DNA damage (Fig. 2).
Fig 2.
Selenomethionine activates P53 via the selenoprotein thioredoxin, which activates Ref-1's ability to alter the redox state of P53 cysteines. P53 activated by SeMet elevates DNA excision repair, but does not induce apoptosis or cell cycle arrest as it does when DNA damage activates P53 by phosphorylation.
Binding to an antibody that detects P53 in its active conformation was ≈3-fold higher after treatment with SeMet, resembling the response to reducing agents in vitro (11–13) but evidently requiring Ref-1 in vivo. P53 transactivation activity was elevated ≈2-fold after SeMet treatment. The biological consequences of P53 activation by SeMet were also distinctive. SeMet induced neither cell cycle arrest nor apoptosis. Instead, DNA excision repair was enhanced 2-fold, as measured by a host-cell reactivation assay that detects repair of transcription-blocking lesions induced in a reporter gene by UV light. Survival was also enhanced 2-fold; both responses required P53. SeMet was unable to enhance survival in cells defective in nucleotide excision repair (NER). The biological relevance of these changes can be assessed by noting that 2- and 3.5-fold reductions in survival after UV are seen in, respectively, the XPV and XPC forms of xeroderma pigmentosum, a hereditary syndrome predisposed to multiple sunlight-induced skin cancers (14).
Several subtleties remain to be explored. As the authors point out, the importance of cysteines 275 and 277 themselves for the effects on P53 activity and DNA repair is not yet demonstrated, because other cysteines may be and probably were reduced. In mouse, the corresponding residues (272 and 274) were marginally important or not involved, respectively, for Trp-53 transactivation activity and suppression of transformation (13). It also remains to be seen whether the tidy distinction between cysteine-activated repair and phosphorylation-activated arrest/apoptosis is general. The DNA repair system involved is not yet completely clear. It would seem to be global genomic repair, the predominant form of nucleotide excision repair, because that system has been linked to P53 up-regulation of P48 (15). Yet, the present work hints that transcription-coupled repair may be involved as well, because (i) the repair experiment assessed lesions in a transcribing gene and (ii) cells defective in transcription-coupled repair were insensitive to SeMet. It would also be interesting to know whether base excision repair (BER), the removal of a damaged base from its sugar, responds to SeMet. P53 has recently been shown to play a role in this pathway (16, 17) by associating with Ref-1 (18, 19). Ref-1 was in fact first identified as a protein with DNA-repair and redox activities that reside on different domains (20, 21), and is also referred to as APE1 for “apurinic/apyrimidinic endonuclease.” After a glycoslyase has removed a damaged base, Ref-1 cuts 5′ to the apurinic/apyrimidinic site to generate a 3′-OH group and the abasic deoxyribose-5-phosphate. This gap is filled by DNA polymerase β. P53 appears to form a complex with β polymerase and possibly damaged DNA, in doing so stabilizing the complex (16, 19). Se may also have an additional role, making SeMet treatment not equivalent to simply up-regulating Ref-1. Whereas SeMet did not induce apoptosis, up-regulating Ref-1 directly results in a moderate increase in apoptosis concomitant with enhancing the transactivation activity of P53 for P21 and Bax (18). Conversely, SeMet increased survival after UV, but up-regulating P53 directly can reduce UV mutations without affecting survival (22).
Details aside, the ability to manipulate DNA repair via diet suggests several applications. It is already known that enhancing DNA repair with a topically applied repair endonuclease reduces the number of new skin precancers in xeroderma pigmentosum patients by two-thirds, and the number of new basal cell carcinomas by one-third (23). SeMet might have a similar therapeutic effect in these patients or in immunosuppressed transplant recipients, who have a 100-fold elevated frequency of sunlight-dependent skin precancers and cancers (24). Seo et al.'s use of UV radiation in the present study may serve as a proxy for endogenous DNA damage, resulting from normal metabolism, that contributes to internal cancers. The observation that the maximum lifespan of diverse mammalian species correlates with DNA excision repair capacity might also be put to use (25).
A common feature of the antioxidant mechanisms discussed so far has been their role in preventing the first precancerous initiated cell. Surprisingly, nonscavenging roles for antioxidants have recently emerged that would be corrective, operating even after the horse is out of the barn (Fig. 1). Twenty years ago, reduced glutathione was reported to induce complete regression of rat liver tumors that had been induced by aflatoxin B1 (26). Apoptosis was on few minds at the time but, curiously, it was noted that the posttreatment livers had a “remodeled” appearance similar to that seen after partial hepatectomy. This finding faded from view, apparently because it was not reproducible using a different tumor in a different rat strain.
Several recent reports, however, have piqued interest in apoptotic effects of antioxidants. Sulfur-containing antioxidants such as N-acetyl cysteine and the structurally-related penicillamine, both used clinically for other purposes, were found to up-regulate P53, induce caspase activity, and induce apoptosis in transformed or tumor-derived cell lines from a diverse variety of tissues (27). Strikingly, primary fibroblasts and keratinocytes were unaffected and caspase induction was as much as 480-fold greater in the transformed cells. P53 was required for the full effect. Vitamin E was ineffective, suggesting that redox potentials are involved rather than radical scavenging. These antioxidants act as if they up-regulate an existing P53-based abnormality detector, which then senses an abnormality present in the transformed cell. This abnormality has not been identified, but in at least one cell lineage it arose early in the transformation process, after immortalization but before the cell was capable of forming a tumor (27). The chief suspect for this abnormality is the P16Ink4a-CyclinD1/CDK4-RB-E2F1-ARF-P53 axis, one or another part of which is aberrant in nearly all human tumors (28).
Similarly, (−)-epigallocatechin-3-gallate (EGCG), the principle chemopreventive antioxidant in green tea, induced apoptosis and cell cycle arrest in a human keratinocyte carcinoma cell line, as well as in prostate carcinoma and lymphoma cell lines, but not in normal human keratinocytes (2). This activity appeared to be P53-independent. In vivo, treating UV-irradiated mice with EGCG after the end of UV treatment but before the appearance of tumors induced apoptosis in skin precancers and squamous cell carcinomas but not in nontumor skin (29). The frequency of squamous cell carcinomas was reduced by 66%.
Caffeic acid phenethyl ester (CAPE) is a plant flavonoid found in honeybee hives. Although an antioxidant in normal cells, it has been reported to deplete intracellular glutathione in rat embryo fibroblasts transformed with adenovirus 5, but not in the parental fibroblast, and leads to apoptosis specifically in the transformed cells (30). With regard to human studies, the compounds furthest along are the thiol-containing antioxidant pyrrolidinedithiocarbamate (PDTC) and the water-soluble vitamin E analogue, Trolox. These were reported to induce apoptosis in colon carcinoma cells by inducing P21 via C/EBPβ (31). Apoptosis was blocked by C/EBPβ antisense transcripts, although overexpressing P21 via C/EBPβ did not itself lead to apoptosis. Systemic administration of these antioxidants in combination with 5-fluorouracil, a standard therapeutic agent for colon carcinoma, completely suppressed the growth of human colon tumor xenografts in mice. The animals' survival suggests that normal colonic and vascular cells were unaffected.
Repair and apoptotic activities such as these would not have been identified in traditional screens for chemotherapeutic agents. These screens do not include normal-cell controls; instead, they seek compounds highly toxic at low doses. The Seo et al. paper (7) will undoubtedly stimulate searches for the mechanism of various surprising antioxidant effects, both preventive and corrective, that are evidently unrelated to scavenging reactive oxygen species.
See companion article on page 14548.
References
- 1.Mayne S. T. & Vogt, T. M. (2000) in PPO Updates: Principles and Practice of Oncology, ed. Rosenberg, S. A. (Lippincott Williams and Wilkins, New York), Vol. 14, pp. 1–12. [Google Scholar]
- 2.Mukhtar H. & Ahmad, N. (1999) Toxicol. Sci. 52,(Suppl.), 111-117. [DOI] [PubMed] [Google Scholar]
- 3.Huang M. T., Smart, R. C., Wong, C. Q. & Conney, A. H. (1988) Cancer Res. 48, 5941-5946. [PubMed] [Google Scholar]
- 4.O'Brien P. (1994) in Free Radicals in Diagnostic Medicine, ed. Armstrong, D. (Plenum, New York), pp. 215–239.
- 5.Miquel J., Quintanilha, A. T. & Weber, H., (1989) CRC Handbook of Free Radicals and Antioxidants in Biomedicine (CRC Press, Boca Raton, FL), Vol. 2.
- 6.Sun Y. & Oberley, L. W. (1996) Free Radical Biol. Med. 21, 335-348. [DOI] [PubMed] [Google Scholar]
- 7.Seo Y. R., Kelley, M. R. & Smith, M. L. (2002) Proc. Natl. Acad. Sci. USA 99, 14548-14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson M. A., Porterfield, B. W., Jacobs, E. T. & Clark, L. C. (1999) Semin. Urol. Oncol. 17, 91-96. [PubMed] [Google Scholar]
- 9.Allan C. B., Lacourciere, G. M. & Stadtman, T. C. (1999) Annu. Rev. Nutr. 19, 1-16. [DOI] [PubMed] [Google Scholar]
- 10.Wu H. H. & Momand, J. (1998) J. Biol. Chem. 273, 18898-18905. [DOI] [PubMed] [Google Scholar]
- 11.Hainaut P. & Milner, J. (1993) Cancer Res. 53, 4469-4473. [PubMed] [Google Scholar]
- 12.Hupp T. R., Meek, D. W., Midgley, C. A. & Lane, D. P. (1993) Nucleic Acids Res. 21, 3167-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rainwater R., Parks, D., Anderson, M. E., Tegtmeyer, P. & Mann, K. (1995) Mol. Cell. Biol. 15, 3892-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews A. D., Barrett, S. F. & Robbins, J. H. (1978) Proc. Natl. Acad. Sci. USA 75, 1984-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang B. J., Ford, J. M., Hanawalt, P. C. & Chu, G. (1999) Proc. Natl. Acad. Sci. USA 96, 424-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J., Ahn, J., Wilson, S. H. & Prives, C. (2001) EMBO J. 20, 914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Offer H., Wolkowicz, R., Matas, D., Blumenstein, S., Livneh, Z. & Rotter, V. (1999) FEBS Lett. 450, 197-204. [DOI] [PubMed] [Google Scholar]
- 18.Gaiddon C., Moorthy, N. C. & Prives, C. (1999) EMBO J. 18, 5609-5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo Y. R., Fishel, M. L., Amundson, S., Kelley, M. R. & Smith, M. L. (2002) Oncogene 21, 731-737. [DOI] [PubMed] [Google Scholar]
- 20.Xanthoudakis S., Miao, G. G. & Curran, T. (1994) Proc. Natl. Acad. Sci. USA 91, 23-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelley M. R. & Parsons, S. H. (2001) Antioxid. Redox Signal. 3, 671-683. [DOI] [PubMed] [Google Scholar]
- 22.Tang J. Y., Hwang, B. J., Ford, J. M., Hanawalt, P. C. & Chu, G. (2000) Mol. Cell 5, 737-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yarosh D., Klein, J., O'Connor, A., Hawk, J., Rafal, E. & Wolf, P. (2001) Lancet 357, 926-929. [DOI] [PubMed] [Google Scholar]
- 24.Lindel B., Sigurgeirsson, B., Gavel, H. & Stern, R. S. (2000) Br. J. Dermatol. 143, 513-519. [PubMed] [Google Scholar]
- 25.Hart R. W. & Setlow, R. B. (1974) Proc. Natl. Acad. Sci. USA 71, 2169-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novi A. M. (1981) Science 212, 541-542. [DOI] [PubMed] [Google Scholar]
- 27.Havre P. A., O'Reilly, S., McCormick, J. J. & Brash, D. E. (2002) Cancer Res. 62, 1443-1449. [PubMed] [Google Scholar]
- 28.Sherr C. & McCormick, F. (2002) Cancer Cell 2, 103-112. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y. P., Lou, Y. R., Xie, J. G., Peng, Q. Y., Liao, J., Yang, C. S., Huang, M. T. & Conney, A. H. (2002) Proc. Natl. Acad. Sci. USA 99, 12455-12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiao C., Crothers, A. M., Grunberger, D., Solomon, G., Preston, G. A. & Barrett, J. C. (1995) Cancer Res. 55, 3576-3583. [PubMed] [Google Scholar]
- 31.Chinery R., Brockman, J. A., Peeler, M. O., Shyr, Y., Beauchamp, R. D. & Coffey, R. J. (1997) Nat. Med. 3, 1233-1241. [DOI] [PubMed] [Google Scholar]