Abstract
Although ischemic tolerance has been described in a variety of primary cell culture systems, no similar in vitro models have been reported with any cell line. A model of ischemic preconditioning in the rat pheochromocytoma PC12 cell line is described here. When compared to nonpreconditioned cells, preexposure of PC12 cells to 6 hours of oxygen and glucose deprivation (OGD) significantly increased cell viability after 15 hours of OGD 24 hours later. Flow cytometry analysis of cells labeled with specific markers for apoptosis, Annexin V, and Hoechst 33342, and of DNA content, revealed that apoptosis is involved in OGD-induced PC12 cell death and that preconditioning of the cells mainly counteracts the effect of apoptosis. Immunocytochemistry of caspase-3, a central executioner in the apoptotic process, further confirmed the activation of apoptotic pathways in OGD-induced PC12 cell death. This model may be useful to investigate the cellular mechanisms involved in neuronal transient tolerance following ischemia.
Keywords: apoptosis, OGD, PC12, preconditioning
An initial sublethal stress that activates endogenous cytoprotective mechanisms can protect cells against a more severe subsequent insult and induce a transient state of tolerance. Currently, there is great interest in many laboratories in the study of tolerance. In the brain, preconditioning by sublethal temporary ischemia, ischemic tolerance, has been examined in various animal models of focal and global cerebral ischemia (reviewed in Dirnagl et al, 2003; Kirino 2002). However, the mechanisms underlying ischemic tolerance are not fully elucidated. In vitro models have been used to improve our understanding of this phenomenon and to obtain information about molecular mechanisms involved in preconditioning and the development of tolerance. Ischemia, which can be mimicked in vitro by combined oxygen—glucose deprivation (OGD), and ischemic tolerance have been modeled in a variety of primary cell culture systems with this form of in vitro ischemia (Bruer et al, 1997; Ginis et al, 1999; Grabb and Choi, 1999; Khaspekov et al, 1998; Liu et al, 2000; Snider et al, 1998; Tremblay et al, 2000; Weih et al, 1999). Development of an ischemic tolerance model in a cell line, however, would facilitate screening of molecular mechanisms to identify drug targets. A cell line model would also facilitate rapid screening of small molecules for drug discovery. In the past few years, the emergence of novel strategies for high-throughput screening of potential therapeutic candidates has accelerated the pace of drug discovery (Chanda and Caldwell, 2003; Davidov et al, 2003). These technological advances combined with computational methods directed at analyzing large data sets are currently the most promising solutions to the critical need for faster detection of new drug targets in the pharmaceutical industry (Thiericke , 2003).
The PC12 cell line derived from rat pheochromocytoma has been extensively used for cell signaling studies (Vaudry et al, 2002). This cell line permits rapid screening of molecular mechanisms with minimal preparation time and the positive findings can then be validated in primary neuronal cultures. A PC12 culture system could also be adapted for cell-based high-throughput screening assays. However, ischemic preconditioning has not been demonstrated in PC12 cells previously.
We show here that a sublethal preconditioning episode of OGD renders PC12 cells resistant to a longer, lethal period of OGD. In addition, we use fluorescence-activated cell sorting (FACS) assay in conjunction with labeling of phosphatidyl-serine binding protein Annexin V and cell-permeant nuclear (condensed chromatin) dye Hoechst 33342 and analysis of DNA content, to show that PC12 cells undergo OGD-induced apoptotic death and that preconditioning mainly reduces apoptosis in this model. This work describes a new model for investigations directed at characterizing the molecular pathways involved in ischemic tolerance.
Materials and methods
Oxygen and Glucose Deprivation and Preconditioning of PC12 Cells
PC12 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were plated at a density of 3×105cells /well in 6-well multiwell Biocoat plates precoated with poly-D-lysine (BD Biosciences, Bedford, MA, USA) and grown for 24 hours in RPMI 1640 culture medium supplemented with 10% horse serum and 5% FBS (ATCC), ‘complete medium,’ at 37°C in a normoxia plus 5% CO2 atmosphere. For the induction of OGD, cells were washed twice in RPMI without glucose (Life Technologies, Carlsbad, CA, USA) switched to RPMI 1640 without glucose supplemented with 2% horse serum and 1% FBS (OGD medium) and placed in modular incubator chambers (Billups-Rothenberg, Del Mar, CA, USA). The chambers were flushed with a gas mixture of 95% N2/5% CO2 for 30 mins at room temperature at 3 L/min. After flushing, the chambers were sealed and placed at 37°C. Oxygen—glucose deprivation was carried out for 15 hours or for the indicated times (O2 levels 2% to 3%). For preconditioning experiments, cells were grown for 24 hours in complete medium. They were washed twice in RPMI without glucose, switched to OGD medium and subjected to 6 hours of OGD with initial flushing as described above. Sister plates not subjected to OGD preconditioning (sham preconditioned) were washed twice with RPMI without glucose and maintained in RPMI 1640 (glucose present) supplemented with 2% horse serum and 1% FBS. Sham preconditioned and OGD preconditioned cells were maintained in glucose-containing medium and normoxia for 24 hours for development of cellular tolerance. Subsequently, these cells were exposed to severe OGD for 15 hours. In addition, ‘nonischemic’ controls were sham preconditioned and maintained in RPMI 1640 supplemented with 2% horse serum and 1% FBS, but were not exposed to OGD.
Assessment of Cell Survival and Cell Death
Immediately following OGD, cell survival and cell death were assessed by mitochondrial reduction of XTT and lactate dehydrogenase (LDH) release, respectively. The 2,3-bis[2-methoxy-4-nitro-5-sulphophenyl]-2H-tetrazolium-5-carboxyanilide inner salt (XTT) assay is based on reduction of XTT (yellow in phosphate-buffered saline (PBS)) by mitochondrial dehydrogenases of viable cells yielding an orange formazan product. We measured reduction of XTT by means of an XTT assay kit (Sigma-Aldrich, St. Louis, MO, USA). The cell culture medium was removed and 100 μL of 200 μg/mL of XTT solution in PBS was added to each well. Cells were incubated for 3 hours at 37°C and absorbance was read at 450 nm to evaluate the amount of formazan product. Results were expressed as the percent of absorbance measured in normoxic controls. Lactate dehydrogenase activity released from cells is an indicator of loss of cell membrane integrity. Lactate dehydrogenase release from PC12 cells was assessed with the LDH assay kit (Sigma-Aldrich). Aliquots of culture medium were collected from sister wells for measurement of LDH leakage. For assessment of total LDH activity, cells were incubated with 100 μL of lysis solution/well for 30 mins at 37°C and lysates were centrifuged to remove cellular debris. Absorbance was read at 490 nm and LDH release was expressed as a percentage of the total LDH (cellular plus medium LDH), which represents the proportion of death caused by OGD.
Staining of Apoptotic Cells and Flourescence-Activated Cell Sorting Analysis
Cells were collected using a papain dissociation system (Worthington Biochemical Corporation, Lakewood, NJ, USA) and 106 cells were labeled with Annexin V-FITC and propidium iodide (PI) (Apoptosis Detection Kit, Trevigen, Gaithersburg, MD, USA) or Hoechst 33342 and PI (Vybrant Apoptosis Assay Kit #5, Molecular Probes, Eugene, OR, USA), according to the manufacturer's specifications. In some experiments, the cells were triple-labeled with Annexin V-FITC, Hoechst 33342 and PI to better resolve the cells at early, middle, and late stages of apoptosis. The cells were analyzed using a dual-laser FACSVantage SE flow cytometer (Becton Dickinson, Mountain View, CA, USA). Annexin V-FITC and PI signals were excited using a 488-nm laser light and their emissions captured using bandpass filters set at 530±30 and 613±20 nm, respectively. Hoechst 33342 was excited using a 351-nm UV laser light and its emission captured with a bandpass filter set at 450±20 nm. Cell Quest Acquisition and Analysis software (Becton Dickinson) was used to acquire and quantify the fluorescence signal intensities and to graph the data as bivariate dot-density plots. In multiple labeling experiments, fluorescence emissions of individual fluorophores were corrected for spectral overlap using electronic compensation. For analysis of DNA content cells were fixed for 30 mins at 4°C with 75% ethanol and stained with 5 μg/mL of PI (Trevigen). Assessment of DNA content was performed with a dual-laser FACSVantage SE flow cytometer (Becton Dickinson).
Caspase-3 Immunocytochemistry
PC12 cells were subjected to 15 hours OGD and fixed in 3% paraformaldehyde in cold PBS, pH 7.4 for 20 mins at 4°C. The cells were then washed three times with PBS for 10 to 15 mins, permeabilized for 30 mins with 0.3% Triton in PBS and washed again with PBS. Next, cells were blocked in 5% normal donkey serum in PBS for 1 hour at room temperature and further washed with PBS. After incubation with anti-caspase-3 antibody (Cell Signaling, Beverly, MA, USA) diluted 1:100 in PBS overnight at 4°C and three washes with PBS, the cells were incubated with peroxidase-conjugated donkey anti-rabbit IgG secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA, USA) diluted 1:2,000 in PBS for 2 hours at room temperature and washed again with PBS. The cells were blocked for endogenous peroxidase reaction by treatment with 0.6% H2O2 in PBS for 30 mins at room temperature, washed with PBS and incubated with the ABC kit (VectaStain ABC Kit, Vector Laboratories, Burlingame, CA, USA) for 1 hour at room temperature. After washing with PBS, immunoreactivity was detected using 0.05% 3.3′-diaminobenzidine tetrahydrochloride (DAB) and 0.003% H2O2in PBS for 2 mins. Controls for nonspecific binding included blocking caspase-3 antibody reactivity with the blocking peptide.
Statistics
All values are given as mean±s.d. Repeated measures ANOVA, followed by Newman—Keuls post hoc tests (GraphPad Prism 4.0 software; San Diego, CA, USA) were used for the analysis of differences between differently treated cells. Caspase-3 activation experiments were analyzed with a Student's paired t-test (GraphPad Prism).
Results
Ischemic Tolerance in PC12 Cells
Figure 1 shows representative images of phase-contrast microscopy of PC12 cells before and after exposure to OGD for 6 hours and after 15 hours. Cell shrinkage can be observed after 15 hours, but not 6 hours, of OGD. Preexposure of cells to 6 hours of OGD followed by 15 hours of OGD 1 day later decreased cell shrinkage. The time-course of PC12 cell survival after OGD was analyzed by reduction of XTT. As shown in Figure 2A no significant decrease in cell viability was observed after 6 hours of OGD. Exposure of the cells to 15 and 24 hours of OGD produced a significant decrease of cell viability to about 40% and 70%, respectively (P<0.01 in both cases; n = 8 to 10). Preexposure of cells to 6 hours, but not 5 hours, of OGD significantly increased cell viability after 15 hours of OGD 1 day later (Figure 2B). Analysis of cell death by LDH release showed similar results. Six hours of OGD did not produce a significant increase in the number of dead cells as compared with controls and significantly decreased cell death induced by 15 hours of OGD 1 day later (Figure 3).
Figure 1.
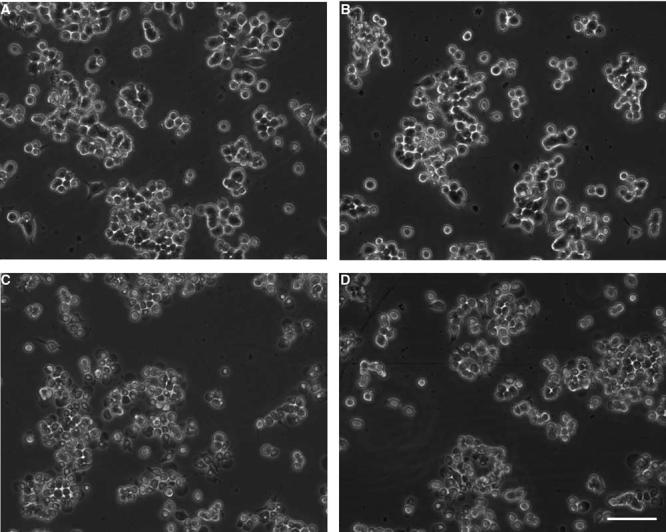
Phase-contrast microscopy images of PC12 cultured cells. (A) Cells before 6 hours of oxygen—glucose deprivation (OGD) (preconditioning). (B) Cells after 6 hours of OGD. (C) Cells subjected to 15 hours of OGD. (D) Preconditioned cells (6 hours of OGD followed 24 hours later by 15 hours of OGD). Cell shrinkage can be observed after 15 hours, but not 6 hours, of OGD. Preexposure of cells to 6 hours of OGD followed by 15 hours of OGD 1 day later decreased cell shrinkage. Bar length = 0.5 mm.
Figure 2.
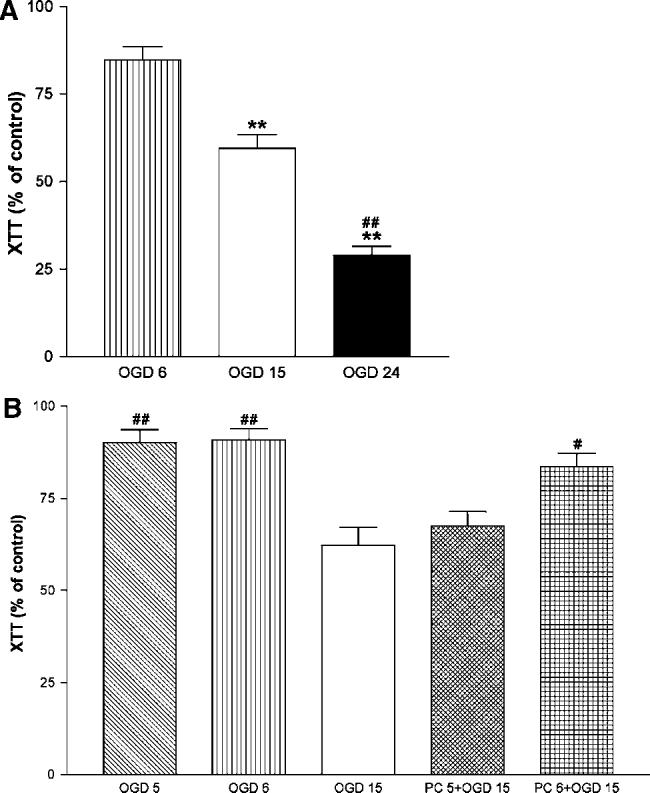
Ischemic tolerance in PC12 cells: Survival after oxygen—glucose deprivation (OGD) determined by XTT reduction immediately following OGD. PC12 cells were incubated for the indicated times in low-serum medium without glucose in hypoxic modular chambers. (A) Effect of 6, 15, and 24 hours of oxygen—glucose deprivation (OGD 6, OGD 15 and OGD 24, respectively). (B) Effects of OGD 5 and OGD 6 on OGD 15, 24 hours later (PC 5 + OGD 15 and PC 6 + OGD 15, respectively). Results represent percentage of control values (no exposure to OGD) expressed as means±s.d. (n = 8 to 10). **P<0.001 versus OGD 6; # and ## P<0.01 and P<0.001 versus OGD 15, respectively (repeated measures ANOVA with Newman— Keuls post hoc tests).
Figure 3.
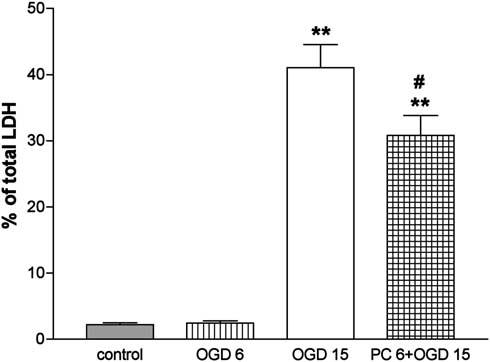
Ischemic tolerance in PC12 cells: Cell death after oxygen—glucose deprivation (OGD) revealed by lactate dehydrogenase (LDH) release. Extracellular LDH activity was expressed as a percentage of total LDH activity (extracellular + intracellular). Effects of 6 and 15 hours of OGD (OGD 6 and OGD 15) and effects of OGD 6 on OGD 15, 24 hours later (PC 6 + OGD 15). Results are expressed as means±s.d. (n = 7). **P<0.001 versus control and OGD 6; #P<0.01 versus OGD 15 (repeated measures ANOVA with Newman—Keuls post hoc tests).
OGD-Induced Apoptosis in PC12 Cells
One of the earliest changes in apoptosis occurs at the plasma membrane and leads to loss of asymmetry. An early and ubiquitous event in apoptosis is the flipping of phosphatidyl serine (PS) from the inner to the outer leaflet of the lipid bilayer allowing its detection by the Ca2+-dependent phosphatidyl-binding protein Annexin V, which can be coupled to biotin or fluorescein for visualization. Chromatin condensation and DNA fragmentation are other early apoptotic events. Nuclei of early apoptotic cells can be stained with nucleic acid dyes such as the fluorophore Hoechst 33342. Both Annexin V and Hoechst 33342 can be used in combination with PI, a cell-impermeant dye that stains cells with compromised membrane integrity. Propidium idodide is excluded from early apoptotic cells; increased membrane permeability that occurs at later stages of apoptosis or during necrosis allows PI to enter the cell. Mixed populations of viable, early apoptotic, late apoptotic, and necrotic cells can be accurately distinguished and quantified based on their different staining patterns (Hamel et al, 1996; Koopman et al,1994;Rimon et al, 1997). Fluorescence-activated cell sorting analysis of Annexin V, Hoechst 33342, and PI-stained cells demonstrated that apoptosis is the main process involved in OGD-induced cell death in PC12 cells. After 6 hours of OGD, most cells (∼95%) were viable with the remaining being either early apoptotic (∼2%) or necrotic (∼3%). After 15 hours of OGD, a pattern of increased Annexin V-positive, PI-negative apoptotic cells emerged together with several patterns of Hoechst 33342 staining in combination with low levels of PI staining. Necrotic cells were highly stained with PI (Figures 4 and 5). Quantitative analysis showed that 15 hours of OGD produced a 10-fold increase in the number of apoptotic cells compared to controls. Necrotic cells also increased significantly after 15 hours of OGD, but less so (∼2 to 3-fold) (Figure 5). Furthermore, preexposure of PC12 cells to 6 hours of OGD, attenuated cell death induced by 15 hours of OGD 1 day later (as previously demonstrated with XTT and LDH analysis) by significantly decreasing the number of apoptotic, but not necrotic, cells (Figure 6).
Figure 4.
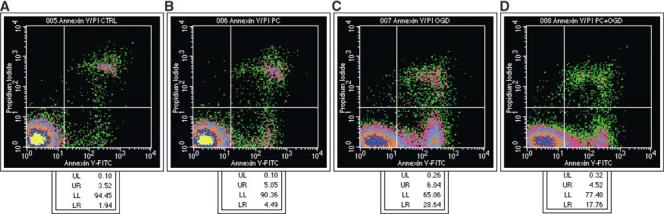
Fluorescence-activated cell sorter (FACS) analysis of ischemic preconditioned PC12 cells. PC12 cells were exposed or not exposed to 6 hours of oxygen—glucose deprivation (OGD), reoxygenated for 24 hours and subjected to 15 hours of OGD. Immediately thereafter, cells were harvested and an aliquot of 106 cells/sample was simultaneously stained for FITC-Annexin V and PI. An aliquot of 20,000 cells was analyzed by FACS and the results were displayed as a bivariate distribution of Annexin V and PI fluorescence intensity. (A—D) The results shown in each panel are representative of eight independent experiments. (A) Control cells. (B) Cells exposed to 6 hours of OGD. (C) Cells subjected to 15 hours of OGD. (D) Preconditioned cells subjected to 15 hours of OGD. Cells in the lower-left quadrant (LL), unstained for both Annexin V and PI, are defined as viable cells. Cells in the lower-right quadrant (LR), stained for Annexin V but negative for PI, are defined as early-medium apoptotic cells. Cells in the upper-right quadrant (UR), positive for both Annexin V and PI, represent the late apoptotic and necrotic populations. The percentage of cells in each quadrant is shown at the bottom of each corresponding panel.
Figure 5.
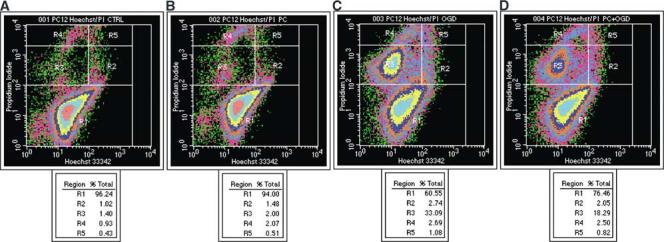
FACS analysis of ischemic and ischemic-preconditioned PC12 cells using PI and Hoechst 33342 labeling. PC12 cells were treated as described in the legend of Figure 2, then simultaneously stained for Hoechst 33342 and PI. Fluorescence signal intensities of 100,000 cells were quantified by FACS and the conjoint signals of Hoechst 33342 and PI staining displayed in a pseudocolored dot-density plot. (A—D) The results shown in each panel are representative of eight independent experiments. (A) Control cells. (B) Cells exposed to 6 hours of OGD. (C) Cells subjected to 15 hours of OGD. (D) Preconditioned cells subjected to 15 hours of OGD. Hoechst 33342 stains all nuclei and PI stains nuclei of cells with a disrupted plasma membrane. Cells in R1, with intact nuclei stained for Hoechst 33342, but unstained for PI are defined as viable cells. Cells in R2 represent early apoptosis where chromatin becomes condensed and stains more intensely with Hoechst 33342. Cells in R3 with fragmented nuclei and a low level of PI staining are defined as middle/late apoptotic cells. Cells in R4with fragmented nuclei that have lost membrane integrity and are therefore highly stained with PI represent the very late apoptosis population. Cells in R5 with loss of membrane integrity and high-level PI staining, but no DNA fragmentation represent necrotic cells. The percentage of cells in each fraction is shown at the bottom.
Figure 6.
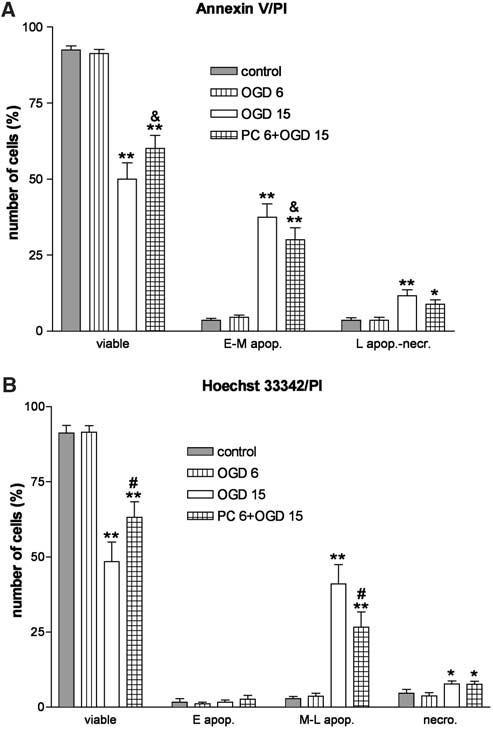
Summary of FACS analysis of ischemic and ischemic preconditioned PC12 cells. Effects of 6 and 15 hours of oxygen—glucose deprivation (OGD 6 and OGD 15) and effects of OGD 6 on OGD 15, 24 hours later (PC 6 + OGD 15) are shown. (A) Annexin/PI staining. (B) Hoechst 33342/PI staining. Results represent number of cells (% of total) and are expressed as means±s.d. (n = 8). * and **P<0.01 and P<0.001 versus respective control; #P<0.01 versus OGD 15; &P<0.05 versus OGD 15 (repeated measures ANOVA with Newman— Keuls post hoc tests).
Because of extensive DNA cleavage occurring during apoptosis, cells at a later stage of apoptosis can be identified on the basis of their total DNA content (Darzynkiewicz et al, 1992). Previously fixed cells are stained with a fluorescent dye that binds DNA quantitatively and are analyzed for their DNA content by flow cytometry. Individual cells in a population can be classified based on their total DNA content using fluorescence intensity. Typically, cells at a late stage of apoptosis will exhibit a ‘sub G1’ peak (fraction M1 in Figure 7). FACS analysis of DNA distributions in PC12 cells labeled with PI showed a negligible ‘sub G1’ fraction in control cells or in cells subjected to 6 hours of OGD (means±s.d., 5.3±0.6 and 4.2±0.3, respectively; n = 3). After 15 hours of OGD there was a 5-fold increase in the mean±s.d. of the ‘sub G1’ fraction (28.2710.5; ANOVA: P<0.05). The effect of 15 hours of OGD was reduced to 3-fold, when cells were preexposed to 6 hours of OGD (mean±s.d., 17.476.9) (Figure 7).
Figure 7.
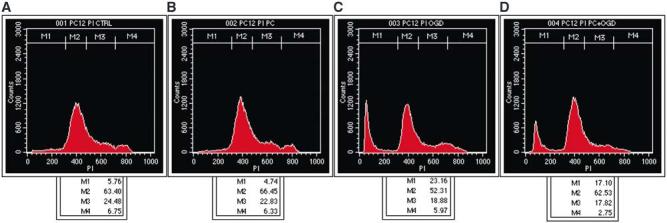
DNA content histograms of PC12 cells. Cells were harvested and fixed in 70% ethanol, followed by staining with 5 μg/mL PI to reveal total DNA content. Data represent 10,000 cell histograms and distribute in the following fractions: the cells in M1 are hypodiploid (‘sub G1’ fraction), typically late apoptotic cells. The M2 fraction represents the diploid cell population including cells in G0/G1 phase, early apoptotic and necrotic cells. The M3 fraction represents cells replicating their DNA (cells in S-phase) and the M4 fraction represents tetraploid cells (G2/M phase). (A) Control cells; (B) cells after 6 hours of OGD; (C) cells subjected to 15 hours of OGD; and (D) preconditioned cells (6 hours of OGD followed 24 hours later by 15 hours of OGD).
Oxygen—glucose deprivation-induced activation of apoptotic pathways in PC12 cells was further confirmed by analysis of caspase-3 activation. Cultures were stained with antibodies directed against cleaved caspase-3. There was a 4-fold to 5-fold increase in the mean±s.d. of positive cells per well when exposed to 15 hours of OGD versus controls (1489±417 versus 349±90; n = 3; P<0.05). No increase in caspase-3 activation was observed after 6 hours of OGD (data not shown).
Discussion
Tolerance to ischemia and hypoxia can be modeled in vitro and has been described in cultured primary cells including cortical neurons (Bruer et al, 1997; Gonzalez-Zulueta et al, 2000; Grabb and Choi, 1999; Liu et al, 2000; Meloni et al, 2002; Sakaki et al, 1995; Snider et al, 1998; Tauskela et al, 1999; Tremblay et al, 2000; Weih et al, 1999), hippocampal neurons (Khaspekov et al, 1998), cerebellar granule neurons (Prass et al, 2002; Ruscher et al, 2002; Wick et al, 2002), astrocytes and brain capillary endothelial cells (Ginis et al, 1999). However, no ischemic tolerance model has yet been developed in any cell line. The present study demonstrates ischemic preconditioning in a PC12 cell line. PC12 cells can differentiate and exhibit features of sympathetic neurons under the influence of nerve growth factor (Greene and Tischler, 1976). Since they were established as a cell line more than 25 years ago, PC12 cells have been the object of intense investigation in neurobiology for the study of signal transduction mechanisms that regulate physiological and pathological functions of neurons (Tischler , 2002). For example, studies of cell differentiation and survival (Agell et al, 2002; Anneren et al, 2003; Miller and Kaplan, 2001; Szeberenyi , 1996; Vaudry et al, 2002), apoptosis (Macdonald et al, 2003; Valavanis et al, 2001), Ca2+ signaling (Ghosh et al, 1994), Parkinson's disease (Elkon et al, 2001; Ryu et al, 2002), Alzheimer's disease (Ge and Lahiri, 2002; Leutz et al, 2002) and Huntington's disease (Peters et al, 2002; Sipione and Cattaneo, 2001) have been conducted in PC12 cell model systems. In addition, the ability of PC12 cells to respond to hypoxia (Seta et al, 2002) satisfies an important requirement for an in vitro ischemia model.
We observed no significant PC12 death for up to 6 hours of OGD exposure. This interval corresponds to the maximal nonlethal stress that can be tolerated by PC12 cells since cellular death increased with longer OGD exposures (data not shown). This period of OGD was thus used as a reproducible level of stress for ischemic preconditioning. Fifteen hours of OGD constituted a suitable lethal ischemic stress for study of PC12 cell cytoprotection in that it induced apoptosis and necrosis in about 40% of the cells. Six hours of preconditioning OGD in PC12 cells followed by 24 hours of normoxia (‘reperfusion’) was found to confer significant cytoprotection from a subsequent 15 hours of OGD. We also observed PC12 cell protection with a 48-hours-reperfusion interval that was similar to that observed after a 24-hours interval (data not shown). Cell death was quantified by LDH release, mitochondrial reduction of XTT, and FACS analysis. These assays gave comparable results. In addition, FACS permitted the relative role of apoptosis and necrosis to be assessed. Our data obtained by FACS analysis of an early-stage marker (Annexin V) combined with an early- to middle-stage marker (Hoechst 33342) of apoptosis and a selective marker for necrosis (PI), showed that apoptosis occurs during OGD and is responsible for most of the observed cell death and that protection afforded by preconditioning prevents, or at least delays, apoptotic cell death. This was further demonstrated with the analysis of total DNA content, which revealed cells with ‘sub-G1’ peak undergoing DNA fragmentation characteristic of apoptotic cells. Immunocytochemistry of caspase-3, a central executioner in the apoptotic process, showed that this enzyme increased approximately four- to five-fold, thus confirming activation of apoptotic pathways in OGD-induced PC12 cell death.
In conclusion, we describe here ischemic tolerance in the PC12 cell line. The model provides a new tool for the identification of pathways involved in ischemic tolerance. Ischemic tolerance can be one example of a more broad, general-stress response of the cell. Therefore, the present model could be applied to the study of the mechanisms involved in tolerance to other stressful stimuli. Cell-based assays with high-throughput capacity can be used as direct screens and models to explore molecular mechanisms involved in cellular function and pathology. In neurobiology, the most predictive cell system is likely to be cultured neurons. However, primary neurons are laborious and time-consuming to grow. Additionally, the scarcity of material makes primary cells difficult to use for FACS analysis and for classical biochemical assays. In addition, primary cultures are impractical for high-throughput screens that require a large number of cells. In contrast, cell lines are suitable for genomic and proteomic approaches and represent an attractive model system in the early stages of the discovery process. Furthermore, immortalized cell lines such as PC12 cells are suitable for high-throughput cellular screen technology for identifying molecular targets and for chemical genetics, a new productive strategy in drug discovery that uses small molecules to disrupt a signal transduction cascade (Croston , 2002; Stockwell , 2002; Zheng and Chan, 2002).
References
- Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal. 2002;14:649–54. doi: 10.1016/s0898-6568(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Anneren C, Lindholm CK, Kriz V, Welsh M. The FRK/RAK-SHB signaling cascade: a versatile signal-transduction pathway that regulates cell survival, differentiation and proliferation. Curr Mol Med. 2003;3:313–24. doi: 10.2174/1566524033479744. [DOI] [PubMed] [Google Scholar]
- Bruer U, Weih MK, Isaev NK, Meisel A, Ruscher K, Bergk A, Trendelenburg G, Wiegand F, Victorov IV, Dirnagl U. Induction of tolerance in rat cortical neurons: hypoxic preconditioning. FEBS Lett. 1997;414:117–21. doi: 10.1016/s0014-5793(97)00954-x. [DOI] [PubMed] [Google Scholar]
- Chanda SK, Caldwell JS. Fulfilling the promise: drug discovery in the post-genomic era. Drug Discov Today. 2003;8:168–74. doi: 10.1016/s1359-6446(02)02595-3. [DOI] [PubMed] [Google Scholar]
- Croston GE. Functional cell-based uHTS in chemical genomic drug discovery. Trends Biotechnol. 2002;20:110–5. doi: 10.1016/s0167-7799(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz MA, Lassota P, Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Davidov E, Holland J, Marple E, Naylor S. Advancing drug discovery through systems biology. Drug Discov Today. 2003;8:175–83. doi: 10.1016/s1359-6446(03)02600-x. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–54. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Elkon H, Melamed E, Offen D. 6-Hydroxydopamine increases ubiquitin-conjugates and protein degradation: implications for the pathogenesis of Parkinson's disease. Cell Mol Neurobiol. 2001;21:771–81. doi: 10.1023/A:1015160323009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge YW, Lahiri DK. Regulation of promoter activity of the APP gene by cytokines and growth factors: implications in Alzheimer's disease. Ann NY Acad Sci. 2002;973:463–7. doi: 10.1111/j.1749-6632.2002.tb04684.x. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Ginty DD, Bading H, Greenberg ME. Calcium regulation of gene expression in neuronal cells. J Neurobiol. 1994;25:294–303. doi: 10.1002/neu.480250309. [DOI] [PubMed] [Google Scholar]
- Ginis I, Schweizer U, Brenner M, Liu J, Azzam N, Spatz M, Hallenbeck JM. TNF-alpha pretreatment prevents subsequent activation of cultured brain cells with TNF-alpha and hypoxia via ceramide. Am J Physiol. 1999;276:C1171–1183. doi: 10.1152/ajpcell.1999.276.5.C1171. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Zulueta M, Feldman AB, Klesse LJ, Kalb RG, Dillman JF, Parada LF, Dawson TM, Dawson VL. Requirement for nitric oxide activation of p21(ras)/extracellular regulated kinase in neuronal ischemic preconditioning. Proc Natl Acad Sci USA. 2000;97:436–41. doi: 10.1073/pnas.97.1.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabb MC, Choi DW. Ischemic tolerance in murine cortical cell culture: critical role for NMDA receptors. J Neurosci. 1999;19:1657–62. doi: 10.1523/JNEUROSCI.19-05-01657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–8. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel W, Dazin P, Israel MA. Adaptation of a simple flow cytometric assay to identify different stages during apoptosis. Cytometry. 1996;25:173–81. doi: 10.1002/(SICI)1097-0320(19961001)25:2<173::AID-CYTO6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Khaspekov L, Shamloo M, Victorov I, Wieloch T. Sublethal in vitro glucose—oxygen deprivation protects cultured hippocampal neurons against a subsequent severe insult. Neuroreport. 1998;9:1273–6. doi: 10.1097/00001756-199805110-00003. [DOI] [PubMed] [Google Scholar]
- Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283–96. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–20. [PubMed] [Google Scholar]
- Leutz S, Steiner B, Marques CA, Haass C, Muller WE, Eckert A. Reduction of trophic support enhances apoptosis in PC12 cells expressing Alzheimer's APP mutation and sensitizes cells to staurosporine-induced cell death. J Mol Neurosci. 2002;18:189–201. doi: 10.1385/JMN:18:3:189. [DOI] [PubMed] [Google Scholar]
- Liu J, Ginis I, Spatz M, Hallenbeck JM. Hypoxic preconditioning protects cultured neurons against hypoxic stress via TNF-alpha and ceramide. Am J Physiol Cell Physiol. 2000;278:C144–153. doi: 10.1152/ajpcell.2000.278.1.C144. [DOI] [PubMed] [Google Scholar]
- Macdonald NJ, Delderfield SM, Zhang W, Taglialatela G. Tumour necrosis factor-alpha- vs. growth factor deprivation-promoted cell death: distinct converging pathways. Aging Cell. 2003;2:245–56. doi: 10.1046/j.1474-9728.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- Meloni BP, Majda BT, Knuckey NW. Evaluation of preconditioning treatments to protect near-pure cortical neuronal cultures from in vitro ischemia induced acute and delayed neuronal death. Brain Res. 2002;928:69–75. doi: 10.1016/s0006-8993(01)03361-3. [DOI] [PubMed] [Google Scholar]
- Miller FD, Kaplan DR. On Trk for retrograde signaling. Neuron. 2001;32:767–70. doi: 10.1016/s0896-6273(01)00529-3. [DOI] [PubMed] [Google Scholar]
- Peters PJ, Ning K, Palacios F, Boshans RL, Kazantsev A, Thompson LM, Woodman B, Bates GP, D'souzaSchorey C. Arfaptin 2 regulates the aggregation of mutant huntingtin protein. Nat Cell Biol. 2002;4:240–5. doi: 10.1038/ncb761. [DOI] [PubMed] [Google Scholar]
- Prass K, Ruscher K, Karsch M, Isaev N, Megow D, Priller J, Scharff A, Dirnagl U, Meisel A. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J Cereb Blood Flow Metab. 2002;22:520–5. doi: 10.1097/00004647-200205000-00003. [DOI] [PubMed] [Google Scholar]
- Rimon G, Bazenet CE, Philpott KL, Rubin LL. Increased surface phosphatidylserine is an early marker of neuronal apoptosis. J Neurosci Res. 1997;48:563–70. doi: 10.1002/(sici)1097-4547(19970615)48:6<563::aid-jnr9>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Freyer D, Karsch M, Isaev N, Megow D, Sawitzki B, Priller J, Dirnagl U, Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291–301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson's disease. J Neurosci. 2002;22:10690–8. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki T, Yamada K, Otsuki H, Yuguchi T, Kohmura E, Hayakawa T. Brief exposure to hypoxia induces bFGF mRNA and protein and protects rat cortical neurons from prolonged hypoxic stress. Neurosci Res. 1995;23:289–96. doi: 10.1016/0168-0102(95)00954-x. [DOI] [PubMed] [Google Scholar]
- Seta K, Kim HW, Ferguson T, Kim R, Pathrose P, Yuan Y, Lu G, Spicer Z, Millhorn DE. Genomic and physiological analysis of oxygen sensitivity and hypoxia tolerance in PC12 cells. Ann NY Acad Sci. 2002;971:379–88. doi: 10.1111/j.1749-6632.2002.tb04500.x. [DOI] [PubMed] [Google Scholar]
- Sipione S, Cattaneo E. Modeling Huntington's disease in cells, flies, and mice. Mol Neurobiol. 2001;23:21–51. doi: 10.1385/MN:23:1:21. [DOI] [PubMed] [Google Scholar]
- Snider BJ, Lobner D, Yamada KA, Choi DW. Conditioning heat stress reduces excitotoxic and apoptotic components of oxygen—glucose deprivation-induced neuronal death in vitro. J Neurochem. 1998;70:120–9. doi: 10.1046/j.1471-4159.1998.70010120.x. [DOI] [PubMed] [Google Scholar]
- Stockwell BR. Chemical genetic screening approaches to neurobiology. Neuron. 2002;36:559–62. doi: 10.1016/s0896-6273(02)01056-5. [DOI] [PubMed] [Google Scholar]
- Szeberenyi J. Gene activation pathways of nerve growth factor signaling: a minireview. Neurobiology (Bp) 1996;4:1–11. [PubMed] [Google Scholar]
- Tauskela JS, Chakravarthy BR, Murray CL, Wang Y, Comas T, Hogan M, Hakim A, Morley P. Evidence from cultured rat cortical neurons of differences in the mechanism of ischemic preconditioning of brain and heart. Brain Res. 1999;827:143–51. doi: 10.1016/s0006-8993(99)01322-0. [DOI] [PubMed] [Google Scholar]
- Thiericke R. High-throughput screening technologies. EXS. 2003;93:71–85. doi: 10.1007/978-3-0348-7997-2_4. [DOI] [PubMed] [Google Scholar]
- Tischler AS. Chromaffin cells as models of endocrine cells and neurons. Ann NY Acad Sci. 2002;971:366–70. doi: 10.1111/j.1749-6632.2002.tb04498.x. [DOI] [PubMed] [Google Scholar]
- Tremblay R, Chakravarthy B, Hewitt K, Tauskela J, Morley P, Atkinson T, Durkin JP. Transient NMDA receptor inactivation provides long-term protection to cultured cortical neurons from a variety of death signals. J Neurosci. 2000;20:7183–92. doi: 10.1523/JNEUROSCI.20-19-07183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanis C, Hu Y, Yang Y, Osborne BA, Chouaib S, Greene L, Ashwell JD, Schwartz LM. Model cell lines for the study of apoptosis in vitro. Methods Cell Biol. 2001;66:417–36. doi: 10.1016/s0091-679x(01)66019-9. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Stork PJ, Lazarovici P, Eiden LE. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002;296:1648–9. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- Weih M, Kallenberg K, Bergk A, Dirnagl U, Harms L, Wernecke KD, Einhaupl KM. Attenuated stroke severity after prodromal TIA: a role for ischemic tolerance in the brain? Stroke. 1999;30:1851–4. doi: 10.1161/01.str.30.9.1851. [DOI] [PubMed] [Google Scholar]
- Wick A, Wick W, Waltenberger J, Weller M, Dichgans J, Schulz JB. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci. 2002;22:6401–7. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XF, Chan TF. Chemical genomics in the global study of protein functions. Drug Discov Today. 2002;7:197–205. doi: 10.1016/s1359-6446(01)02118-3. [DOI] [PubMed] [Google Scholar]


