Abstract
Nearly 500 alkaloids have been detected in skin extracts from frogs of the family Dendrobatidae. All seem to have been sequestered unchanged into skin glands from alkaloid-containing arthropods. Ants, beetles, and millipedes seem to be the source of decahydroquinolines, certain izidines, coccinellines, and spiropyrrolizidine oximes. But the dietary source for a major group of frog-skin alkaloids, namely the pumiliotoxins (PTXs), alloPTXs, and homoPTXs, remained a mystery. In hopes of revealing an arthropod source for the PTX group, small arthropods were collected from eight different sites on a Panamanian island, where the dendrobatid frog (Dendrobates pumilio) was known to contain high levels of two PTXs. The mixed arthropod collections from several sites, each representing up to 20 arthropod taxa, contained PTX 307A and/or alloPTX 323B. In addition, the mixed arthropod collections from several sites contained a 5,8-disubstituted indolizidine (205A or 235B), representing another class of alkaloids previously unknown from an arthropod. An ant alkaloid, decahydroquinoline 195A, was detected in the mixed arthropod collections from several sites. Thus, “combinatorial bioprospecting” demonstrates that further collection and analysis of individual taxa of leaf-litter arthropods should reveal the taxa from which PTXs, alloPTXs, and 5,8-disubstituted indolizidines are derived.
Alkaloids are basic compounds, with nitrogen usually present in a ring, and have a limited distribution in nature; the great majority are of plant origin (1). The first examples of “animal” alkaloids were the samandarines from the European fire salamander (2). The samandarines apparently are synthesized by the salamander (G. Habermehl, personal communication cited in ref. 3). Over 500 alkaloids of 22 different structural classes have now been detected in skin extracts from certain genera in four different families of frogs and toads (3). Initially, such alkaloids were called “dendrobatid” alkaloids after the family Dendrobatidae, from which they first were isolated (4). They were thought to be synthesized by the frogs. However, when raised in captivity, dendrobatid frogs had no detectable alkaloids in their skin (5, 6). When fed alkaloids, captive-raised frogs readily accumulated the alkaloids unchanged into skin (7). It now seems that all dendrobatid alkaloids are of dietary origin (8, 9), which for such frogs consists of small, even tiny arthropods. Indeed, the common evolutionary event leading to skin alkaloids in dendrobatid frogs, Madagascan mantellid frogs (10), and South American bufonid toads (11) is probably the overexpression of an uptake system for sequestration of dietary alkaloids into skin glands. Australian myobatrachid frogs of the genus Pseudophryne synthesize their own unique indolic pseudophrynamines while sequestering pumiliotoxins (PTXs) from a dietary source (12).
The likely sources for several classes of the frog alkaloids have been identified. Myrmicine ants seem to be the source of the 3,5-disubstituted pyrrolizidines, the 3,5-disubstituted indolizidines, the 4,6-disubstituted quinolizidines, the 2,5-disubstituted pyrrolidines, the 2,6-disubstituted piperidines, and the 2,5-disubstituted decahydroquinolines (DHQs) (13, 14). It is likely that the 3,5-disubstituted lehmizidines (15), the histrionicotoxins, and the gephyrotoxins will prove to be of ant origin, based on certain structural analogies to known ant alkaloids, namely an unbranched carbon skeleton and often a terminal acetylene. The coccinellines and structurally related tricylics are likely of beetle origin, whereas the spiropyrrolizidine oximes are likely of millipede origin (14). However, the sources for the steroidal batrachotoxins, the cardiotonic PTXs, and the analgetic epibatidine remained a mystery.
The PTXs and alloPTXs occur widely among all lineages of frogs/toads that contain sequestered lipophilic alkaloids (16). Thus, the dietary source for these alkaloids must be widely distributed in a variety of tropical and semitropical habitats. Yet providing arthropods from leaf litter, by using a funnel technique, to dendrobatid frogs (Dendrobates auratus) being raised in terraria resulted in no detectable accumulation of a PTX that was a major alkaloid in wild-caught frogs from that leaf-litter site in Panamá (8). Histrionicotoxins, a gephyrotoxin and a DHQ, all probably of ant origin, were detected, as were spiropyrrolizidines, precoccinelline, and other tricyclics. In a subsequent study, the arthropod-containing fresh leaf litter from that same site was provided to D. auratus being raised in outside, screened cages (14). Again, no PTXs were detected in skin extracts, although ant, beetle, and millipede alkaloids were found.
Analysis of arthropod extracts, primarily ants, obtained during field work on dendrobatid and mantellid frogs over the past 10 years has not revealed any PTXs, and discovery of the dietary source of PTXs represented a major challenge. It was decided to address this challenge by making an extensive forceps-mediated collection of small leaf-litter arthropods from a site where skin extracts from frogs contained high levels of PTXs. Populations of the small dendrobatid frog, Dendrobates pumilio, from Isla Bastimentos, Bocas, Panamá, were chosen, because frogs from that area contained high levels of PTXs 307A and 323A (ref. 17, see gas chromatogram in ref. 4). However, it was uncertain whether such an extensive collection would reveal PTXs in any of the many taxa of small leaf-litter arthropods. Therefore, a combinatorial approach was adopted, whereby all of the small arthropods from each of eight sites on Isla Bastimentos were collected together for analysis. With three collectors and eight collection sites, this combinatorial bioprospecting provided 22 mixed collections of arthropods, each containing up to 20 different major taxa. Analysis by gas chromatography-mass spectrometry (GC-MS) led to detection of a PTX, an alloPTX, two 5,8-disubstituted indolizidines, a DHQ, and a spiropyrrolizidine. For structures see Fig. 1. The results indicate that analysis of individually collected arthropod taxa at these sites should allow identification of arthropod sources for PTXs, alloPTXs, and 5,8-disubstituted indolizidines.
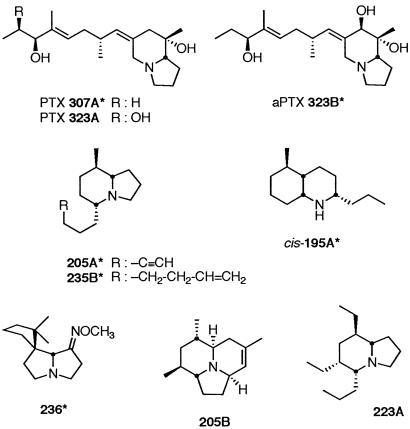
Fig 1.
Alkaloid structures. *, Detected in both frog-skin and arthropod extracts.
Materials and Methods
Field Collection and Analysis.
Collections of frogs (D. pumilio) and leaf-litter arthropods were made on March 17, 19, and 20–22, 2000 (by J.W.D., M.A.D., and A.E.), at eight sites on the western end of Isla Bastimentos (see Table 1). Sites I (collected twice) and II–V were very close to one another on the north coast of Isla Bastimentos to the east of the town of Bastimentos. Site VI was on the coast of a promontory, Punta San Juan, facing the town of Bastimentos to the north. Sites VII and VIII were on the south coast, several kilometers away from the other sites.
Table 1.
Presence of alkaloids in skin extracts of the frog D. pumilio and in mixed extracts of microsympatric arthropods in sites I–VIII on Isla Bastimentos, Panamá
| Site I. North coast forest below lighthouse, cleared understory. Leaf litter: abundant. |
| Frogs: Major: 5,8-I 205A, 235B. Minor: PTX 237A, 251D, 307A; DHQ 195A; 5,6,8-I 223A; Tricyclic 205B. |
| Arthropods: aPTX 323B; DHQ 195A; 5,8-I 205A. |
| Site II. North coast forest with Cyclanthus pata and cacao. Leaf litter: abundant. |
| Frogs: Major: 5,8-I 235B. Minor: PTX 307A, 323A; aPTX 323B; DHQ 195A; 5,8-I 233D; Tricyclic 205B; SpiroP 236. |
| Arthropods: PTX 307A; aPTX 323B; DHQ 195A; 5,8-I 235B. |
| Site III. North coast lightly forested slope, abundant Heliconia. Leaf litter: sparse. |
| Frogs: Major: PTX 307A, 323A; DHQ 195A; 5,8-I 235B. Minor: aPTX 323B; 5,8-I 205A, 207A; 5,6,8-I 223A; Tricyclic 205B. |
| Arthropods: PTX 307A, DHQ 195A. |
| Site IV. North coast forest with Rutacea and some Heliconia. Leaf litter: abundant. |
| Juvenile frogs: DHQ 195A; 5,8-I 235B. Trace: Tricyclic 205B. |
| Arthropods: aPTX 323B; DHQ 195A; 5,8-I 235B; SpiroP 236. |
| Site V. North coast hillside forest with many palms. Leaf litter: abundant. |
| Frogs: Major: 5,8-I 235B. Minor: PTX 307A, 323A; alloPTX 323B; DHQ 195A; 5,8-I 233D, 5,6,8-I 223A; Tricyclic 205B; SpiroP 236, 252A. |
| Arthropods: PTX 307A; DHQ 195A, 5,8-I 205A; SpiroP 236. |
| Site VI. Coastal forest, cleared understory, facing north to Bastimentos. Leaf litter: sparse. |
| Frogs: Major: PTX 307A, 323A; DHQ 195A; 5,8-I 235B. Minor: aPTX 323B; 5,8-I 205A, 233D, 235B; 5,6,8-I 223A, 235E; Tricyclic 205B. |
| Arthropods: DHQ 195A; 5,8-I 235B. |
| Site VII. South coast secondary forest. Leaf litter: abundant. |
| Frogs: Major: 5,8-I 205A, 235B; 5,6,8-I 223A. Minor: DHQ 195A; 3,5-I 195B; 5,6,8-I 237C. |
| Arthropods: 5,8-I 235B; SpiroP 236. |
| Site VIII. South coast forested trail near crest. Leaf litter: sparse. |
| Frogs: Major: DHQ 195A; 5,6,8-I 223A. Minor: aPTX 267A; 3,5-P 251K; 5,8-I 205A. |
| Arthropods: No alkaloids detected. |
A detailed analysis of all alkaloids, including some previously undetected trace alkaloids, will be published elsewhere. aPTX, alloPTX; 3,5-P, 3,5-disubstituted pyrrolizidine; 3,5-I and 5,8-I disubstituted indolizidines; 5,6,8-I, 5,6,8-trisubstituted indolizidine; SpiroP, spiropyrrolizidine. The coordinates of sites are: site I, N 9° 21.6′, W 82° 12.1′; site II, N 9° 21.2′, W 82° 12.5′; site III, N 9° 21.2′, W 82° 12.6′; site IV, N 9° 12.1′, W 82° 12.7′; site, V N 9° 21.1′, W 82° 12.8′; site, VI N 9° 20.5′, W 82° 12.4′; site VII, N 9° 20.4′, W 82° 10.8′; site VIII, N 9° 19.2′, W 82° 9.2′.
Two collections made at site I, one on March 17, 2002, and one on March 19, 2002.
Present as a trace alkaloid in frogs from this site.
No adults were collected. The levels of alkaloids in the juveniles were very low.
At each site for 1–1.5 h, leaf litter was placed on white cloth bags, and all small arthropods were collected with forceps and placed together into methanol in small (3.5-ml) plastic vials for analysis. Millipedes and centipedes were placed in separate vials. The analysis of the millipede extracts will be reported elsewhere. Specimens of D. pumilio, which were abundant at each site, were collected and killed, and five skins per site were placed in methanol for analysis. Only juveniles (snout-vent length, 10–12 mm) were collected at site IV. Voucher specimens were deposited in the herpetological collection housed at Florida International University.
Each methanol extract of mixed arthropods was carefully concentrated to a small volume and analyzed by GC-MS using a Finnigan GCQ mass spectrometer with a 30-m Rtx-5 column (0.25 mm i.d.) programmed from 100 to 280°C at 10°/min (13). The analysis revealed indolizidine 235B in one collection of arthropods from site VII (see Table 1). After further concentration, another analysis revealed alkaloids in 14 more collections.
Each methanol extract from five frog skins was prepared and partitioned as described (8) to yield a methanolic alkaloid fraction. Major and minor alkaloids (see Fig. 2) were detected with a flame-ionization detector after GC on a 6-foot 1.5% OV-1 packed column (2 mm i.d.), programmed from 150 to 280°C at 10°/min. Injection volume was 2 μl, corresponding to 2 mg wet weight frog skin (8). The alkaloids then were subjected to analysis by GC-MS using the GCQ mass spectrometer.
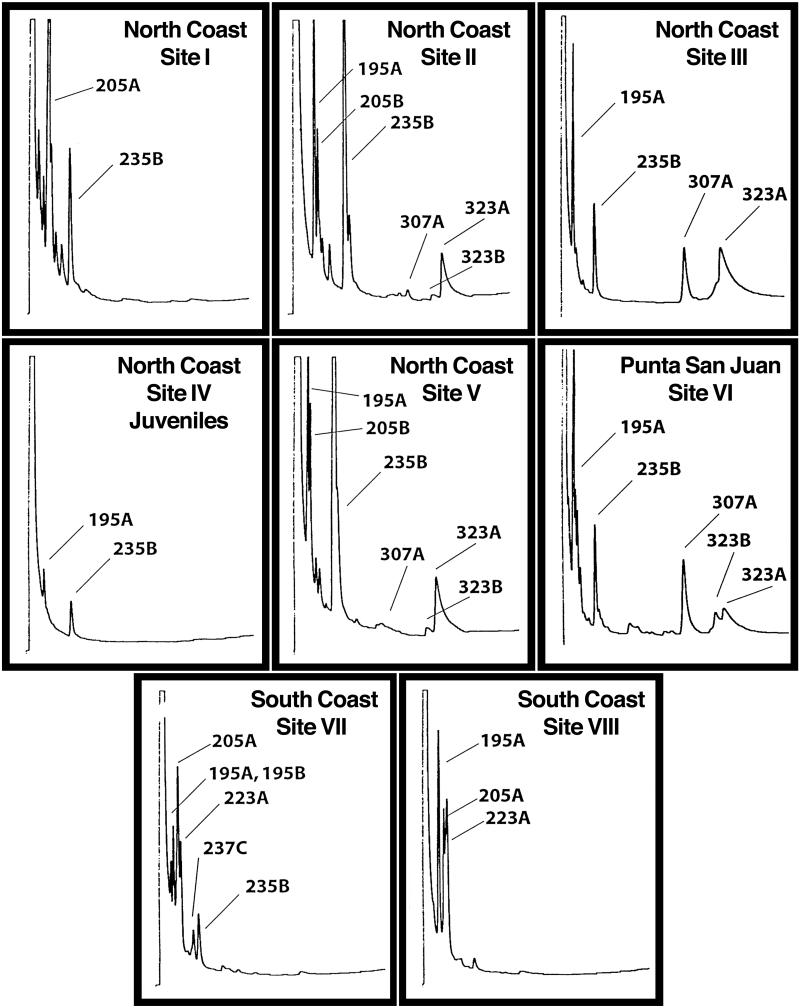
Fig 2.
GC profiles of alkaloids in skins of dendrobatid frogs (D. pumilio) from different sites on Isla Bastimentos, Bocas, Panamá, by flame-ionization detection (see Materials and Methods for conditions).
Results
The nature of the arthropods in each collection and the alkaloids detected are presented in Table 2. In addition to arthropods, four gastropods and nine worms were present. The presence of such invertebrates was not associated with the detection of any alkaloid class. Alkaloids were detected in 15 of the 22 mixed arthropod collections. Combined skin extracts from five frogs were analyzed to obtain a profile deemed typical for frogs at each site (see Fig. 2 for GC scans). Major and minor alkaloids of frog skin for each site and the alkaloids detected in arthropods from each site are compared in Table 1. The present results can be summarized as follows:
PTX 307A and alloPTX 323B was present in several collections; a unique arthropod source was not apparent.
5,8-Disubstituted indolizidines 205A and 235B were present in several collections; a unique arthropod source was not apparent.
DHQ 195A, an alkaloid known to occur in ants (13, 14), was present in two collections in which no ants appeared to be present (Table 2) but was detected in ants (Solenopsis sp.) from one site.
Spiropyrrolizidine 236, a presumed millipede alkaloid (14), was present in two collections in which no millipedes appeared to be present (Table 2) but was detected in millipede collections (data not shown).
Table 2.
Detection of alkaloids in combined extracts of leaf-litter arthropods from dendrobatid frog sites
| Site
|
Collector
|
Alkaloids
|
Arthropods | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ant | Beetle | Diplura | Diptera | Hemiptera | Isopod | Larva | Mite | Orthoptera | Pseudoscorpion | Spider | Springtail | Thrip | Tick | Weevil | Miscellaneous | |||
| I (3/17/00) | A.E./M.A.D. | 5,8-I 205A | 4 (3) | 4 (3) | — | — | 1 (1) | 13 (1) | 12 (3) | 1 (1) | — | 1 (1) | 5 (5) | — | — | — | 2 (2) | 3 millipede |
| J.W.D. | DHQ 195A | — | 1 (1) | — | — | — | 12 (1) | 1 (1) | 1 (1) | — | — | 1 (1) | 4 (1) | — | 1 (1) | — | 2 termite | |
| I (3/20/00) | A.E./M.A.D. | DHQ 195A | 6 (4) | 1 (1) | 1 (1) | — | — | 12 (1) | 4 (2) | 6 (2) | — | 3 (1) | 4 (4) | 3 (2) | — | 2 (2) | 3 (1) | 1 millipede, 1 wasp |
| J.W.D. | aPTX 323B | 32 (6) | 2 (2) | — | — | — | 22 (1) | 6 (5) | 9 (3) | — | 4 (1) | 6 (6) | 3 (1) | 4 (1) | 1 (1) | — | 1 cricket | |
| II | A.E. | 5,8-I 235B PTX 307A | 12 (7) | 2 (2) | 1 (1) | — | 1 (1) | 15 (1) | 3 (3) | 5 (3) | 1 (1) | 7 (1) | 23 (17) | 7 (3) | — | 5 (2) | — | 1 bee |
| M.A.D. | PTX 307A aPTX 323B | 24 (8) | 10 (4) | — | — | 7 (2) | 32 (2) | 3 (3) | 5 (3) | — | 5 (1) | 24 (13) | 9 (3) | 1 (1) | 5 (3) | 1 (1) | — | |
| J.W.D. | DHQ 195A PTX 307A | 78 (7) | 1 (1) | 2 (1) | 1 (1) | 2 (2) | 11 (1) | 6 (4) | 4 (2) | — | 1 (1) | 9 (1) | 1 (1) | — | 2 (1) | — | 6 wasp | |
| III | A.E./M.A.D. | — | 16 (6) | 4 (4) | 4 (2) | — | 1 (1) | 13 (1) | 5 (5) | 1 (1) | — | 6 (1) | 5 (3) | 8 (5) | — | 1 (1) | — | 2 centipede, 3 millipede |
| J.W.D. | DHQ 195A PTX 307A | 3 (2) | 2 (2) | 3 (2) | — | — | 4 (1) | 2 (2) | — | — | 1 (1) | 2 (2) | — | — | — | — | 1 centipede, 1 roach | |
| IV | A.E. | 5,8-I 235B aPTX 323B | 8 (1) | 5 (3) | 2 (1) | — | 2 (2) | 21 (1) | 10 (3) | 3 (3) | — | 8 (2) | 9 (1) | 4 (1) | 2 (1) | — | — | — |
| M.A.D. | — | 5 (4) | 3 (3) | — | 1 (1) | — | 2 (1) | — | 2 (1) | — | — | 2 (1) | 5 (2) | — | — | — | 1 roach | |
| J.W.D. | 5,8-I 235B, DHQ 195A, 236 | 9 (1) | — | 1 (1) | — | — | 5 (1) | 13 (5) | 3 (1) | — | 1 (1) | 3 (3) | 2 (2) | — | 1 (1) | — | 1 roach, 1 wasp | |
| V | A.E./M.A.D. | 236 | 15 (5) | 6 (5) | — | 1 (1) | 2 (2) | 8 (1) | 13 (6) | 8 (3) | 1 (1) | 5 (1) | 17 (10) | 4 (3) | — | — | — | 1 millipede, 1 termite |
| J.W.D. | 5,8-I 205A DHQ 195A, PTX 307A, 236 | — | — | — | — | 1 (1) | 4 (1) | 2 (2) | 1 (1) | — | — | 6 (4) | 2 (1) — | — | — | — | 1 millipede, 1 roach, 1 termite | |
| VI | A.E. | — | 45 (8) | 4 (4) | 3 (3) | — | — | 8 (1) | 10 (9) | 1 (1) | — | 1 (1) | 11 (10) | 2 (2) | — | 2 (2) | — | 1 bee, 1 roach |
| M.A.D. | — | 28 (1) | 3 (3) | — | — | — | — | 3 (3) | — | — | 1 (1) | 4 (4) | 6 (5) | — | 1 (1) | — | 1 cricket, 11 termite | |
| J.W.D. | 5,8-I 235B, DHQ 195A | 30 (2) | 1 (1) | 2 (1) | — | — | 4 (1) | — | 2 (2) | — | 1 (1) | 6 (6) | — | — | 2 (2) | — | 1 cricket, 1 roach, 2 termite, 8 wasp | |
| VII | A.E./M.A.D. | 5,8-I 235B | 15 (6) | 3 (3) | 1 (1) | 1 (1) | — | 3 (1) | 14 (11) | 3 (1) | — | 3 (1) | 8 (8) | 12 (4) | 18 (2) | 1 (1) | — | — |
| J.W.D. | 236 | 12 (3) | — | — | — | 1 (1) | 8 (1) | — | — | — | — | 8 (8) | 1 (1) | — | — | — | ||
| VIII | A.E. | — | 28 (10) | 23 (8) | — | — | — | 13 (1) | 5 (4) | 3 (1) | — | 2 (1) | 14 (9) | 5 (3) | 1 (1) | 1 (1) | 1 (1) | 1 roach, 2 wasp |
| M.A.D. | — | 5 (3) | 5 (3) | 2 (2) | 1 (1) | 1 (1) | 14 (1) | 2 (1) | 3 (3) | — | 4 (1) | 9 (7) | — | — | — | 1 (1) | 1 cricket, 1 roach | |
| J.W.D. | — | 27 (2) | 4 (3) | 1 (1) | — | — | 6 (1) | 6 (5) | — | — | 3 (1) | 8 (7) | 1 (1) | — | — | — | 3 cricket, 7 wasp | |
Number of specimens in each combined collection is given with the number of apparent morphospecies in parentheses. Over 200 morphospecies were assigned. Some may represent castes of the same species. Four gastropods and eight worms are included.
Includes one homoptera.
One gastropod present.
One worm present.
Includes one opilione.
Includes three alates.
Includes one alate.
Two of the major frog-skin alkaloids were not detected in the arthropod collections, namely PTX 323A and the 5,6,8-trisubstituted indolizidine 223A (Fig. 1, revised structure from ref. 18). Several of the minor frog-skin alkaloids were not detected in the arthropod collections: PTXs 237A and 251D; alloPTX 267A; 5,8-disubstituted indolizidines 207A and 233D; 5,6,8-trisubstituted indolizidines 235E and 237C; two ant alkaloids (13, 14), namely 3,5-disubstituted indolizidine 195B and 3,5-disubstituted pyrrolizidine 251K; the presumed millipede alkaloid (14) spiropyrrolizidine 252A; and the presumed beetle alkaloid (19) tricyclic 205B.
Discussion
The results indicate that further collection and analysis of individual taxa of leaf-litter arthropods at sites on Isla Bastimentos should reveal the source(s) of the PTXs, alloPTXs, and 5,8-disubstituted indolizidines present in skin extracts of the dendrobatid frog found at these sites. Combinatorial bioprospecting now needs to be applied at other tropical sites, namely those where batrachotoxins, histrionicotoxins, or epibatidine are found as major or significant components in the skin of dendrobatid frogs at such sites.
It proved disappointing that attempts to associate the occurrence of specific alkaloids with a particular taxon or morphospecies of arthropod present in the different mixed collections failed. A morphospecies is defined as morphologically distinct but not identified to the species level. Analysis of the present results has raised a number of significant questions as to the putative source of certain alkaloids as follows.
PTXs and AlloPTXs.
PTX 307A and the closely related alloPTX 323B (a 7-hydroxy derivative of 307A) were found in several of the mixed arthropod collections. Both PTX 307A and alloPTX 323B occurred together only in one collection (Tables 1 and 2). PTX 307A was detected in five of the collections as follows: (i) all three collections from site II, where it was a minor alkaloid in the frogs, (ii) one collection (J.W.D.) from site III, where it was a minor alkaloid in the frogs, and (iii) one collection (J.W.D.) from site V, where it was a minor alkaloid in the frogs. Ants were present in four of the five collections where PTX 307A was detected. However, no association of a particular taxon or morphospecies with the occurrence of PTX 307A was possible. PTX 307A was not detected in collections from site VI, where it was a major alkaloid in the frogs. Interestingly, PTX 323A (a 16-hydroxy analog of 307A) accompanied PTX 307A as either a major or minor alkaloid in frogs from four of the sites (Table 1) but was never detected in the arthropod collections.
The occurrence of alloPTX 323B in three of the arthropod collections also could not be linked to any taxon. It was a minor (never a major) alkaloid in frogs from sites II, III, V, and VI. It was detected in the arthropod collections from site II, but it also was found in collections from two other sites where only trace amounts of 323B were found in the frogs (Table 1).
5,8-Disubstituted Indolizidines.
The 5,8-disubstituted indolizidines, a relatively common class of alkaloids in extracts from dendrobatid frogs (3), were of unknown origin. Unlike the pyrrolidines, piperidines, izidines, and DHQs of myrmicine ants, such 5,8-disubstituted indolizidines contain a branched carbon skeleton. Two such 8-methyl-5-substituted indolizidines were detected in the mixed arthropod collections. Indolizidine 205A was detected in collections from site I, where it was a major alkaloid in frogs from that site (Fig. 2), and site V, where it was only a trace alkaloid in frogs (Table 1). It was not detected in collections from other sites, although it was either a major or minor alkaloid in frogs from four other sites (Table 1). Indolizidine 235B was detected in collections from four sites (Table 1). It was either a major or minor alkaloid in frogs from all those sites. No association between a taxon or morphospecies and occurrence of 5,8-indolizidines was apparent. Only juvenile frogs were collected at site IV. The levels of alkaloids were very low compared with adult frogs (see Fig. 2). The major alkaloids in the juveniles were indolizidine 235B and DHQ 195A.
DHQs.
DHQs have been detected in myrmicine ants (13, 14, 20) including extracts from ants that were microsympatric in Panamá with a dendrobatid frog (D. auratus) that contained the same DHQ 195A as a major alkaloid (14). An extract from ants (Solenopsis sp.), collected crossing the trail at site I, contained 195A, a minor alkaloid in frogs from the same site. DHQ 195A was detected in one arthropod collection from site I and in collections from sites II–IV and VI, where ants were present (Table 2). However, 195A occurred in one arthropod collection from site I and one from site V in which no ants appeared to be present. The ants found in the mixed collections were assigned to 36 morphospecies (data not presented). From 2 to 10 morphospecies of ants were present in each mixed collection (Table 2). There were no clear insights as to whether the presence of any of the ant morphospecies correlated with the detection of 195A. The “trail ant” (Solenopsis sp., assigned as morphospecies no. 5) from site I, in which 195A was detected, was not present in any of the arthropod collections where 195A was detected, but five specimens of that morphospecies did occur in one arthropod collection from site I; 195A was not detected in that collection. A total of 15 ant morphospecies was represented in the five collections that contained 195A, but only seven occurred in more than one collection. All of these seven morphospecies also occurred in collections where 195A was not detected. Thus, a unique source of 195A in the mixed arthropod collections is not apparent. DHQ 195A was either a major or a minor alkaloid in frog extracts from all the sites including extracts from juveniles at site IV (Table 1).
Spiropyrrolizidines.
The spiropyrrolizidine O-methyloxime 236 had not been detected from an arthropod source but was proposed to be a millipede alkaloid based on having a ring system identical to that of the millipede alkaloid nitropolyzonamine (21), which has a nitro group instead of the O-methyloxime substituent of 236 (22). It was found in three of nine mixed millipede collections (data not shown). Alkaloid 236 was detected in four of the mixed arthropod collections. However, no millipede was found in two of those four mixed collections.
Conclusions
The present combinatorial bioprospecting approach was developed because it was uncertain whether a forceps-mediated collection of all morphospecies of small leaf-litter arthropods would result in detection of alkaloids that were found sequestered in skin of microsympatric dendrobatid frogs. The analysis of each morphospecies separately would have been a major task: A total of over 200 morphospecies (data not presented) from over 20 major taxa were present in the mixed arthropod collections. The results of the combinatorial approach indicate that further forceps-mediated collections of specific leaf-litter arthropods will likely identify the source of certain frog-skin alkaloids. Although a large variety of beetles were present in the collections, no tricyclic coccinelline-like alkaloids were detected, not even the tricyclic 205B (Fig. 1) that was present as a minor alkaloid in frogs from five sites.
The failure to detect some of the major/minor frog-skin alkaloids in potential leaf-litter prey items could result from many factors including seasonal variations in the availability of such arthropods and the occurrence of random hatches or migrations (i.e., some of the frog-skin alkaloids may have been sequestered and retained from arthropods eaten months or even years before this study). Wild-caught dendrobatid frogs retain alkaloids in captivity for several years (6). It was disappointing that efforts to link the presence of a particular taxon or morphospecies with occurrence of alkaloids proved inconclusive. Certain taxa (pseudoscorpions, spiders, and springtails) were unlikely candidates, because they occurred in all or most mixed collections including those lacking alkaloids. The presence of gastropods or worms did not associate with any class of alkaloids detected in the mixed collections. Unless cross-contamination of extracts or lack of detection of taxa occurred, the inconclusive nature of results suggests that certain alkaloids occur in more than one arthropod taxon. For example, DHQ 195A, found in ant extracts in previous studies (13, 14) and in the one ant extract of the present study, was detected in two mixed collections that apparently had no ants. In addition, the presumed millipede alkaloid 236, found in three millipede collections (data not shown), was also found in two arthropod collections that had no millipedes. There is the possibility that, rather than different arthropod taxa having developed the biosynthetic enzymes to form, for example the PTXs, there is a symbiotic microorganism or even a microscopic mite that produces PTXs and can use more than one arthropod taxon as a host. Further forceps-mediated studies should reveal whether more than one arthropod taxon contains PTXs or representatives of other alkaloid classes. If so, it would strongly suggest a symbiotic source, and the presence of microscopic symbionts would need to be assessed.
The current study has provided further evidence indicating that availability of alkaloid-containing arthropod prey items is responsible for the variable profile of alkaloids in dendrobatid frogs, not genetic factors. The remarkable differences in amounts and profiles (Fig. 2 and Table 1) of alkaloids in frogs from different sites that are close together in northwestern Isla Bastimentos attest to the importance of location and the incredible biodiversity of alkaloid-containing arthropods as prey items at different locations. Early efforts to correlate aposematic coloration with toxicity in D. pumilio populations were done when it was thought the frogs were producing their own toxic alkaloids (17). In retrospect, it is not surprising that bright coloration and toxicity did not correlate well. Recent efforts by Summers and Clough (23) to present a correlation of the vividness of coloration in dendrobatid frogs with artificial toxicity values were based on an erroneous concept, namely that the availability of different alkaloid-containing arthropods as prey items to dendrobatid frogs at a particular site has remained constant during the evolution of aposematic coloration. Indeed, the profile of toxic and of relatively nontoxic alkaloids in each frog represents only a summation of alkaloids sequestered from dietary prey during the few years of the frog's life and provides no insight into what the frog's ancestors had available to them during centuries of evolution. In our own studies, we have seen remarkable changes in the profile of alkaloids in dendrobatid frogs from the same site occur during only a decade. For example, alkaloid 236 was not detected in an Isla Bastimentos population of D. pumilio collected in 1972 but had become a significant, albeit minor, alkaloid in collections at that same site in 1981. Similarly, alkaloid 235B was a trace alkaloid in 1972 but is now either a major or a minor alkaloid in frogs (sites I–VI) from the same general area on Isla Bastimentos. Clearly, aposematic coloration developed during ancestral times, for which we have no insight into what dietary alkaloids were available to the frogs.
The proposal that ant specialization was a key factor in evolution of alkaloid sequestration (24) and in some cases aposematic coloration may be true, because ants do produce alkaloids, and in recent times ants do represent a major part of the diet of some dendrobatid frogs including D. pumilio (24–26). In the present study, only DHQ 195A of the known ant alkaloids (14) was detected as a major/minor alkaloid in frogs from all sites on Isla Bastimentos. The only other ant alkaloids found in significant amounts in the present frog-skin extracts were the 3,5-disubstituted indolizidine 195B, as a minor alkaloid in frogs from site VII, and two isomers of the 3,5-disubstituted pyrrolizidine 251K, as minor alkaloids in frogs from site VIII. Clearly, the dietary source of most alkaloids, presently found in skins of certain dendrobatid, mantellid, and bufonid frogs/toads, still remains a challenge for further research on leaf-litter arthropods.
Acknowledgments
We thank Dr. Mahabir Gupta (Universidad de Panamá) for his generous assistance with this project. The Instituto Nacional de Recursos Naturales Renovables of Panamá is gratefully acknowledged for permission to collect frog specimens and arthropods necessary for these studies. Ralph Saporito, Valerie C. Clark, and others are thanked for constructive comments on the manuscript.
Abbreviations
PTX, pumiliotoxin
DHQ, decahydroquinoline
References
- 1.Pelletier S. W., (1983) Alkaloids: Chemical and Biological Perspectives (Wiley, New York), pp. 1–31.
- 2.Schöpf C. (1961) Experientia 17, 285-295. [DOI] [PubMed] [Google Scholar]
- 3.Daly J. W., Garraffo, H. M. & Spande, T. F. (1999) in Alkaloids: Chemical and Biological Perspectives, ed. Pelletier, S. W. (Pergamon, New York), pp. 1–161.
- 4.Daly J. W., Myers, C. W. & Whittaker, N. (1987) Toxicon 25, 1023-1095. [DOI] [PubMed] [Google Scholar]
- 5.Daly J. W., Myers, C. W., Warnick, J. E. & Albuquerque, E. X. (1980) Science 208, 1383-1385. [DOI] [PubMed] [Google Scholar]
- 6.Daly J. W., Secunda, S. I., Garraffo, H. M., Spande, T. F, Wisnieski, A., Nishihara, C. & Cover, J. F., Jr. (1992) Toxicon 30, 887-898. [DOI] [PubMed] [Google Scholar]
- 7.Daly J. W., Secunda, S. I., Garraffo, H. M., Spande, T. F., Wisnieski, A. & Cover, J. F., Jr. (1994) Toxicon 32, 657-663. [DOI] [PubMed] [Google Scholar]
- 8.Daly J. W., Garraffo, H. M., Spande, T. F., Jaramillo, C. & Rand, S. (1994) J. Chem. Ecol. 20, 943-955. [DOI] [PubMed] [Google Scholar]
- 9.Daly J. W. (1998) J. Nat. Prod. 61, 162-172. [DOI] [PubMed] [Google Scholar]
- 10.Garraffo H. M., Caceres, J., Daly, J. W., Spande, T. F., Andriamaharavo, N. R. & Andriantsiferana, M. (1993) J. Nat. Prod. 56, 1016-1038. [DOI] [PubMed] [Google Scholar]
- 11.Garraffo H. M., Spande, T. F., Daly, J. W., Baldessari, A. & Gros, E. G. (1993) J. Nat. Prod. 56, 357-373. [DOI] [PubMed] [Google Scholar]
- 12.Smith B. P., Tyler, M. J., Kaneko, T., Garraffo, H. M., Spande, T. F. & Daly, J. W. (2002) J. Nat. Prod. 65, 439-447. [DOI] [PubMed] [Google Scholar]
- 13.Jones T. H., Gorman, J. S. T., Snelling, R. R., DeLabie, H. C., Blum, M. S., Garraffo, H. M., Jain, P., Daly, J. W. & Spande, T. F. (1999) J. Chem. Ecol. 25, 1179-1193. [Google Scholar]
- 14.Daly J. W., Garraffo, H. M., Jain, P., Spande, T. F., Snelling, R. R., Jaramillo, G. & Rand, A. S. (2000) J. Chem. Ecol. 26, 73-85. [Google Scholar]
- 15.Garraffo H. M., Jain, P., Spande, T. F., Daly, J. W., Jones, T. H., Smith, L. J. & Zottig, V. E. (2001) J. Nat. Prod. 64, 421-427. [DOI] [PubMed] [Google Scholar]
- 16.Daly J. W. (1995) Proc. Natl. Acad. Sci. USA 92, 9-13.7816854 [Google Scholar]
- 17.Daly J. W. & Myers, C. W. (1967) Science 156, 970-973. [DOI] [PubMed] [Google Scholar]
- 18.Toyooka N., Fukutome, A., Nemoto, H., Daly, J. W., Spande, T. F., Garraffo, H. M. & Kaneko, T. (2002) Org. Lett. 4, 1715-1717. [DOI] [PubMed] [Google Scholar]
- 19.Tokuyama T., Garraffo, H. M., Spande, T. F. & Daly, J. W. (1998) Anal. Assoc. Quim. Argentina 86, 291-298. [Google Scholar]
- 20.Spande T. F., Jain, P., Garraffo, H. M., Pannell, L. K., Yeh, H. J. C., Daly, J. W., Fukumoto, S., Imamura, K., Tokuyama, T., Torres, J. A., et al. (1999) J. Nat. Prod. 62, 5-21. [DOI] [PubMed] [Google Scholar]
- 21.Smolanoff J., Kluge, A. F., Meinwald, J., McPhail, A., Miller, R. W., Hicks, K. & Eisner, T. (1975) Science 188, 734-735. [DOI] [PubMed] [Google Scholar]
- 22.Hutchinson K. D., Silverton, J. V. & Daly, J. W. (1994) Tetrahedron 50, 6129-6136. [Google Scholar]
- 23.Summers K. & Clough, M. E. (2001) Proc. Natl. Acad. Sci. USA 98, 6227-6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caldwell J. P. (1996) J. Zool. 240, 75-101. [Google Scholar]
- 25.Donnelly M. A. (1991) Copeia 3, 723-730. [Google Scholar]
- 26.Toft C. A. (1995) Herpetologica 51, 202-216. [Google Scholar]