Abstract
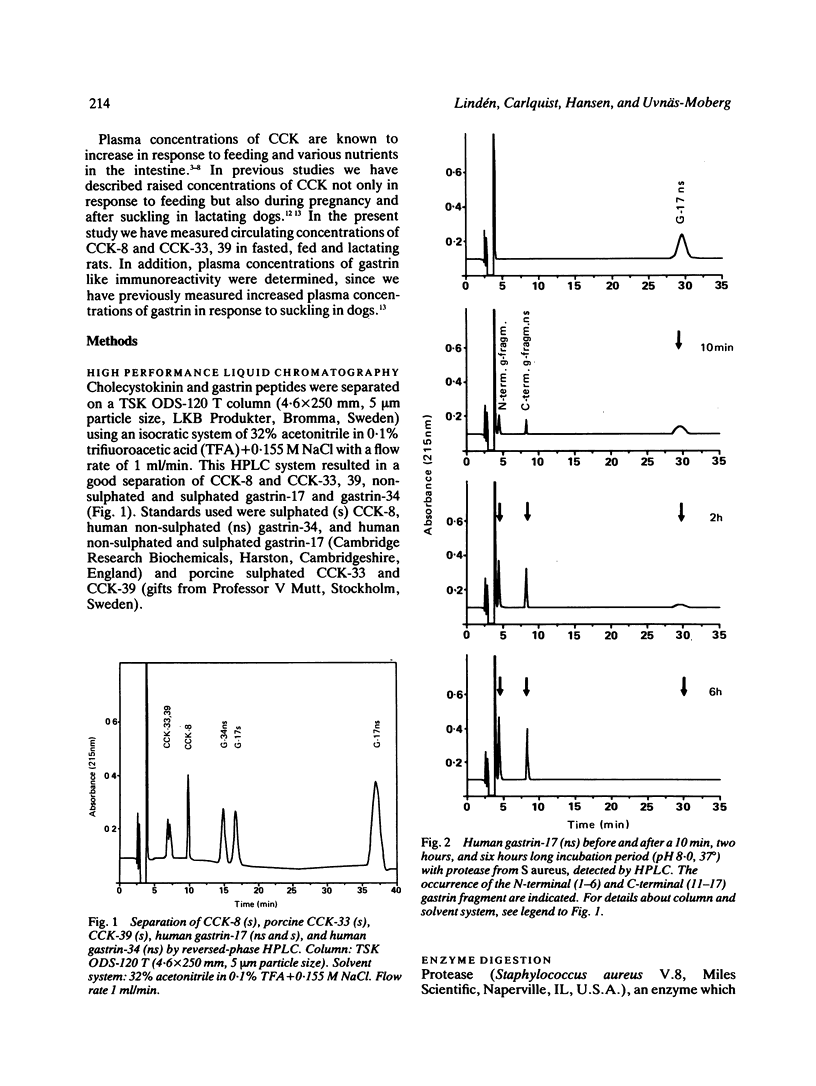
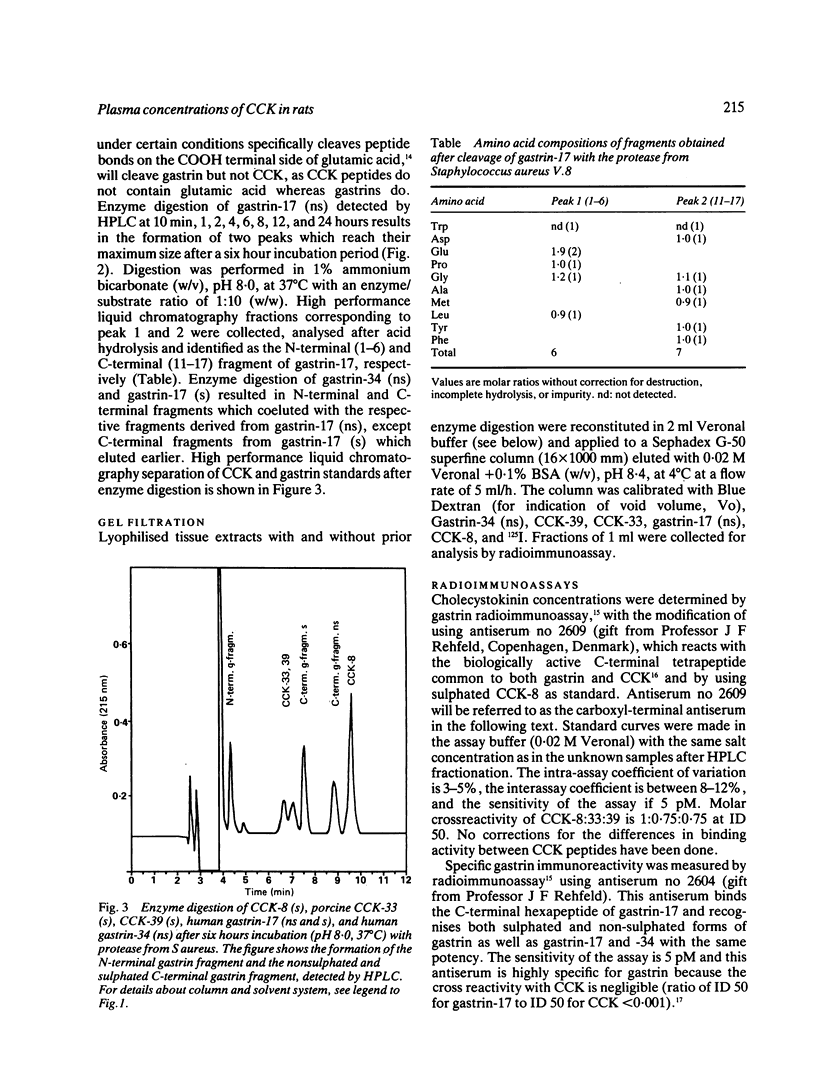
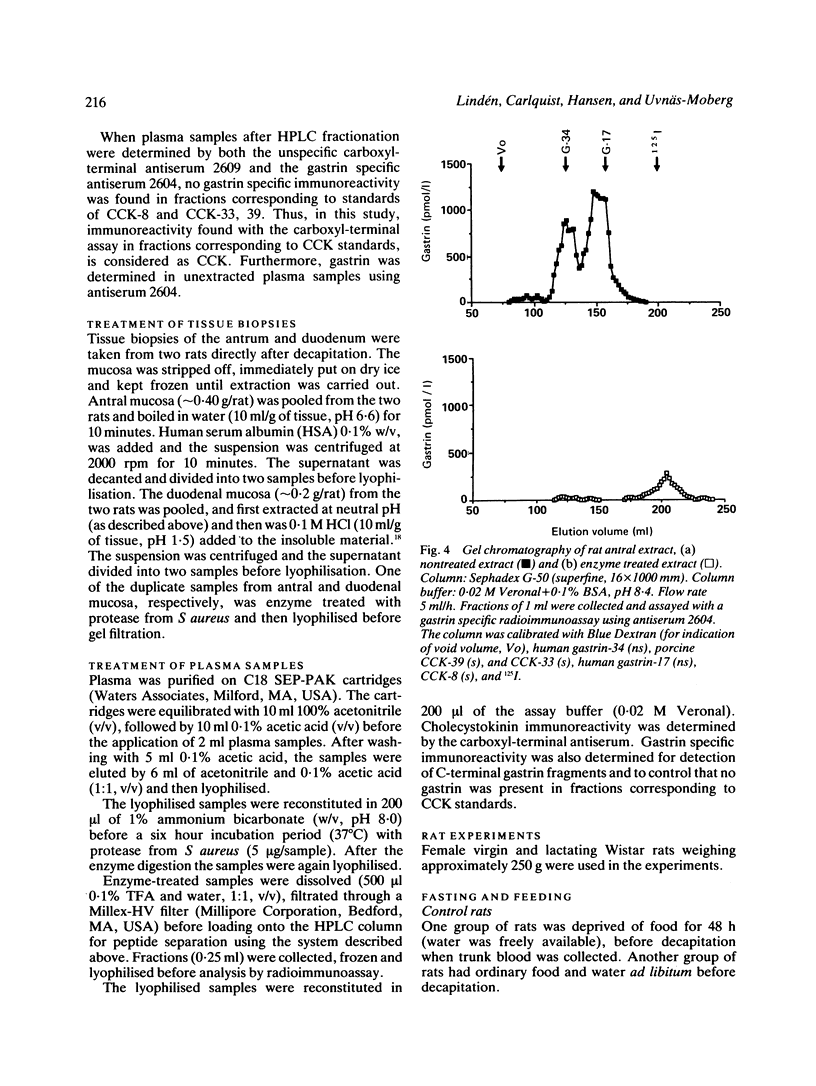
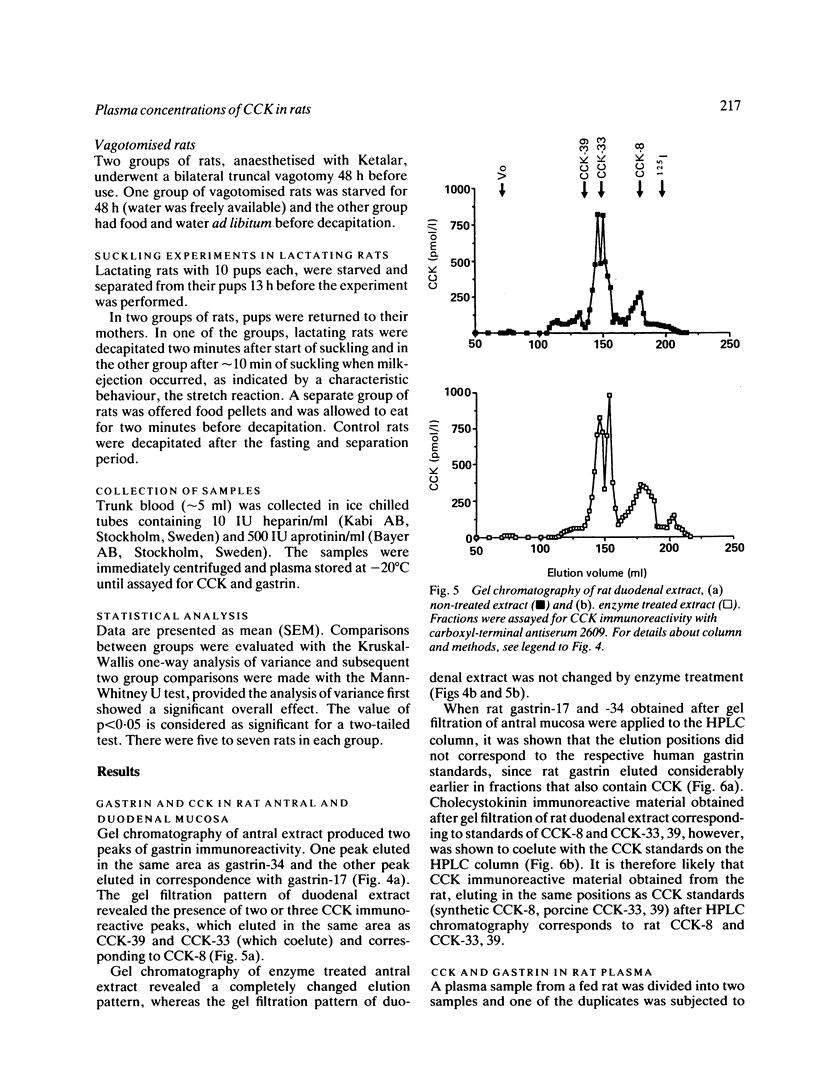
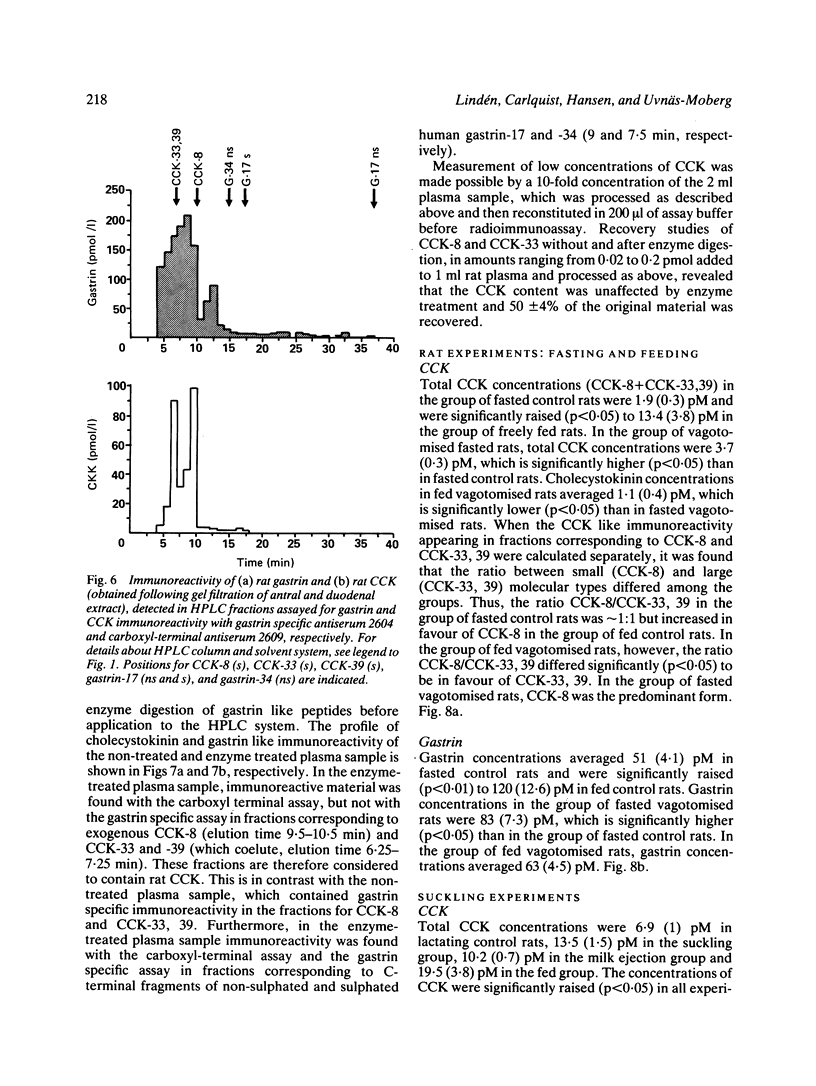
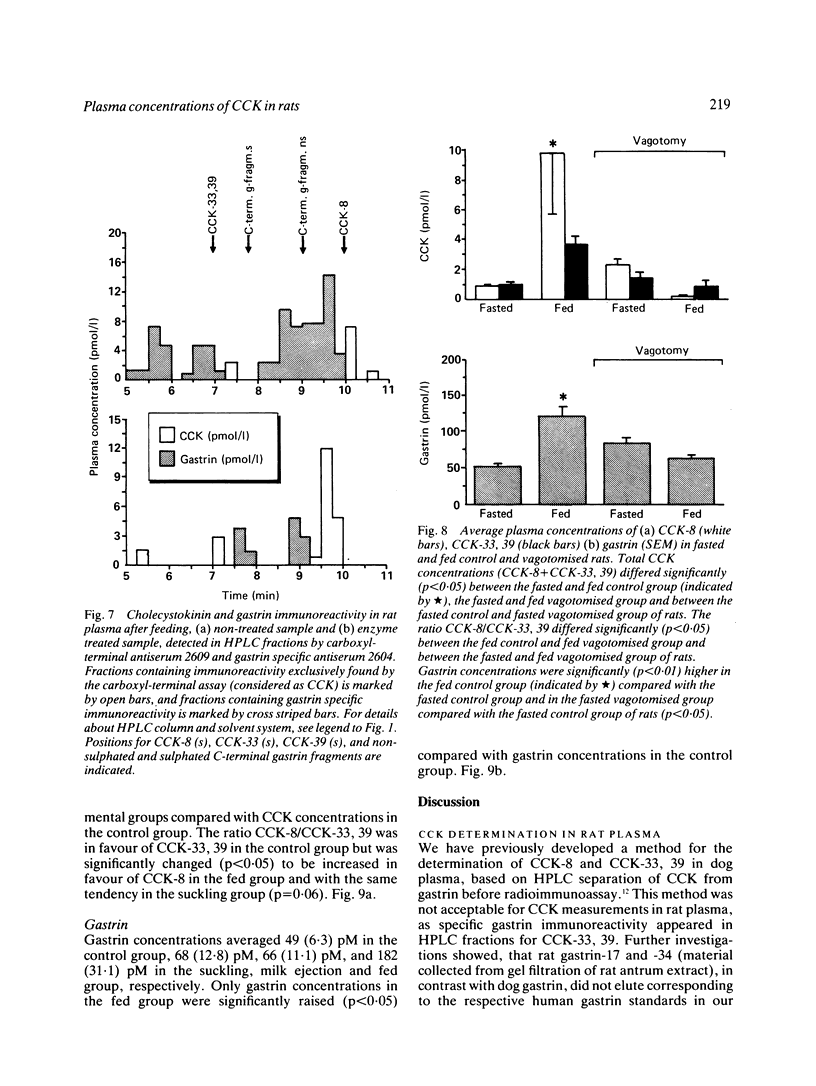
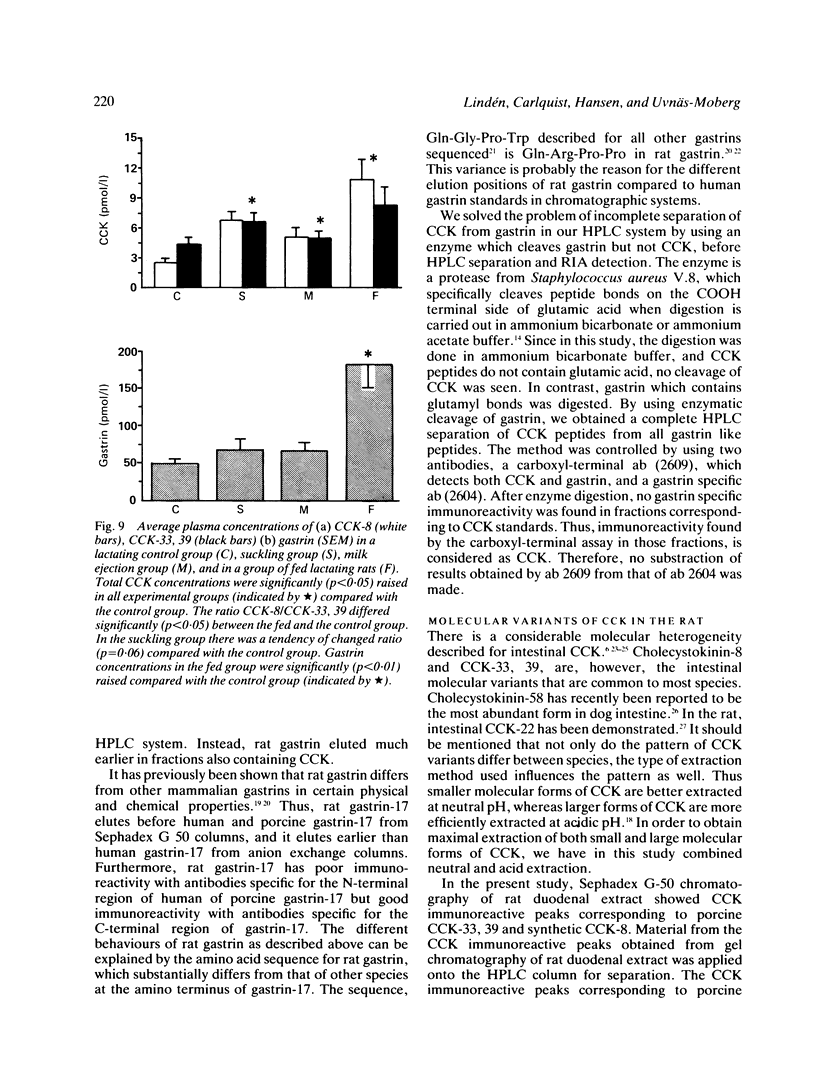
A new specific method for determination of cholecystokinin, CCK-8, and CCK-33, 39 in rat plasma is described. Plasma CCK radioimmunoassay (RIA) is difficult, because of cross-reactivity with gastrin. In the rat, problems because of difficulties in separating gastrin from CCK by high performance liquid chromatography (HPLC) exist. These were solved by enzyme digestion of gastrin before HPLC separation of molecular variants of CCK from gastrin fragments. Cholecystokinin immunoreactive forms in the HPLC fractions were determined by an antibody, which recognises the carboxyl terminus of CCK and gastrin. Fasting concentrations of small (CCK-8) and large (CCK-33, 39) molecular forms of CCK averaged 1.9 (0.3) pM and were raised to 13.4 (3.8) pM in rats fed ad libitum. Cholecystokinin in lactating rats rose two-fold after suckling, compared with 2.8 fold in response to feeding. The basal ratio between CCK-8 and CCK-33, 39 was approximately 1:1, but increased in favour of CCK-8 after feeding and in response to suckling. Gastrin like immunoreactivity measured in unextracted plasma was found to rise after feeding, but was unchanged in response to suckling.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacarese-Hamilton A. J., Adrian T. E., Bloom S. R. Distribution and heterogeneity of immunoreactive cholecystokinin (CCK) in the mucosa of the porcine gastrointestinal tract. Regul Pept. 1984 Nov;9(4):289–298. doi: 10.1016/0167-0115(84)90081-8. [DOI] [PubMed] [Google Scholar]
- Calam J., Ellis A., Dockray G. J. Identification and measurement of molecular variants of cholecystokinin in duodenal mucosa and plasma. Diminished concentrations in patients with celiac disease. J Clin Invest. 1982 Jan;69(1):218–225. doi: 10.1172/JCI110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debas H. T., Farooq O., Grossman M. I. Inhibition of gastric emptying is a physiological action of cholecystokinin. Gastroenterology. 1975 May;68(5 Pt 1):1211–1217. [PubMed] [Google Scholar]
- Eng J., Du B. H., Pan Y. C., Chang M., Hulmes J. D., Yalow R. S. Purification and sequencing of a rat intestinal 22 amino acid C-terminal CCK fragment. Peptides. 1984 Nov-Dec;5(6):1203–1206. doi: 10.1016/0196-9781(84)90188-8. [DOI] [PubMed] [Google Scholar]
- Eysselein V. E., Böttcher W., Kauffman G. L., Jr, Walsh J. H. Molecular heterogeneity of canine cholecystokinin in portal and peripheral plasma. Regul Pept. 1984 Oct;9(3):173–185. doi: 10.1016/0167-0115(84)90070-3. [DOI] [PubMed] [Google Scholar]
- Eysselein V. E., Eberlein G. A., Hesse W. H., Singer M. V., Goebell H., Reeve J. R., Jr Cholecystokinin-58 is the major circulating form of cholecystokinin in canine blood. J Biol Chem. 1987 Jan 5;262(1):214–217. [PubMed] [Google Scholar]
- Fölsch U. R., Schafmayer A., Ebert R., Becker H. D., Creutzfeldt W. Elevated plasma cholecystokinin concentrations in exocrine pancreatic atrophy in the rat. Digestion. 1984;29(1):60–64. doi: 10.1159/000199010. [DOI] [PubMed] [Google Scholar]
- Håkanson R., Vallgren S., Ekelund M., Rehfeld J. F., Sundler F. The vagus exerts trophic control of the stomach in the rat. Gastroenterology. 1984 Jan;86(1):28–32. [PubMed] [Google Scholar]
- Izzo R. S., Brugge W. R., Praissman M. Immunoreactive cholecystokinin in human and rat plasma: correlation of pancreatic secretion in response to CCK. Regul Pept. 1984 Sep;9(1-2):21–34. doi: 10.1016/0167-0115(84)90004-1. [DOI] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Williams J. A. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984 Sep;87(3):542–549. [PubMed] [Google Scholar]
- Lilja P., Wiener I., Inoue K., Fried G. M., Greeley G. H., Jr, Thompson J. C. Release of cholecystokinin in response to food and intraduodenal fat in pigs, dogs and man. Surg Gynecol Obstet. 1984 Dec;159(6):557–561. [PubMed] [Google Scholar]
- Linden A., Eriksson M., Carlquist M., Uvnäs-Moberg K. Plasma levels of gastrin, somatostatin, and cholecystokinin immunoreactivity during pregnancy and lactation in dogs. Gastroenterology. 1987 Mar;92(3):578–584. doi: 10.1016/0016-5085(87)90004-7. [DOI] [PubMed] [Google Scholar]
- Lindén A., Hansen S., Bednar I., Forsberg G., Södersten P., Uvnäs-Moberg K. Sexual activity increases plasma concentrations of cholecystokinin octapeptide and offsets hunger in male rats. J Endocrinol. 1987 Oct;115(1):91–95. doi: 10.1677/joe.0.1150091. [DOI] [PubMed] [Google Scholar]
- Lindén A., Uvnäs-Moberg K. Plasma levels of cholecystokinin (CCK-8 and CCK-33-39) in response to feeding and during pregnancy in dogs. Scand J Gastroenterol. 1987 Sep;22(7):859–864. doi: 10.3109/00365528708991926. [DOI] [PubMed] [Google Scholar]
- Maton P. N., Selden A. C., Chadwick V. S. Differential distribution of molecular forms of cholecystokinin in human and porcine small intestinal mucosa. Regul Pept. 1984 Jan;8(1):9–19. doi: 10.1016/0167-0115(84)90024-7. [DOI] [PubMed] [Google Scholar]
- Maton P. N., Selden A. C., Chadwick V. S. Large and small forms of cholecystokinin in human plasma: measurement using high pressure liquid chromatography and radioimmunoassay. Regul Pept. 1982 Oct;4(5):251–260. doi: 10.1016/0167-0115(82)90118-5. [DOI] [PubMed] [Google Scholar]
- Nilsson G. Increased plasma gastrin levels in connection with inhibition of gastric acid responses to sham feeding following bulbar perfusion with acid in dogs. Scand J Gastroenterol. 1975;10(3):273–277. [PubMed] [Google Scholar]
- Reeve J. R., Jr, Dimaline R., Shively J. E., Hawke D., Chew P., Walsh J. H. Unique amino terminal structure of rat little gastrin. Peptides. 1981 Winter;2(4):453–458. doi: 10.1016/s0196-9781(81)80104-0. [DOI] [PubMed] [Google Scholar]
- Reeve J. R., Jr, Eysselein V., Walsh J. H., Ben-Avram C. M., Shively J. E. New molecular forms of cholecystokinin. Microsequence analysis of forms previously characterized by chromatographic methods. J Biol Chem. 1986 Dec 15;261(35):16392–16397. [PubMed] [Google Scholar]
- Rehfeld J. F., Holst J. J., Jensen S. L. The molecular nature of vascularly released cholecystokinin from the isolated perfused porcine duodenum. Regul Pept. 1982 Jan;3(1):15–28. doi: 10.1016/0167-0115(82)90003-9. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. I. Development of sequence-specific radioimmunoassays for porcine triacontatriapeptide cholecystokinin. J Biol Chem. 1978 Jun 10;253(11):4016–4021. [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978 Jun 10;253(11):4022–4030. [PubMed] [Google Scholar]
- Ryder S., Eng J., Straus E., Yalow R. S. Alkaline extraction and characterization of cholecystokinin-immunoreactivity from rat gut. Gastroenterology. 1981 Aug;81(2):267–275. [PubMed] [Google Scholar]
- Schaffer M. H., Agarwal K. L., Noyes B. E. Rat gastrin's amino acid sequence determined from the nucleotide sequence of the mRNA. Peptides. 1982 Jul-Aug;3(4):693–696. doi: 10.1016/0196-9781(82)90172-3. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K. Release of gastrointestinal peptides in response to vagal activation induced by electrical stimulation, feeding and suckling. J Auton Nerv Syst. 1983 Oct;9(1):141–155. doi: 10.1016/0165-1838(83)90137-6. [DOI] [PubMed] [Google Scholar]
- Walsh J. H., Lamers C. B., Valenzuela J. E. Cholecystokinin-octapeptidelike immunoreactivity in human plasma. Gastroenterology. 1982 Mar;82(3):438–444. [PubMed] [Google Scholar]
- Wolfe M. M., McGuigan J. E. Immunochemical characterization of gastrinlike and cholecystokininlike peptides released in dogs in response to a peptone meal. Gastroenterology. 1984 Aug;87(2):323–334. [PubMed] [Google Scholar]


