Abstract
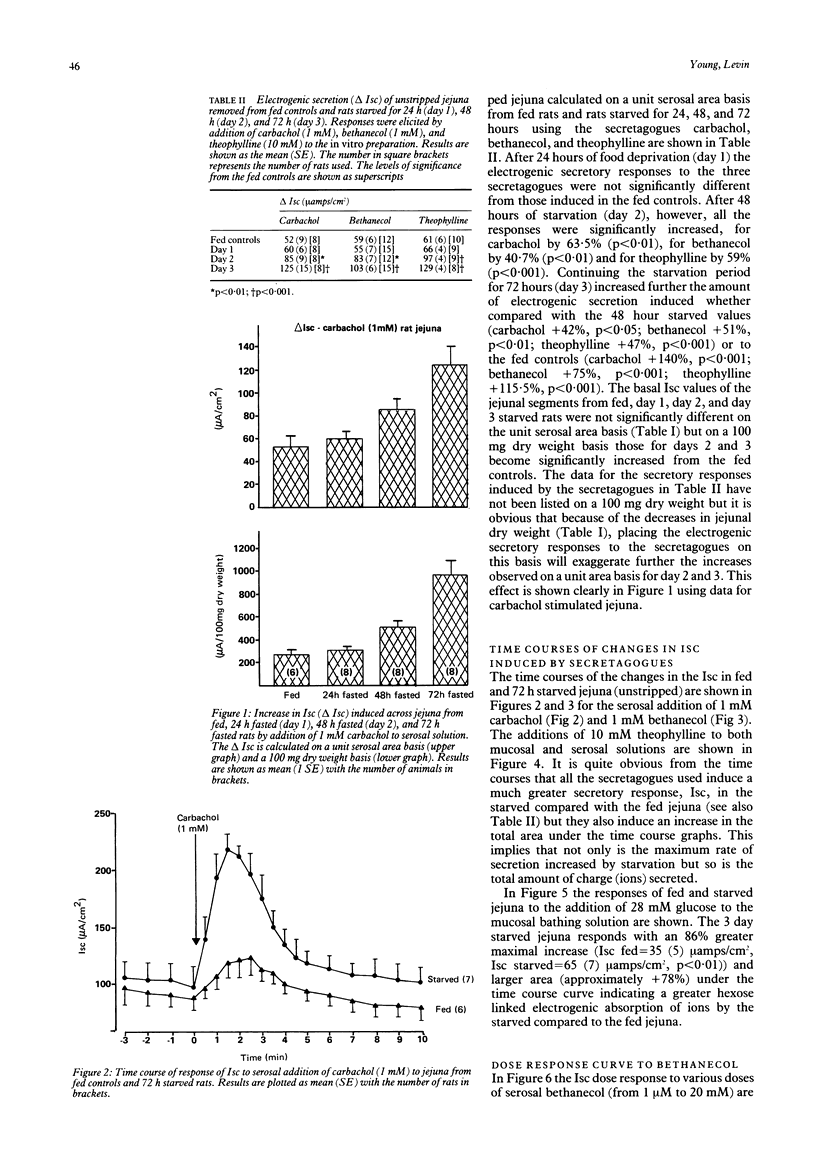
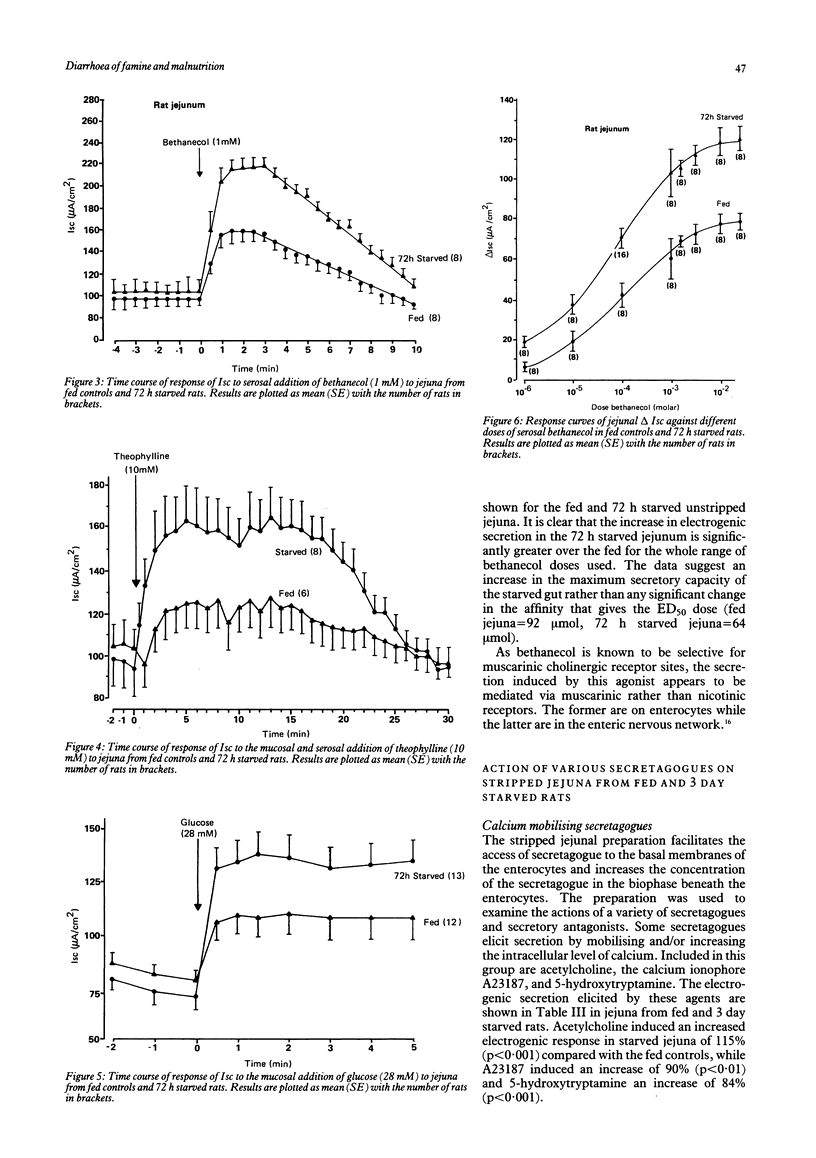
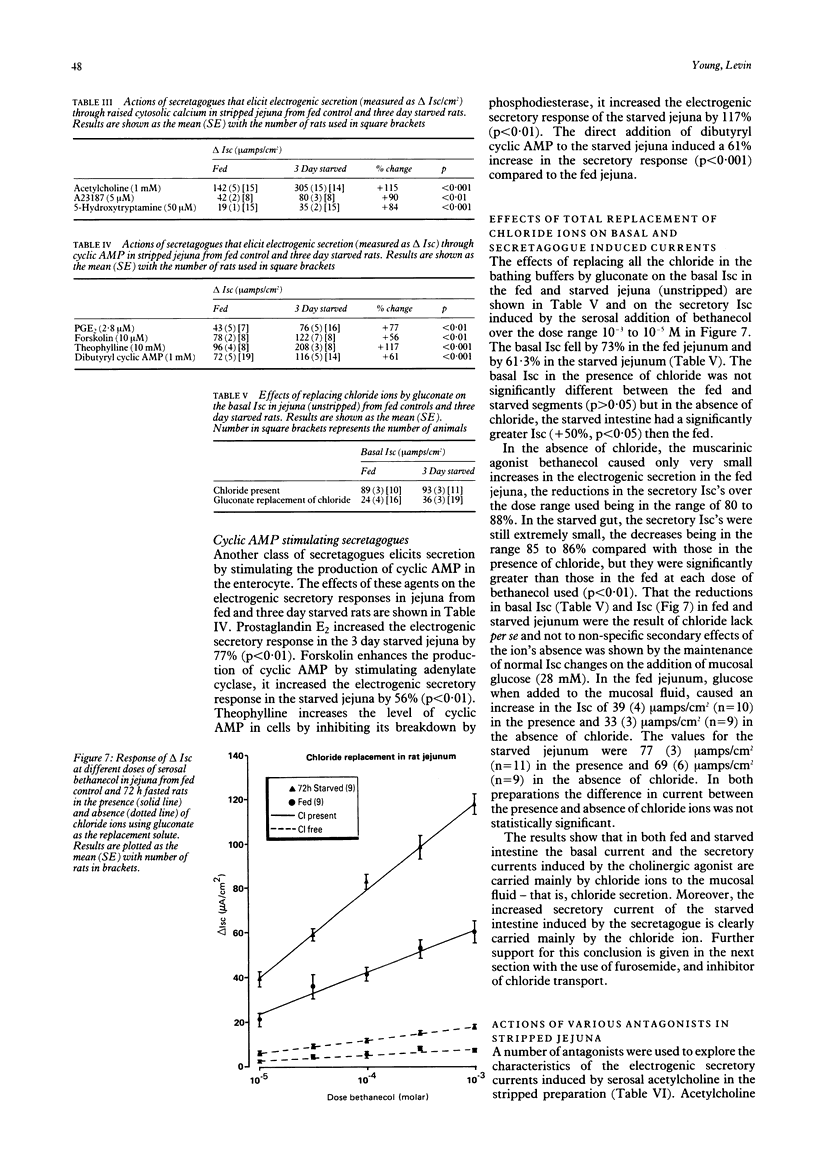
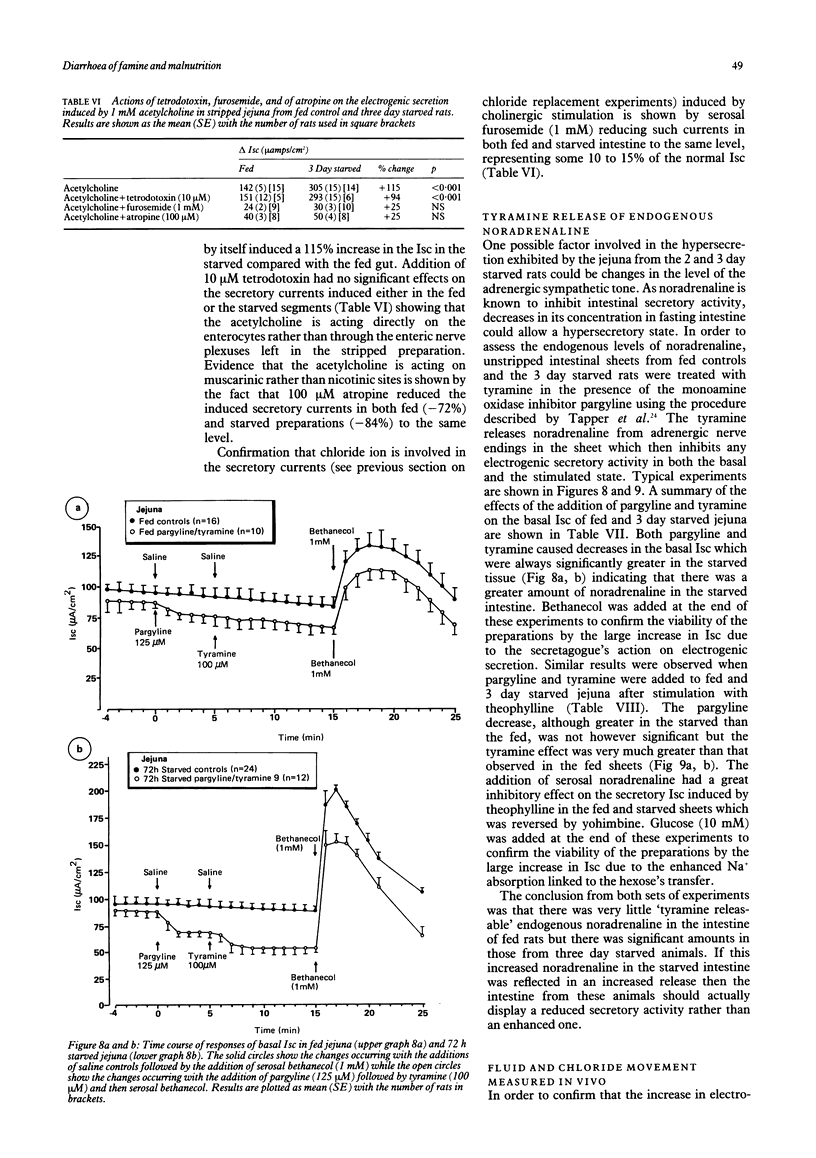
The effects of progressive starvation for up to three days on the secretory functions of the intestine were investigated using in vitro and in vivo preparations of rat jejunum and secretagogues whose action was either through cyclic AMP or Ca++. Initial starvation for 24 h (day 1) did not significantly alter the basal net electrogenic ion secretion measured in vitro as the short circuit current (Isc, muamps/cm2) or the change in electrogenic ion secretion (delta Isc) induced by the secretagogues. By day 2 of starvation, however, the maximum delta Isc transient induced by the cholinergic and other secretagogues (delta Isc = Isc max-basal Isc) was greatly increased (up to a maximum of 117%) compared with the fed controls on an area basis. The delta Isc were even greater on day 3 of starvation. If a tissue weight basis was used to normalise the data the increase became even more marked. The enhancement in secretion was not caused by a decrease in absorptive capacity as glucose, added mucosally, gave larger increases in absorptive currents in the starved than in the fed jejuna. Bethanecol dose-delta Isc response curves in fed and starved jejuna showed an increase in the maximum electrogenic secretion in the starved but no apparent change in the affinity of their cholinergic receptors mediating the enhanced secretion. The starvation-induced increase in secretion elicited by bethanecol was blocked by atropine, indicating that the receptors were muscarinic, but was unaffected by tetrodotoxin indicating that the enteric neural innervation was not essential for its expression. Noradrenaline released by tyramine was greater in the starved than the fed jejunum, suggesting that a decreased sympathetic tone was unlikely to be the major cause of the starvation induced secretory enhancement. Measurement of jejunal fluid movements in vivo showed that in fed controls and throughout the three days of starvation there was an unchanged net fluid absorption in the basal, unstimulated state. By day 2 and day 3 of starvation, however, bethanecol stimulated fluid secretion was very much greater than that of the fed controls. This increase in fluid secretion was concomitant with significant increases in the concentration of chloride in the lumenal fluid. Starvation thus appears to make the rat jejunum hypersensitive to cholinergic and other secretagogues, increasing the electrogenic secretion of chloride in vitro and that of chloride and fluid in vivo. These results obtained with the rat model give a new insight into possible mechanisms by which the diarrhoea of human famine and malnutrition may be expressed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adibi S. A., Allen E. R. Impaired jejunal absorption rates of essential amino acids induced by either dietary caloric or protein deprivation in man. Gastroenterology. 1970 Sep;59(3):404–413. [PubMed] [Google Scholar]
- Bolton J. E., Field M. Ca ionophore-stimulated ion secretion in rabbit ileal mucosa: relation to actions of cyclic 3',5'-AMP and carbamylcholine. J Membr Biol. 1977 Jun 30;35(2):159–173. doi: 10.1007/BF01869947. [DOI] [PubMed] [Google Scholar]
- Brown H. O., Levine M. L., Lipkin M. Inhibition of intestinal epithelial cell renewal and migration induced by starvation. Am J Physiol. 1963 Nov;205(5):868–872. doi: 10.1152/ajplegacy.1963.205.5.868. [DOI] [PubMed] [Google Scholar]
- Debnam E. S., Levin R. J. Effects of fasting and semistarvation on the kinetics of active and passive sugar absorption across the small intestine in vivo. J Physiol. 1975 Nov;252(3):681–700. doi: 10.1113/jphysiol.1975.sp011165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnam E. S., Thompson C. S. The effect of fasting on the potential difference across the brush-border membrane of enterocytes in rat small intestine. J Physiol. 1984 Oct;355:449–456. doi: 10.1113/jphysiol.1984.sp015430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Asarkof N., Pike G. Calcium dependence of serotonin-induced changes in rabbit ileal electrolyte transport. J Clin Invest. 1980 Aug;66(2):341–352. doi: 10.1172/JCI109862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Sheerin H. E., Henderson A., Smith P. L. Catecholamine effects on cyclic AMP levels and ion secretion in rabbit ileal mucosa. Am J Physiol. 1975 Jul;229(1):86–92. doi: 10.1152/ajplegacy.1975.229.1.86. [DOI] [PubMed] [Google Scholar]
- HELWEG-LARSEN P., HOFFMEYER H., KIELER J., HESS THAYSEN E., HESS THAYSEN J., THYGESEN P., HERTEL WULFF M. Famine disease in German concentration camps; complications and sequels, with special reference to tuberculosis, mental disorders and social consequences. Acta Psychiatr Neurol Scand Suppl. 1952;83:1–460. [PubMed] [Google Scholar]
- HOOPER C. S., BLAIR M. The effect of starvation on epithelial renewal in the rat duodenum. Exp Cell Res. 1958 Feb;14(1):175–181. doi: 10.1016/0014-4827(58)90224-6. [DOI] [PubMed] [Google Scholar]
- Hopper A. F., Wannemacher R. W., Jr, McGovern P. A. Cell population changes in the intestinal epithelium of the rat following starvation and protein-depletion. Proc Soc Exp Biol Med. 1968 Jul;128(3):695–698. doi: 10.3181/00379727-128-33103. [DOI] [PubMed] [Google Scholar]
- Hubel K. A. Intestinal ion transport: effect of norepinephrine, pilocarpine, and atropine. Am J Physiol. 1976 Jul;231(1):252–257. doi: 10.1152/ajplegacy.1976.231.1.252. [DOI] [PubMed] [Google Scholar]
- Levin R. J. The intestinal absorption of some essential and non-essential amino acids in fed and fasting rats. Life Sci. 1970 Jan 22;9(2):61–68. doi: 10.1016/0024-3205(70)90245-6. [DOI] [PubMed] [Google Scholar]
- Roediger W. E. Metabolic basis of starvation diarrhoea: implications for treatment. Lancet. 1986 May 10;1(8489):1082–1084. doi: 10.1016/s0140-6736(86)91341-3. [DOI] [PubMed] [Google Scholar]
- Smith M. W. Expression of digestive and absorptive function in differentiating enterocytes. Annu Rev Physiol. 1985;47:247–260. doi: 10.1146/annurev.ph.47.030185.001335. [DOI] [PubMed] [Google Scholar]
- Steiner M., Farrish G. C., Gray S. J. Intestinal uptake of valine in calorie and protein deprivation. Am J Clin Nutr. 1969 Jul;22(7):871–877. doi: 10.1093/ajcn/22.7.871. [DOI] [PubMed] [Google Scholar]
- Strombeck D. R. The production of intestinal fluid by cholera toxin in the rat. Proc Soc Exp Biol Med. 1972 May;140(1):297–303. doi: 10.3181/00379727-140-36444. [DOI] [PubMed] [Google Scholar]
- Theodorsson-Norheim E. Kruskal-Wallis test: BASIC computer program to perform nonparametric one-way analysis of variance and multiple comparisons on ranks of several independent samples. Comput Methods Programs Biomed. 1986 Aug;23(1):57–62. doi: 10.1016/0169-2607(86)90081-7. [DOI] [PubMed] [Google Scholar]
- Thompson C. S., Debnam E. S. Starvation-induced changes in the autoradiographic localisation of valine uptake by rat small intestine. Experientia. 1986 Aug 15;42(8):945–948. doi: 10.1007/BF01941773. [DOI] [PubMed] [Google Scholar]
- Wahawisan R., Wallace L. J., Gaginella T. S. Muscarinic receptors on rat ileal villus and crypt cells. J Pharm Pharmacol. 1986 Feb;38(2):150–153. doi: 10.1111/j.2042-7158.1986.tb04533.x. [DOI] [PubMed] [Google Scholar]
- Young J. B., Landsberg L. Suppression of sympathetic nervous system during fasting. Science. 1977 Jun 24;196(4297):1473–1475. doi: 10.1126/science.867049. [DOI] [PubMed] [Google Scholar]


