Abstract
Ultrastructural examination of biopsies showing Helicobacter pylori associated chronic gastritis reveals close attachment between gastric surface epithelial cells and the organism. The finding of 'adhesion pedestals', which represents a cellular response to the presence of the organism, is analogous to the response of intestinal cells to enteropathogenic E coli. Thus the development of bacterial attachment sites in H pylori associated gastritis might be an indication of pathogenicity. We have therefore explored the relationship between the proportion of organisms forming attachment sites and histological indices of disease 'activity'. Antral biopsies from 40 patients with H pylori positive gastritis were examined histologically and ultrastructurally, and the percentage of attached organisms compared with subjective assessments of epithelial degeneration, mucin depletion, polymorphonuclear and chronic inflammatory cell infiltration. We found a significant increase in the proportion of attached bacteria in cases showing histological epithelial degeneration, and a significant decrease in cases showing intraepithelial polymorph infiltration. The direct relationship between bacterial attachment and cellular degeneration lends further support to a pathogenic effect. Reduced attachment in the face of polymorph infiltration might indirectly reflect aspects of the immune response--namely, blocking of adhesion by IgA, with complement activation and generation of leucotactic factors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode G., Malfertheiner P., Ditschuneit H. Pathogenetic implications of ultrastructural findings in Campylobacter pylori related gastroduodenal disease. Scand J Gastroenterol Suppl. 1988;142:25–39. [PubMed] [Google Scholar]
- Cantey J. R., Blake R. K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977 Mar;135(3):454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- Chen X. G., Correa P., Offerhaus J., Rodriguez E., Janney F., Hoffmann E., Fox J., Hunter F., Diavolitsis S. Ultrastructure of the gastric mucosa harboring Campylobacter-like organisms. Am J Clin Pathol. 1986 Nov;86(5):575–582. doi: 10.1093/ajcp/86.5.575. [DOI] [PubMed] [Google Scholar]
- Clausen C. R., Christie D. L. Chronic diarrhea in infants caused by adherent enteropathogenic Escherichia coli. J Pediatr. 1982 Mar;100(3):358–361. doi: 10.1016/s0022-3476(82)80429-0. [DOI] [PubMed] [Google Scholar]
- Das S. S., Karim Q. N., Easmon C. S. Opsonophagocytosis of Campylobacter pylori. J Med Microbiol. 1988 Oct;27(2):125–130. doi: 10.1099/00222615-27-2-125. [DOI] [PubMed] [Google Scholar]
- Dixon M. F., Wyatt J. I., Burke D. A., Rathbone B. J. Lymphocytic gastritis--relationship to Campylobacter pylori infection. J Pathol. 1988 Feb;154(2):125–132. doi: 10.1002/path.1711540204. [DOI] [PubMed] [Google Scholar]
- Drumm B., Sherman P., Cutz E., Karmali M. Association of Campylobacter pylori on the gastric mucosa with antral gastritis in children. N Engl J Med. 1987 Jun 18;316(25):1557–1561. doi: 10.1056/NEJM198706183162501. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Moulds J. J., Graham D. Y. N-acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988 Nov;56(11):2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin C. S., Armstrong J. A., Marshall B. J. Campylobacter pyloridis, gastritis, and peptic ulceration. J Clin Pathol. 1986 Apr;39(4):353–365. doi: 10.1136/jcp.39.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S. F., Wyatt J. I., Rathbone B. J. Simplified techniques for identifying Campylobacter pyloridis. J Clin Pathol. 1986 Nov;39(11):1279–1279. doi: 10.1136/jcp.39.11.1279-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J. A., Gill V., Swan J. C., Ognibene F., Shelhamer J., Parrillo J. E., Masur H. Prospective evaluation of a monoclonal antibody in diagnosis of Pneumocystis carinii pneumonia. Lancet. 1986 Jul 5;2(8497):1–3. doi: 10.1016/s0140-6736(86)92555-9. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Armstrong J. A., McGechie D. B., Glancy R. J. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Med J Aust. 1985 Apr 15;142(8):436–439. doi: 10.5694/j.1326-5377.1985.tb113443.x. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., McGechie D. B., Rogers P. A., Glancy R. J. Pyloric Campylobacter infection and gastroduodenal disease. Med J Aust. 1985 Apr 15;142(8):439–444. doi: 10.5694/j.1326-5377.1985.tb113444.x. [DOI] [PubMed] [Google Scholar]
- Morris A., Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987 Mar;82(3):192–199. [PubMed] [Google Scholar]
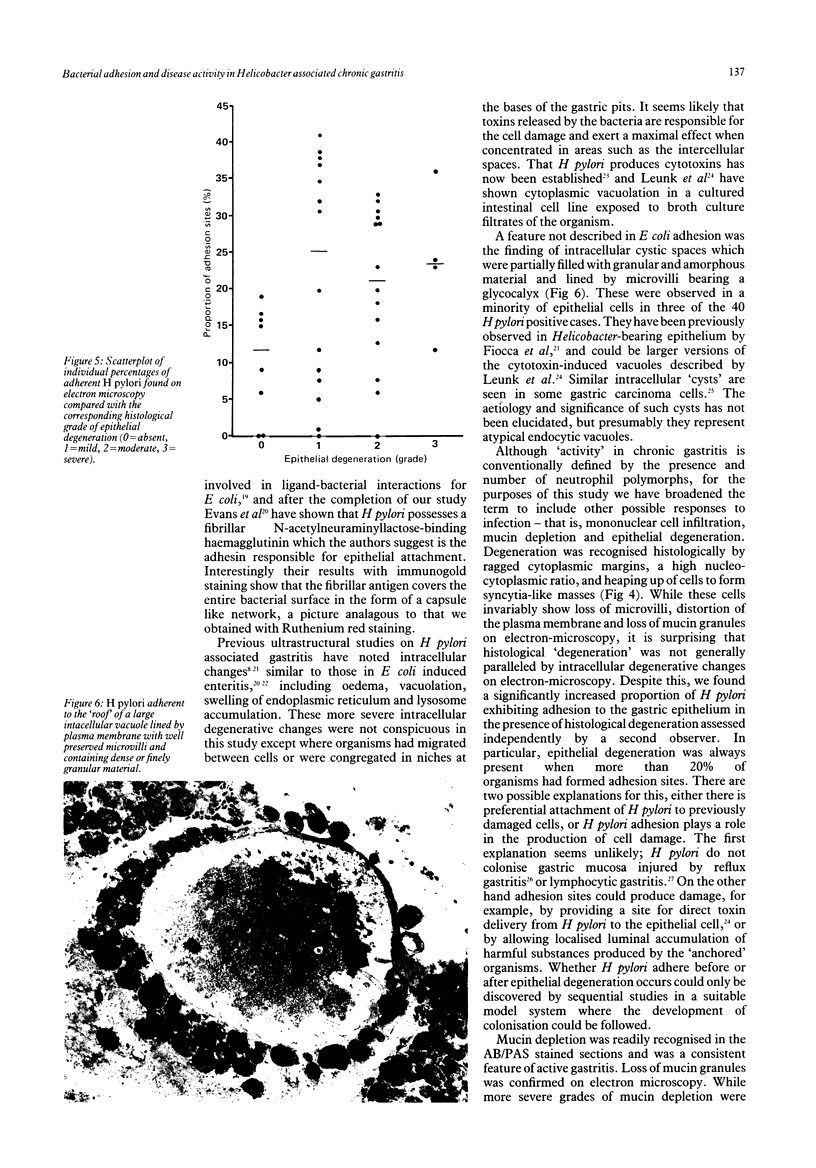
- Nevalainen T. J., Järvi O. H. Intracellular cysts in gastric carcinoma. Acta Pathol Microbiol Scand A. 1976 Nov;84(6):517–522. doi: 10.1111/j.1699-0463.1976.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Rauws E. A., Langenberg W., Houthoff H. J., Zanen H. C., Tytgat G. N. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988 Jan;94(1):33–40. [PubMed] [Google Scholar]
- Rosenstein I. J., Stoll M. S., Mizuochi T., Childs R. A., Hounsell E. F., Feizi T. New type of adhesive specificity revealed by oligosaccharide probes in Escherichia coli from patients with urinary tract infection. Lancet. 1988 Dec 10;2(8624):1327–1330. doi: 10.1016/s0140-6736(88)90868-9. [DOI] [PubMed] [Google Scholar]
- Rothbaum R., McAdams A. J., Giannella R., Partin J. C. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982 Aug;83(2):441–454. [PubMed] [Google Scholar]
- Steer H. W. The gastro-duodenal epithelium in peptic ulceration. J Pathol. 1985 Aug;146(4):355–362. doi: 10.1002/path.1711460409. [DOI] [PubMed] [Google Scholar]
- Tricottet V., Bruneval P., Vire O., Camilleri J. P., Bloch F., Bonte N., Roge J. Campylobacter-like organisms and surface epithelium abnormalities in active, chronic gastritis in humans: an ultrastructural study. Ultrastruct Pathol. 1986;10(2):113–122. doi: 10.3109/01913128609014587. [DOI] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]