Abstract
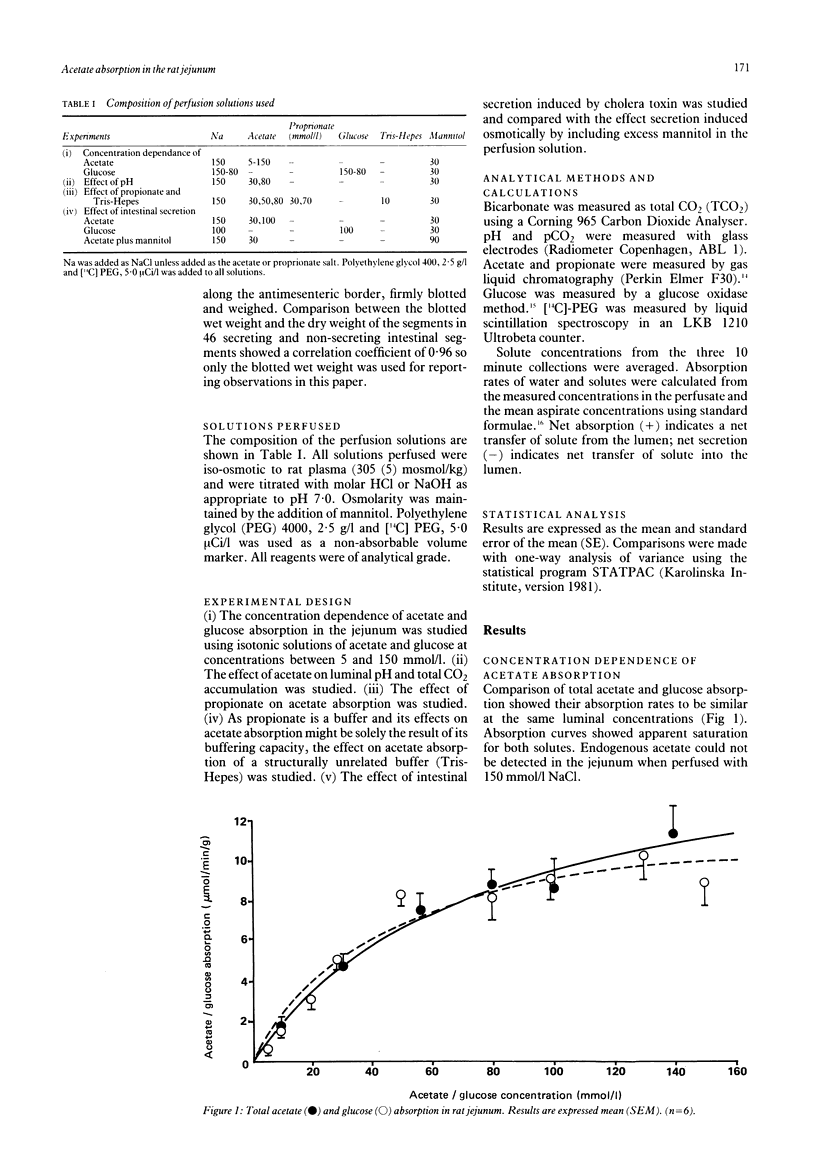
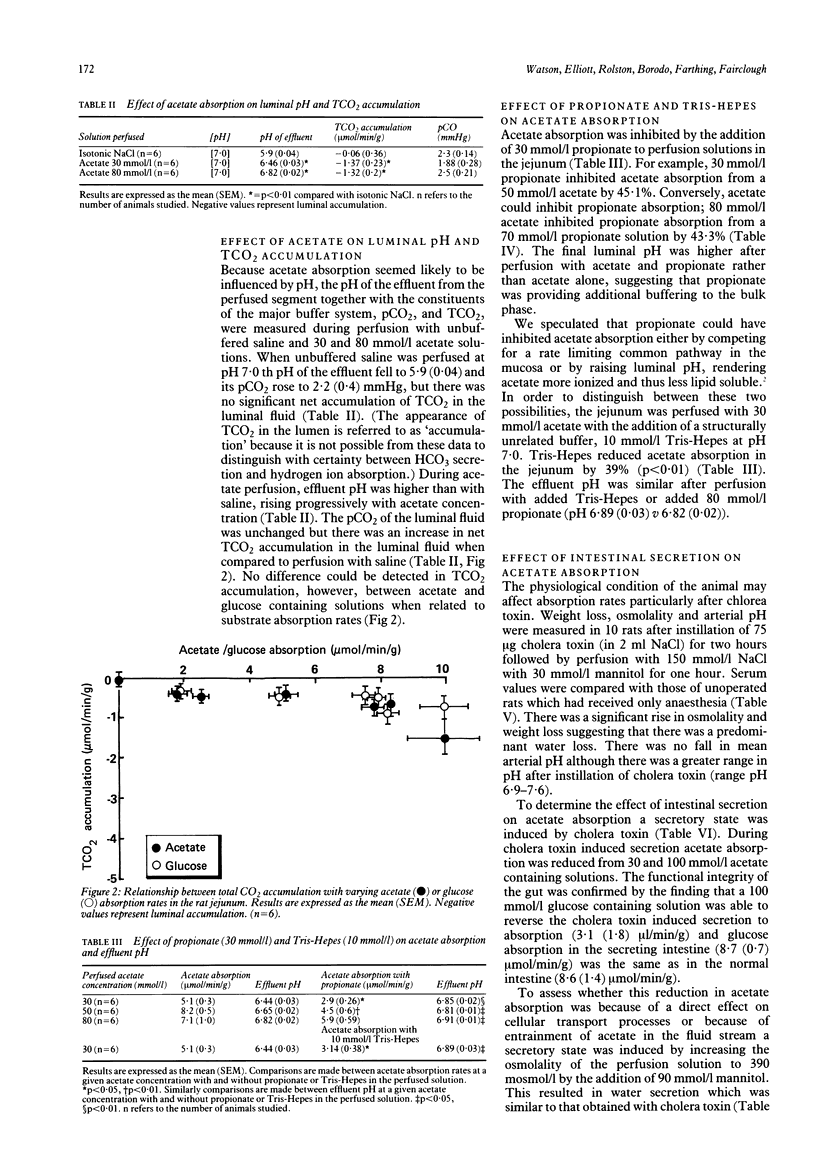
Acetate absorption was studied in rat jejunum using steady state perfusion in vivo. Absorption conformed to apparent saturation kinetics and was similar in magnitude to glucose absorption. When compared with normal saline, acetate perfusion was associated with luminal alkalinisation. There was no difference in total CO2 secretion when similar rates of acetate and glucose absorption were compared, suggesting that total CO2 secretion was the result of mucosal metabolism. Absorption of acetate and propionate were mutually inhibitory. Acetate absorption was also inhibited by Tris-Hepes pH 7.0. When the gut was pretreated with cholera toxin to induce a secretory state, acetate absorption was reduced by 41.9%. This effect could be reproduced if similar water secretion was osmotically induced by the addition of mannitol. These data suggest that acetate is absorbed, at least, partially by non-ionic diffusion in the rat jejunum and that its absorption is reduced in the secreting intestine by solvent drag.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argenzio R. A., Whipp S. C. Inter-relationship of sodium, chloride, bicarbonate and acetate transport by the colon of the pig. J Physiol. 1979 Oct;295:365–381. doi: 10.1113/jphysiol.1979.sp012974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman R. E. Simple, rapid method for determination of propionic acid and other short-chain fatty acids in serum. Clin Chem. 1978 May;24(5):800–803. [PubMed] [Google Scholar]
- Jørgensen K. E., Sheikh M. I. Characteristics of uptake of short chain fatty acids by luminal membrane vesicles from rabbit kidney. Biochim Biophys Acta. 1986 Sep 11;860(3):632–640. doi: 10.1016/0005-2736(86)90563-8. [DOI] [PubMed] [Google Scholar]
- Lloyd J. B., Whelan W. J. An improved method for enzymic determination of glucose in the presence of maltose. Anal Biochem. 1969 Sep;30(3):467–470. doi: 10.1016/0003-2697(69)90143-2. [DOI] [PubMed] [Google Scholar]
- Lucas M. Determination of acid surface pH in vivo in rat proximal jejunum. Gut. 1983 Aug;24(8):734–739. doi: 10.1136/gut.24.8.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano L., Reale E., Rechkemmer G., von Engelhardt W. Structure of zonulae occludentes and the permeability of the epithelium to short-chain fatty acids in the proximal and the distal colon of guinea pig. J Membr Biol. 1984;82(2):145–156. doi: 10.1007/BF01868939. [DOI] [PubMed] [Google Scholar]
- Naupert C., Rommel K. Absorption of short and medium chain fatty acids in the jejunum of the rat. Z Klin Chem Klin Biochem. 1975 Dec;13(12):553–562. doi: 10.1515/cclm.1975.13.12.553. [DOI] [PubMed] [Google Scholar]
- Redfors S., Sjövall H. The importance of nervous and humoral factors in the control of vascular resistance, blood flow distribution and net fluid absorption in the cat small intestine during hemorrhage. Acta Physiol Scand. 1984 Aug;121(4):305–315. doi: 10.1111/j.1748-1716.1984.tb07461.x. [DOI] [PubMed] [Google Scholar]
- Rolston D. D., Borodo M. M., Kelly M. J., Dawson A. M., Farthing M. J. Efficacy of oral rehydration solutions in a rat model of secretory diarrhea. J Pediatr Gastroenterol Nutr. 1987 Jul-Aug;6(4):624–630. doi: 10.1097/00005176-198707000-00023. [DOI] [PubMed] [Google Scholar]
- Ruppin H., Bar-Meir S., Soergel K. H., Wood C. M., Schmitt M. G., Jr Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980 Jun;78(6):1500–1507. [PubMed] [Google Scholar]
- Rübsamen K., von Engelhardt W. Absorption of Na, H ions and short chain fatty acids from the sheep colon. Pflugers Arch. 1981 Aug;391(2):141–146. doi: 10.1007/BF00657005. [DOI] [PubMed] [Google Scholar]
- Sallee V. L., Dietschy J. M. Determinants of intestinal mucosal uptake of short- and medium-chain fatty acids and alcohols. J Lipid Res. 1973 Jul;14(4):475–484. [PubMed] [Google Scholar]
- Schmitt M. G., Jr, Soergel K. H., Wood C. M. Absorption of short chain fatty acids from the human jejunum. Gastroenterology. 1976 Feb;70(2):211–215. [PubMed] [Google Scholar]
- Schmitt M. G., Jr, Soergel K. H., Wood C. M., Steff J. J. Absorption of short-chain fatty acids from the human ileum. Am J Dig Dis. 1977 Apr;22(4):340–347. doi: 10.1007/BF01072192. [DOI] [PubMed] [Google Scholar]
- Sladen G. E., Dawson A. M. An evaluation of perfusion techniques in the study of water and electrolyte absorption in man: the problem of endogenous secretions. Gut. 1968 Oct;9(5):530–535. doi: 10.1136/gut.9.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. W., Wiggins P. M., Lee S. P., Tasman-Jones C. Diffusion of butyrate through pig colonic mucus in vitro. Clin Sci (Lond) 1986 Mar;70(3):271–276. doi: 10.1042/cs0700271. [DOI] [PubMed] [Google Scholar]
- Watford M., Lund P., Krebs H. A. Isolation and metabolic characteristics of rat and chicken enterocytes. Biochem J. 1979 Mar 15;178(3):589–596. doi: 10.1042/bj1780589. [DOI] [PMC free article] [PubMed] [Google Scholar]


