Abstract
Carbohydrate–protein recognition is central to many biological processes. Enzymes that act on polysaccharide substrates frequently contain noncatalytic domains, “carbohydrate-binding modules” (CBMs), that target the enzyme to the appropriate substrate. CBMs that recognize specific plant structural polysaccharides are often able to accommodate both the variable backbone and the side-chain decorations of heterogeneous ligands. “CBM29” modules, derived from a noncatalytic component of the Piromyces equi cellulase/hemicellulase complex, provide an example of this selective yet flexible recognition. They discriminate strongly against some polysaccharides while remaining relatively promiscuous toward both β-1,4-linked manno- and cello-oligosaccharides. This feature may reflect preferential, but flexible, targeting toward glucomannans in the plant cell wall. The three-dimensional structure of CBM29-2 and its complexes with cello- and mannohexaose reveal a β-jelly-roll topology, with an extended binding groove on the concave surface. The orientation of the aromatic residues complements the conformation of the target sugar polymer while accommodation of both manno- and gluco-configured oligo- and polysaccharides is conferred by virtue of the plasticity of the direct interactions from their axial and equatorial 2-hydroxyls, respectively. Such flexible ligand recognition targets the anaerobic fungal complex to a range of different components in the plant cell wall and thus plays a pivotal role in the highly efficient degradation of this composite structure by the microbial eukaryote.
Protein–carbohydrate interactions play a critical role in biology. These macromolecular associations are particularly important in cellular signaling, host–pathogen interactions, and in the microbial recycling of carbon from the plant cell wall (1). Microbial enzymes involved in these processes frequently possess a modular structure in which noncatalytic carbohydrate-binding modules (CBMs) target the biocatalysts to specific polysaccharides and enhance catalytic efficiency by increasing the effective concentration of the enzyme on the surface of insoluble substrates (2, 3). As with the catalytic modules of these enzymes (4), CBMs are grouped into a number of discrete families based upon amino acid sequence similarity (5). The 30 CBM families currently known may be found as part of a continuously updated carbohydrate-active enzyme database at http://afmb.cnrs-mrs.fr/CAZY/index.html.
The three-dimensional (3D) structures of CBMs from 12 families (3) have shown that these protein modules are composed almost exclusively of β-strands arranged in a “jelly-roll” motif whose topography reflects the macroscopic nature of the target substrate. Modules that interact with crystalline cellulose, for example, display a planar hydrophobic surface that is thought to interact with adjacent chains on the surface of the crystal lattice (6). Conversely, CBMs that bind soluble or amorphous cellulose or xylan possess clefts that interact with a single chain of their poly/oligosaccharide ligands (7, 8). Subtle differences in the structure of CBMs can lead to very diverse ligand specificity, exemplified by families 4 and 6, in which highly related proteins bind a range of ligands that include cellulose, xylan, laminarin, and mannan. Thus, CBMs in complex with their respective ligands present excellent systems for dissecting the molecular determinants that define the structural basis for protein–carbohydrate recognition, which is central to numerous essential biological processes.
The plant cell wall degrading capacity of gut anaerobic fungi is vested in a multienzyme complex, termed the “cellulosome” (9), that binds to plant polysaccharides. The catalytic components of the Piromyces equi cellulosome do not contain CBMs (9, 10), suggesting that the capacity of this enzyme complex to attach to the plant cell wall is mediated by one or a small number of noncatalytic proteins. Recently, the first of these noncatalytic components was identified. The protein, known as NCP1, was shown to consist of three N-terminal copies of a fungal dockerin (11) linked to two closely related CBMs. The two CBMs define a previously uncharacterized sequence family, CBM29 (12). Both are highly discriminatory for gluco- and manno-configured ligands. Soluble glucomannan, galactomannan, β-glucan, and hydroxyethylcellulose (HEC), as well as insoluble forms of cellulose and mannan, are all targets for CBM29, with the two copies of the module in NCP1 displaying significantly higher affinity against glucomannan than either of the individual modules (12). NCP1 is, therefore, likely to play a pivotal role in the efficient degradation of the plant cell wall by the Piromyces cellulosome, and its unusual ligand specificity presents an excellent model system for studying protein–carbohydrate recognition. Here, we report the structural and thermodynamic basis of the unusual specificity of the C-terminal CBM (CBM29-2) from P. equi NCP1 in native and both cellohexaose and mannohexaose complexed forms. We propose a model for CBM29 ligand recognition in which the major molecular determinant is the orientation of the aromatic residues in the binding site complementing the conformation of the target sugar polymers. The paucity of direct hydrogen bonds facilitates the promiscuity in ligand recognition required to accommodate chemical variation in the polysaccharide backbone.
Materials and Methods
Sources of Sugars.
Manno-oligosaccharides, “galactomannopentaose” (mannopentaose with galactosyl moieties α-1,6 linked to both mannoses 3 and 4), low viscosity carob galactomannan (in which α1,6-linked galactopyranose groups are present on 20% of the mannose residues) and low viscosity konjac glucomannan (composed of a random sequence of glucose and mannose present in a 3:2 molar ratio) were obtained from Megazyme (Bray, Ireland). HEC (in which the average degree of substitution is 1 per glucose moiety and the molar polymerization of hydroxyethyl groups is 2) was obtained from Aldrich. Cello-oligosaccharides were obtained from Seikagaku Kogyo, Tokyo.
Construction of pVM1 and Purification of CBM29-2.
The pET22b derivative, pVM1, encoding CBM29-2 had been constructed previously (12). CBM29-2 comprised residues 336–479 of P. equi NCP1 attached to a C-terminal His-6 tag (12). Standard culture conditions (12, 13) were used to express native and seleno-l-methionine-containing CBM29-2 in Escherichia coli BL21(DE3):pLysS and E. coli B834 harboring pVM1, respectively. CBM29-2 was purified to electrophoretic homogeneity by metal ion affinity chromatography (14) using Talon resin (CLONTECH), FPLC anion-exchange chromatography employed a UNO Q-12 column (Bio-Rad), and gel filtration on a prepacked Superdex 75 column (AP Biotech, U.K.). Buffers used to purify CBM29-2 containing seleno-l-methionine were supplemented with 10 mM mercaptoethanol.
Isothermal Titration Calorimetry.
Isothermal titration calorimetry (ITC) was performed at 25°C in 50 mM sodium phosphate buffer, pH 7.0, with a Microcal Omega titration calorimeter, as described (8). With all ligands except cellotetraose, mannopentaose, and galactomannopentaose, the c-value (product of the molar concentration of binding sites × the association constant) was significantly greater than 1, allowing accurate deconvolution of the binding data (15).
Crystallization of CBM29-2.
Purified CBM29-2 at 20 mg/ml was screened for crystallization with the Clear Strategy Screen (16). Tetragonal bipyramidal crystals were obtained after 2 days from a drop that comprised 2 μl of protein at 10 mg/ml and 1 μl of 100 mM Na/Hepes buffer, pH 7.5, containing 150 mM KSCN, 20% (vol/vol) ethylene glycol, and 16–21% (wt/vol) PEG3350. Selenomethionine-containing crystals were obtained as for native CBM29-2, with the inclusion of 10 mM DTT. SeMet CBM29-2 belongs to space group P43212, with unit cell dimensions a = b = 93.3 Å; c = 82.9 Å and contains two molecules of protein in the asymmetric unit. Crystals of CBM29 in complex with cellohexaose were grown from 100 mM Na/Hepes buffer, pH 7.5, containing 20% (wt/vol) PEG3350, 0.2 M Li2SO4, and 10 mM cellohexaose and have space group C2, with unit cell dimensions a = 107.7 Å, b = 43.0 Å, c = 35.5 Å, and β = 105.4o. Mannohexaose complex crystals were grown from drops composed of equal volumes of 3.0 M ammonium sulfate and protein (10 mg/ml), in addition to 10 mM mannohexaose. They belong to space group C2, with unit cell dimensions a = 51.1 Å, b = 42.7 Å, c = 60.1 Å, and β = 93.7o. Cryoprotectant mother liquors were prepared from the growth conditions with glycerol incorporated to 20–30% (vol/vol) as appropriate.
Structure Solution and Refinement.
Multiwavelength data were collected on beamline ID14-4 at the European Synchrotron Radiation Facility (ESRF), Grenoble by using an Area Detector Systems Corporation (ADSC) Quantum-4 charge-coupled device. A single crystal of CBM29-2, mounted in a rayon fiber loop was used for data collection at 100 K. The fluorescence spectrum, spanning the selenium K-absorption edge, determined wavelengths for a three-wavelength multiwavelength anomalous diffraction experiment (λ1 = 0.9794 Å, λ2 = 0.9790 Å, and λ3 = 0.9322 Å). At each wavelength, 400 images of 0.4o were collected, and the data was processed with the HKL suite (17).
Scaled multiwavelength anomalous diffraction data were used as input to the SOLVE (18) in space groups P41212 and P43212. The higher native Fourier coefficient confirmed the space group as P43212. Solvent flattening was performed by using DM (19), and two independent partial models of CBM29 were traced by using QUANTA (Accelrys, San Diego, CA). The resultant noncrystallographic symmetry (NCS) operator, derived from the model coordinates, was used to improve the electron density map further by NCS averaging. Five percent of the observations were set aside for cross-validation analysis, and the structure was refined with REFMAC (20) by using the “high-energy” remote 2.34 Å data (Rmerge 0.086, mean I/σI 54, 99.8% complete). All other computing used the CCP4 suite (21) unless otherwise stated. Water molecules were first placed automatically with refmac/arp (20, 22) and verified manually before coordinate deposition.
Model Building and Refinement of the CBM29 Cellohexaose and CBM29 Mannohexaose Complexes.
Data, to 1.15 Å, were collected from a single crystal at 100K of the CBM29-(Glc)6 complex on beamline ID14-2 at the ESRF. They have an overall Rmerge of 0.031 (0.075), are 98% complete to 1.3 Å (≈50% between 1.3 and 1.15 Å), with a mean I/σI of 34 (10), with the outer resolution shell statistics in parentheses. The structure was solved by molecular replacement with the program AMORE (23) by using a single molecule of SeMet CBM29 as the search model with data between 35 and 3.0 Å and a sphere of Patterson integration of 10 Å. Initial rigid body refinement was followed by cycles of maximum-likelihood refinement with REFMAC and manual rebuilding. A single molecule of cellohexaose was clear (statistically disordered in one subsite, described below) and a single cobalt ion (present in the purification and confirmed by fluorescence analysis at the ESRF, not shown) tetrahedrally coordinated to Glu-98, Glu-99, His-181, and His-183 was also included. Water molecules were included as described above. Data for the CBM29-(Man)6 complex were collected to 1.5 Å from a single crystal at 100K on beamline PX9.6 of the Daresbury Synchrotron Radiation Source (SRS) with an ADSC charge-coupled device. They have an overall Rmerge of 0.085 (0.23), are 99% complete to 1.52 Å (93% between 1.57 and 1.52 Å), and have a mean I/σI of 14 (3.9), with the outer resolution shell statistics in parentheses. The structure was solved by molecular replacement with the refined 1.15 Å structure of the CBM29-(Glc)6 complex (lacking the histidine-tag and cobalt ion) as the search model and refined as described above.
Results
Ligand Specificity of CBM29-2.
The interactions of CBM29-2 with soluble poly- and oligosaccharides were quantified by using ITC and the energetic contributions to binding dissected, Table 1. CBM29-2 interacts with cello-oligosaccharides, displaying highest affinity for the hexasaccharide and showing a similar pattern of binding with manno-oligosaccharides, albeit with lower affinity. With both manno- and gluco-oligosaccharides, the largest increase in affinity was from the tetraose to the pentaose sugar, consistent with a minimum of five saccharide moieties required to span the three aromatic residues in the binding cleft. CBM29-2 binds galactomannopentaose with ≈10-fold-lower affinity than mannopentaose (not shown).
Table 1.
Affinity of CBM29-2 for oligo- and polysaccharides determined by ITC
| Ligand | Ka, ×103 M−1 | ΔG (kcal mol−1) | ΔH (kcal mol−1) | TΔS (kcal mol−1) | n |
|---|---|---|---|---|---|
| Mannohexaose | 2.5 (±0.0) | −4.63 (±0.01) | −8.67 (±0.10) | −4.04 (±0.10) | 1.48 (±0.14) |
| Mannopentaose | 0.8 (±0.1) | – | – | – | – |
| Mannotetraose | NB | – | – | – | – |
| Cellohexaose | 12.5 (±2.5) | −5.57 (±0.12) | −7.89 (±0.18) | −2.32 (±0.06) | 1.06 (±0.11) |
| Cellopentaose | 5.9 (±0.1) | −5.14 (±0.05) | −7.23 (±0.37) | −2.09 (±0.13) | 1.30 (±0.10) |
| Cellotetraose | 0.6 (±0.2) | – | – | – | – |
| Cellotriose | NB | – | – | – | – |
| Glucomannan (Konjac) | 374.8 (±104.5) | −7.57 (±0.17) | −16.58 (±0.36) | −9.01 (±0.46) | 0.99 (±0.08) |
| Galactomannan (Carob) | 92.1 (±10.8) | −6.77 (±0.07) | −13.54 (±1.34) | −6.77 (±1.27) | 1.02 (±0.07) |
| HEC | 14.4 (±1.8) | −5.66 (±0.08) | −9.62 (±0.33) | −3.96 (±0.40) | 1.01 (±0.03) |
Number of binding sites on protein.
Values in parentheses are SDs from at least triplicate titrations.
Affinity values are estimates as c (molar concentration of binding sites × association constant) significantly below 1.
NB, no binding detectable by ITC.
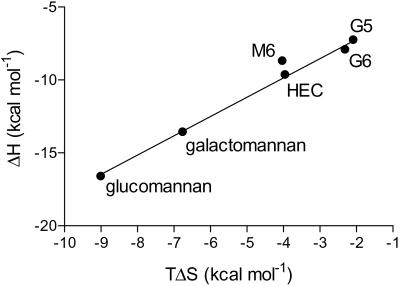
CBM29-2 interacts preferentially with glucomannan, with a binding constant for the mixed sugar polymer 26- and 4-fold higher than that achieved with HEC and galactomannan, respectively. The protein binds ≈40-fold more tightly to carob galactomannan than mannohexaose, whereas the affinity of the CBM for HEC and cellohexaose was similar. The molar concentration of CBM29-2-binding sites on glucomannan and galactomannan were broadly similar (approximately one protein molecule every 8–9 sugar residues), but there is, on average, just one binding site every 40 residues on HEC. The thermodynamics of binding of CBM29-2 to all ligands reveals that binding is enthalpically driven, as observed for the majority of CBMs studied (for example, 8, 13, and 24). The ratios of enthalpy to entropy for the binding of each ligand to CBM29-2 were very similar, except for mannohexaose, which interacted particularly weakly with the protein primarily because of a more unfavorable entropy, Fig. 1. CBM29-2 thus displays unusual binding characteristics in that it is both highly discriminating against a range of polysaccharides (12) yet also retains relaxed specificity toward carbohydrates derived from β-1,4-linked mannose, glucose, or combinations of the two. To investigate these properties, the 3D structure of CBM29-2 was determined in native- and ligand-complexed forms.
Fig 1.
Enthalpy/entropy plot of CBM29-2 interactions with oligo- and polysaccharide ligands. The entropy and enthalpy associated with the binding of glucomannan, galactomannan, hydroxyethyl cellulose (HEC), cellopentaose (G5), cellohexaose (G6), and mannohexaose (M6) to CBM29-2 are shown. The slope, fitted without M6, was 1.3.
3D Structure of CBM29-2 and Its Similarity to Other CBMs.
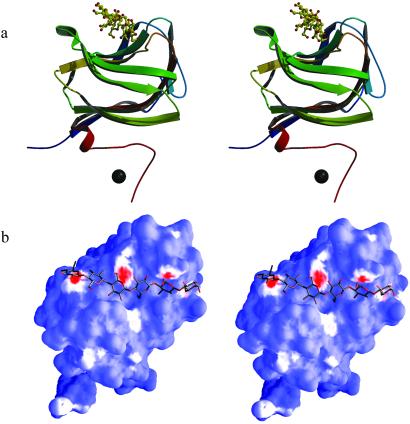
Multiple-wavelength anomalous dispersion techniques allowed the determination of the selenomethionyl CBM29-2 by x-ray crystallography at 2.3 Å resolution. The final structure encompasses residues 4–144 (A molecule) and 3–148 (B molecule), has a crystallographic R value of 0.19 with Rfree = 0.24, and shows deviations from stereochemical target values of 0.018 Å for bonds and 1.6° for angles, respectively. The structure presents a classic β-jelly-roll, with five β-strands forming each of the two faces in Fig. 2a and is similar to the topologies described for CBM families 4, 6, 15, and 22 (7, 8, 13, 24), despite displaying no significant sequence similarity to these proteins. Structural similarity searches using distance matrices, as implemented in DALI (25), reveal that CBM29-2 is most similar to the xylan-binding CBM22 from Clostridium thermocellum Xyn10B (PDB ID code .pdb), with 132 matching Cα positions giving an rms deviation of 3.0 Å; the cellulose-binding CBM4 from Cellulomonas fimi Cel9B (PDB ID code .pdb), with 122 equivalent Cα positions, gave an rms deviation of 2.7 Å. These CBMs also show significant, if more distant, structural similarity with jelly-roll catalytic domains of some glycoside hydrolases such as those from family GH16 [the κ-carrageenase (PDB ID code .pdb), for example, displays 125 equivalent Cα positions, giving an rms deviation of 2.9 Å] hinting at some evolutionary ancestor for these domains. As with most CBMs, the concave “upper” face of the jelly-roll of CBM29-2 is lined with aromatic residues and forms the binding site for oligosaccharides, described below.
Fig 2.
Three-dimensional structure of the cellohexaose complex of CBM29. (a) Topological protein cartoon, color-ramped from N to C terminus, with the ligand in “ball-and-stick” representation and the cobalt ion as a sphere, prepared by using MOLSCRIPT/BOBSCRIPT (29, 30). (b) “Hydrophobic surface” figure generated with the SURFGEN algorithm (available at www.biop.ox.ac.uk). Hydrophobic interaction surfaces are color-ramped from red (most hydrophobic) through white to blue (least hydrophobic).
Structures of CBM29-2 in Complex with Cello- and Mannohexaose.
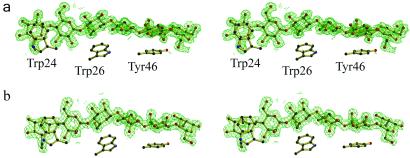
The structure of CBM29-2 in complex with cellohexaose was determined at 1.15 Å resolution. The structure includes residues 1–153, the latter 8 residues, encoded by the vector, comprises a polyhistidine tag which is coordinated to a cobalt ion derived from the affinity purification matrix. The structure has an Rcryst = 0.13, Rfree = 0.16, and shows deviations from stereochemical target values of 0.016 Å and 1.9° for bonds and angles, respectively. The initial electron density maps revealed unambiguous density (see Fig. 3a) for the hexasaccharide along the concave upper surface of the jelly-roll. All of the pyranosides are observed in the undistorted 4C1 chair conformation. Three aromatic residues line this cleft, Trps 24 and 26 from the first β-strand and Tyr-46 from the second, forming the classic hydrophobic platform observed for many CBMs (Fig. 2b). All three aromatic amino acids interact with the α-face of the glucosyl moieties of residues 6, 4, and 2, respectively, which appear to be more hydrophobic by virtue of both axial H-5 and H-3 hydrogens and the aliphatic C5-C6 bond. H-5 “points” into the π-electron cloud of the aromatic ring, hinting at “ring-current” hydrogen-bonding possibilities. The minimum ligand required to harness the binding energy from all three aromatics is a pentasaccharide, consistent with the ITC-derived binding data.
Fig 3.
Observed electron density (maximum-likelihood/σA weighted 2Fobs−Fcalc syntheses) for the CBM29 oligosaccharide complexes. (a) Cellohexaose and (b) mannohexaose both contoured at ≈0.5 electrons per Å3. The locations of the three aromatic residues described in the text are shown for reference. These figures, in divergent stereo, were prepared with BOBSCRIPT (30).
There are few direct hydrogen bonds between CBM29-2 and cellohexaose (Fig. 4a). Glucoside-6 makes no direct hydrogen bonds to the CBM, the O5 of glucoside-5 makes a 3.1 Å hydrogen bond to the NH1 of Arg-112, whereas its O2 makes a direct H-bond to the main chain carbonyl oxygen of Trp-24. Glucoside-4 interacts from its O4 to the side chain of Arg-112 and from its O3 to Arg-112 and Glu-78; whereas its O2 makes a solvent-mediated interaction with Asp-114, it also makes a steric clash (3.3 Å from O2 to the Cγ) with the side chain of Lys-85, which is consequently forced into multiple conformations beyond Cγ. Glucoside-3 interacts predominately by means of its C6-O6 group, which is buried into the protein surface where it makes a direct hydrogen bond to the carbonyl group of Gln116. O5 accepts a hydrogen bond from the side-chain amide of Gln-116. Glucoside-2 is the second site where the O2 hydroxyl (that differentiates gluco-configured ligands from manno-configured ones) makes a direct interaction with the protein, from O2 to the main-chain carbonyl of Ala-118, whereas O3 interacts predominantly with Lys-74. Despite these interactions, glucoside-2 actually shows two distinct conformations resolvable at 1.15 Å; the second is either less occupied or has more conformational mobility, perhaps reflecting an oligosaccharide that terminates in this site rather than continuing to site 1. This latter conformation also makes an additional direct hydrogen bond from O2 to Lys-74, mimicking that seen in the mannohexaose complex described below. Glucoside-1 shows just one direct hydrogen bond to the protein, from its buried C6-O6 hydroxyl again to the main-chain carbonyl of Ala-118, which sits in an unusual turn formed by Pro-119. This configuration would appear to be crucial to defining the topography of the interacting surface for the reducing end of the oligosaccharide. The reducing end of O1 is observed as its α-anomer probably because it is thus able to make a direct hydrogen bond to Arg-59 from a symmetry-related molecule. Water-linked interactions between ligand and protein contribute approximately seven additional H-bonds from hydroxyls in subsites 1–5.
Fig 4.
Schematic representation of the direct (nonsolvent mediated) interactions between CBM29 and ligand cellohexaose (a) and mannohexaose (b). The statistical disorder of the glucosyl moiety in subsite 2 is shown in blue, and hydrogen bonds only observed in the mannohexaose complex (subsites 5 and 2) are shown in red.
The mannohexaose CBM29-2 complex at 1.5 Å resolution features residues 2–142, and a single molecule of mannohexaose has Rcryst = 0.15, Rfree = 0.18, and shows deviations from stereochemical target values of 0.017 Å and 1.9° for bonds and angles, respectively. Again, unambiguous electron density is observed for a hexasaccharide ligand (see Fig. 3b), occupying identical “subsites” as observed for the cellohexaose with all pyranosides displaying the undistorted 4C1 chair conformation. Mannosides 4–6 of the hexasaccharide are slightly more displaced from the protein core than their cello-oligosaccharide counterparts, but this movement is accompanied with appropriate side-chain conformational changes of ≈1.3 Å in the position of Trp-24. The hydrophobic packing between pyranosides 6, 4, and 2 with Trp-24, Trp-26, and Tyr-46, respectively, are identical in the mannohexaose and cellohexaose complexes, and given the role of aromatic residues in sugar binding, it is likely that these interactions contribute greatly to the association of CBM29-2 with both manno- and gluco-derived ligands.
The paucity of direct hydrogen bonds between protein and cellohexaose is repeated for the mannohexaose-CBM29-2 complex (see Fig. 4b). Again, mannoside-6 makes no direct hydrogen bonds, its sole contact with CBM29-2 being the van der Waals interaction with Trp-24. As with cellohexaose, mannoside-5 contributes direct H bonds from O5 to the NH1 of Arg-112 and from O2 to the main-chain carbonyl of Trp-24. Mannoside-5 is able to make an additional interaction from its axial O2 to the NH2 atom of Arg-112, one of only two subsites where a direct manno-O2–protein interaction exists which is not mirrored by a similar interaction in the corresponding cello-oligosaccharide. Mannoside-4 contributes direct hydrogen bonds from O4 to Arg-112 and O3 to Glu-78, exactly as in the cellohexaose complex. Mannoside-3 makes hydrogen bonds from O5 and O6 to Gln-116 and from O3 to Glu-78 as with a gluco-configured ligand and is the second subsite where a hydrogen bond exists for the axial O2 of mannose alone—from O2 to the NE2 of Gln-116. The second mannoside makes almost identical hydrogen bonds to those observed in the cellohexaose complex. The O2 interaction with Lys-74 is observed only in the second conformation for the glucosyl moiety, where the equatorial gluco-O2 lies in an equivalent position to the axial manno-O2 of a mannosyl moiety. As with the cellohexaose complex, water-mediated interactions contribute to binding, with approximately nine water-mediated H-bonds between ligand and protein.
Discussion
CBM29-2 is somewhat promiscuous in its choice of partners, yet it shows discrimination for homo- and heteropolysaccharides of β-1,4-linked d-mannose or d-glucose (12). The first discriminator of ligand specificity is likely to be the location of the three aromatic residues in the binding cleft which exhibit specificity for saccharides displaying the “twisted” conformation along the chain. This conformation has been shown for cellohexaose in solution (26) and when bound to CBM4 (27) and CBM17 (28), not a twofold screw axis, as occurs in crystalline cellulose. It is not known, however, whether manno-oligosaccharides adopt similarly twisted conformations in solution. For both complexes, sugars 5, 3, and 1 point their hydroxymethyl substituents, C6-O6, toward the protein surface to make direct H bonds to the protein, and, likewise, the O3 interactions of the two ligands are similar. It is interesting to note that, as the C6O6 moiety of mannose residues at subsites 6, 4, and 2 point toward the solvent, the binding cleft of CBM29-2 can accommodate regions of galactomannan in which every other backbone sugar is decorated. Because it is every second 6-hydroxyl that is directed into solvent in this manner CBM29-2 still binds weakly to substrates in which adjacent mannose residues are substituted, such as galactomannopentaose. With soluble oligosaccharides, CBM29-2 interacts more tightly with cellohexaose than mannohexaose, even though binding of the latter is enthalpically more favorable. These data are consistent with the crystal structures of the two complexes which reveal two direct hydrogen bonds between CBM29-2 and mannohexaose that are not observed in the cellohexaose complex. For mannoside 5, the inward pointing O2 is able to make an additional interaction with Arg-112 and, likewise, mannoside 3 is able to make a direct interaction with Gln-116 again by virture of its axial O2. Furthermore, in the fourth sugar site, the Cγ of Lys-85 clashes with the equatorial O2 of the glucoside, forcing disorder beyond this point; in the mannohexaose complex, a singly positioned Lys-85 is able to make a solvent-mediated hydrogen bond to the mannosyl moiety. Such direct hydrogen bonding and steric factors suggest that subsites 5, 4, and 3 of CBM29-2 may favor binding of mannose over glucose.
Enthalpy–entropy compensation plots for the binding of ligands to CBM29-2 generate a straight line with slope of 1.3 (Fig. 1). The sole exception to this result is mannohexaose, which appears to be unusually unfavorable in terms of entropy. The reason for this result is unclear. It is possible that more ordered waters are sequestered upon mannohexaose binding, and that their enthalpic gain does not overcome the entropic loss associated with the ordering of water molecules from bulk solvent. Mannohexaose certainly appears to make two to three more solvent-mediated contacts than cellohexaose, but these are difficult to quantify based upon x-ray data alone. It may be possible that, in solution, manno-oligosaccharides display more flexibility than gluco-oligosaccharides, and hence, their binding to the CBM has a greater entropic penalty through increased conformational restriction. In contrast to many other CBMs, including CBM15 and 22 (8, 13), CBM29-2 binds more tightly to polymeric mannans than manno-oligosaccharides. The reasons are unclear. One possibility is that there is some degree of cooperativity between CBM29 molecules that occupy adjacent sites on galactomannan (and glucomannan). These effects are not observed with HEC (which, instead, displays similar affinity to cellohexaose), where the ligand-binding sites are more distantly spaced. These unusual properties clearly require further investigation.
CBM29s in NCP1 play a key role in the targeting of the multicomponent fungal cellulosome of P. equi to the noncrystalline parts of the cell wall. The structure of CBM29-2 reveals a scaffold fine tuned for the recognition of noncrystalline polysaccharides of glucose and mannose. Aromatic residues, appropriately oriented, provide a hydrophobic template for the binding of β-1–4-linked sugars. Discrete recognition of the O3 and O6 hydroxyls builds upon this template, conferring further specificity toward either glucose or mannose-based ligands. Interaction with the 2-hydroxyl, whose chirality defines whether the pyranoside is glucose or mannose, is flexible, thus allowing the relaxed recognition of both gluco- and manno-configured ligands. The high affinity for glucomannans suggests that different subsites on the CBM prefer either glucose or mannose, but the flexible multifaceted approach of CBM29 modules targets the fungal cellulosome to a range of different components in the plant cell wall. Thus, the CBM29s in NCP1 most likely play a pivotal role in the highly efficient degradation of this composite structure by the microbial eukaryote.
Acknowledgments
We thank the Biotechnology and Biological Sciences Research Council and the University of York for funding, and the Daresbury Synchrotron Radiation Source and European Synchrotron Radiation Facility, Grenoble, for provision of data collection facilities. D.N. is a European Molecular Biology Organization Fellow and G.J.D. is a Royal Society University Research Fellow.
Abbreviations
CBM, carbohydrate-binding module
HEC, hydroxyethylcellulose
Data deposition: The structure factors reported in this paper have been deposited in the Protein Data Bank, www.rcsb.org [PDB ID codes (SeMet), 1 gwm (cellohexaose complex), and (mannohexaose complex)].
References
- 1.Brett C. T. & Waldren, K., (1996) Physiology and Biochemistry of Plant Cell Walls (Chapman & Hall, London).
- 2.Gill J., Rixon, J. E., Bolam, D. N., McQueen-Mason, S., Simpson, P. J., Williamson, M. P., Hazlewood, G. P. & Gilbert, H. J. (1999) Biochem. J. 342, 473-480. [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert H. J., Bolam, D. N., Szabo, L., Xie, H., Williamson, M. P., Simpson, P. J., Jamal, S., Boraston, A. B., Kilburn, D. G. & Warren, R. A. J. (2001) in Carbohydrate Bioengineering: Interdisciplinary Approaches, eds. Teeri, T. T., Svensson, B., Gilbert, H. J. & Feizi, T. (R. Soc. Chem., Cambridge, U.K.).
- 4.Henrissat B. & Bairoch, A. (1996) Biochem. J. 316, 695-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutinho P. M. & Henrissat, B. (1999) in Recent Advances in Carbohydrate Engineering, eds. Gilbert, H. J., Davies, G. J., Henrissat, B. & Svensson, B. (R. Soc. Chem., Cambridge, U.K.), pp. 3–12.
- 6.Tormo J., Lamed, R., Chirino, A. J., Morag, E., Bayer, E. A., Shoham, Y. & Steitz, T. A. (1996) EMBO J. 15, 5739-5751. [PMC free article] [PubMed] [Google Scholar]
- 7.Brun E., Johnson, P. E., Creagh, L., Tomme, P., Webster, P., Haynes, C. A. & McIntosh, L. P. (2000) Biochemistry 39, 2445-2458. [DOI] [PubMed] [Google Scholar]
- 8.Szabo L., Jamal, S., Xie, H., Charnock, S. J., Bolam, D. N., Gilbert, H. J. & Davies, G. J. (2001) J. Biol. Chem. 276, 49061-49065. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert H. J., Hazlewood, G. P., Laurie, J. I., Orpin, C. G. & Xue, G. P. (1992) Mol. Microbiol. 6, 2065-2072. [DOI] [PubMed] [Google Scholar]
- 10.Ali B. R. S., Zhou, L. Q., Graves, F. M., Freedman, R. B., Black, G. W., Gilbert, H. J. & Hazlewood, G. P. (1995) FEMS Microbiol. Lett. 6, 2065-2072. [DOI] [PubMed] [Google Scholar]
- 11.Fanutti C., Ponyi, T., Black, G. W., Hazlewood, G. P. & Gilbert, H. J. (1995) J. Biol. Chem. 270, 29314-29322. [DOI] [PubMed] [Google Scholar]
- 12.Freelove A. C., Bolam, D. N., White, P., Hazlewood, G. P. & Gilbert, H. J. (2001) J. Biol. Chem. 276, 43010-43017. [DOI] [PubMed] [Google Scholar]
- 13.Charnock S. J., Bolam, D. N., Turkenburg, J. P., Gilbert, H. J., Ferreira, L. M., Davies, G. J. & Fontes, C. M. (2000) Biochemistry 39, 5013-5021. [DOI] [PubMed] [Google Scholar]
- 14.Xie H., Bolam, D. N., Nagy, T., Szabo, L., Cooper, A., Simpson, P. J., Lakey, J. H., Williamson, M. P. & Gilbert, H. J. (2001) Biochemistry 40, 5700-5707. [DOI] [PubMed] [Google Scholar]
- 15.Wiseman T., Williston, S., Brandts, J. F. & Lin, L. N. (1989) Anal. Biochem. 179, 131-137. [DOI] [PubMed] [Google Scholar]
- 16.Brzozowski A. M. & Walton, J. (2001) J. Appl. Crystallogr. 34, 97-101. [Google Scholar]
- 17.Otwinowski Z. & Minor, W. (1997) Methods Enzymol. 276, 307-326. [DOI] [PubMed] [Google Scholar]
- 18.Terwilliger T. C. & Berendzen, J. (1999) Acta Crystallogr. D 55, 849-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowtan K. D. & Main, P. (1996) Acta Crystallogr. D 49, 148-157. [DOI] [PubMed] [Google Scholar]
- 20.Murshudov G. N., Vagin, A. A. & Dodson, E. J. (1997) Acta Crystallogr. D 53, 240-255. [DOI] [PubMed] [Google Scholar]
- 21.Collaborative Computational Project Number 4 (1994) Acta Crystallogr. D 50, 760-763.15299374 [Google Scholar]
- 22.Lamzin V. S. & Wilson, K. S. (1993) Acta Crystallogr. D 49, 129-147. [DOI] [PubMed] [Google Scholar]
- 23.Navaza J. & Saludijan, P. (1997) Methods Enzymol. 276, 581-594. [DOI] [PubMed] [Google Scholar]
- 24.Czjzek M., Bolam, D. N., Mosbah, A., Allouch, J., Fontes, C. M., Ferreira, L. M., Bornet, O., Zamboni, V., Darbon, H., Smith, N. L., et al. (2001) J. Biol. Chem. 276, 48580-48587. [DOI] [PubMed] [Google Scholar]
- 25.Holm L. & Sander, C. (1993) J. Mol. Biol. 233, 123-138. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama H., Hisamichi, K., Usui, T., Sakai, K. & Ishiyama, J. (2000) J. Mol. Struct. 556, 173-177. [DOI] [PubMed] [Google Scholar]
- 27.Boraston A. B., Nurizzo, D., Notenboom, V., Ducros, V., Rose, D. R., Warren, R. A. J., Kilburn, D. G. & Davies, G. J. (2002) J. Mol. Biol. 319, 1143-1156. [DOI] [PubMed] [Google Scholar]
- 28.Notenboom V., Boraston, A. B., Chiu, P., Freelove, A. C. J., Kilburn, D. G. & Rose, D. R. (2001) J. Mol. Biol. 314, 797-806. [DOI] [PubMed] [Google Scholar]
- 29.Kraulis P. J. (1991) J. Appl. Crystallogr. 24, 946-950. [Google Scholar]
- 30.Esnouf R. M. (1997) J. Mol. Graphics 15, 133-138. [DOI] [PubMed] [Google Scholar]