Abstract
Nonribosomal peptide synthetases (NRPSs) assemble structurally complex peptides from simple building blocks such as amino and carboxyl acids. Product release by macrocyclization or hydrolysis is catalyzed by a thioesterase domain that is an integrated part of the NRPS enzyme. A second thioesterase of type II (TEII) encoded by a distinct gene associated with the NRPS cluster was previously shown by means of gene disruption to be important for efficient product formation. However, the actual role of TEIIs in nonribosomal peptide synthesis remained obscure. Here we report the biochemical characterization of two such TEII enzymes that are associated with the synthetases of the peptide antibiotics surfactin (TEIIsrf) and bacitracin (TEIIbac). Both enzymes were shown to efficiently regenerate misacylated thiol groups of 4′-phosphopantetheine (4′PP) cofactors attached to the peptidyl carrier proteins (PCPs) of NRPSs. For TEIIsrf, a KM of 0.9 μM and a kcat of 95 min−1 was determined for acetyl-PCP hydrolysis. Both enzymes could also hydrolyze aminoacyl or peptidyl PCPs, intermediates of nonribosomal peptide synthesis. However, this reaction is unlikely to be of physiological relevance. Similar intermediates of the primary metabolism such as CoA derivatives and acetyl-acyl carrier proteins of fatty acid synthesis were also not significantly hydrolyzed, as investigated with TEIIsrf. These findings support a model in which the physiological role of TEIIs in nonribosomal peptide synthesis is the regeneration of misacylated NRPS, which result from the apo to holo conversion of NRPS enzymes because of the promiscuity of dedicated 4′PP transferases that use not only free CoA, but also acyl-CoAs as 4′PP donors.
Keywords: polyketide synthase, peptide antibiotic
Many nonribosomally synthesized peptides are important natural products that find application in medicine and biotechnology. Examples for these structurally diverse molecules are antibiotics like penicillin and vancomycin, immunosuppressive agents like cyclosporin A, and cytostatic compounds like epothilone. The biosynthesis of these products is carried out on large multimodular enzymes, the nonribosomal peptide synthetases (NRPSs), in a stepwise assembly from the amino acid monomers (1–3). Each module is dedicated to the activation, optional modification, and incorporation of one monomer into the product and harbors all necessary enzymatic activities in form of specialized domains to perform the single chemical reactions. Central to this biosynthetic strategy and usually a component of each module are the peptidyl carrier protein (PCP) domains. PCPs bind the monomers and intermediates of the growing peptide chain as thioesters through the thiol moiety of their prosthetic group 4′-phosphopantetheine (4′PP) and facilitate on this 20-Å long tether their directed transport through the NRPS assembly line. Conversion of each PCP from the inactive apo form into the active holo form is performed by 4′PP transferases in a posttranslational reaction, referred to as the priming step, which uses CoA as the source of 4′PP (4). Only when all PCPs are equipped with a 4′PP from the priming step, the NRPSs can synthesize their dedicated products in cycles of initiation, elongation, and termination steps (3).
Another component associated with most of the analyzed NRPS biosynthetic gene clusters is a gene predicted to code for a type II thioesterase (TEII) (5–7). The role of TEIIs in nonribosomal peptide synthesis has been obscure to date. These enzymes are not essential; however, they are important for effective synthesis, because deletion of the genes led to a drastic reduction in product yields, but did not completely abolish it (8, 9). From these data and their sequence homology to TEIIs associated with vertebrate fatty acid synthesis, like the medium-chain S-acyl fatty acid synthetase thioester hydrolase found in rat mammary gland (≈25% identity) (8, 10), a function of TEIIs in termination of nonribosomal peptide synthesis or as a “cleaning enzyme” was proposed (8, 11). The “termination” model could recently be ruled out, when another thioesterase, the type I thioesterase (TeI) domain was demonstrated to contain all information for the termination of synthesis by macrocyclization or hydrolysis of the nascent chain (12–15). This domain is an integrated part of the last module of an NRPS and shares only about 10% identity with the TEIIs. The cleaning model assumed that NRPSs would occasionally activate and load a wrong amino acid onto a PCP domain, which could then not be further processed because of specificity restrictions of other domains (16). TEII-catalyzed deaminoacylation of the blocked NRPS would then restore product formation.
We hypothesized that TEIIs might have yet another physiological function in an initial deblocking of holo-NRPSs after the priming step. 4′PP transferases were shown to accept as cosubstrates not only free CoA, but also various acyl-CoA derivatives like acetyl-CoA (17). In these cases, transfer of the corresponding acyl-4′PP onto PCPs would result in inactive NRPSs, because substrates without an α-amino group cannot be elongated. Indeed, a significant fraction of the CoA pool in the cell is in the form of various acyl-CoAs (18). We therefore carried out biochemical experiments to test the validity of both the cleaning after aminomisacylation and the deblocking after mispriming models. Comparison of the catalytic properties of a TEII to hydrolyze the thioester bond of aminoacyl- and peptidyl-PCP substrates on the one hand and of an acetyl-PCP substrate on the other hand showed a strong preference of the TEII for the latter and thus supports the deblocking after mispriming model. This model should also hold true for polyketide synthases (PKSs), whose biosynthetic strategy also uses multiple 4′PP-dependent carrier domains and where knockouts of the associated TEII genes have shown the same phenotype as in the NRPS systems (19, 20).
Materials and Methods
Construction of TEII Expression Plasmids and Protein Purification.
Recombinant genes coding for TEIIsrf, TEIIbac, and TEIItyc were amplified from chromosomal DNA of Bacillus subtilis ATCC 21332, Bacillus licheniformis ATCC 10716, and Bacillus brevis ATCC 8185, respectively. PCR was performed with Vent polymerase (NEB, Schwalbach, Germany) using the following synthetic oligonucleotides (MWG Biotech, Ebersberg, Germany): for amplification of the srf-TE gene, 5′-ATA CCA TGG GCC AAC TCT TCA AAT CAT TTG-3′ and 5′-AAA GGA TCC CGG TTG AAT GAT CGG ATG-3′; for amplification of the bacT gene, 5′-AAA CCA TGG AAT TAT TTT GCC TGC CTT AC-3′ and 5′-TAT AGA TCT AAA CGT CCG GCT GGT TG-3′; and for amplification of the tycF gene, 5′-TAT CCA TGG AGC TAT TTT GCT TTC CGT A-3′ and 5′-ATA GGA TCC GAA AGA GGA ATG GAT TGC CA-3′. PCR-amplified fragments were digested with NcoI and BamHI or BglII and ligated into the NcoI and BglII sites of pQE60 (Qiagen, Hilden, Germany), resulting in the plasmids pTEIIsrf, pTEIIbac, and pTEIItyc. Identity of the plasmids was confirmed by restriction digest and DNA sequence analysis. The strain Escherichia coli M15/pREP4 was transformed with these plasmids for protein expression. Expression and purification of the recombinant proteins was performed as described (21). Pooled fractions were dialyzed three times against assay buffer (50 mM Hepes/100 mM NaCl/1 mM EDTA, pH 7.0). The first two dialyzing steps additionally contained 2 mM DTT. Protein concentration was determined from the absorbance at 280 nm by using the calculated extinction coefficients.
Acetyl-S-4′PP-Enzyme Hydrolysis Assay.
Apo-PCPtycC3, apo-ProCAT, or apo-acyl carrier protein (ACP) (1 μM) were incubated in a total volume of 1.3 ml with 20 μM [1-14C]-acetyl-CoA (50 mCi/mmol, ICN), 50 nM Sfp, and 10 mM MgCl2 in assay buffer (50 mM Hepes/100 mM NaCl/1 mM EDTA, pH 7.3) at 37°C. After complete modification, the reaction mixture was split, and TEIIsrf or TEIIbac was added to one aliquot at 500 nM. Samples (100 μl) were removed at defined time points and mixed with 800 μl trichloroacetic acid (10% wt/vol) and 15 μl BSA solution (25 mg/ml). Denaturated proteins were pelleted by centrifugation, washed with 800 μl trichloroacetic acid (10% wt/vol), and dissolved in 400 μl formic acid. Enzyme-bound radioactivity was analyzed by liquid scintillation counting (Tri-Carb 2100 TR, Packard).
Proline Quench Assay.
Holo-ProCAT (1 μM) was incubated in a total volume of 1.5 ml with 10 mM MgCl2, 5 mM ATP, and 4.1 μM [14C]-Pro (246 mCi/mmol, Hartmann Analytics, Braunschweig, Germany) in assay buffer at 37°C. After complete charging, 1 mM nonlabeled Pro was added, and the reaction mixture was split. TEIIsrf was added to one aliquot at 500 nM. Samples (100 μl) were removed at defined time points and prepared and analyzed as described above.
Tripeptide Formation Assay.
On a 600-μl scale, 500 nM holo-TycA, 500 nM holo-ProCAT-LeuCAT, and 0–5 μM TEIIsrf were incubated with 5 mM ATP, 10 mM MgCl2, 1 mM Phe, 1 mM Pro, 85 μM Leu, and 17.5 μM [1-14C]-Leu (57 mCi/mmol) in assay buffer (pH 7.0) at 37°C. At various time points 100-μl samples were removed, and the reaction was stopped by addition of 50 μl of a 1-butanol/chloroform (4:1) mixture. Reaction mixtures were then completely evaporated, resuspended in 30 μl methanol, of which 3 μl was applied to a TLC plate (silica gel 60 F254). After development in 1-butanol/water/acetic acid/ethyl acetate (1:1:1:1 vol/vol/vol/vol), the TLCs were analyzed by autoradiography and/or radioactive scanning by using the radioactive scanner RITA Star (Raytest, Straubenhardt, Germany). Qualitative assays were performed with 500 nM TEIIsrf and without unlabeled Leu.
Mispriming Simulation.
On a 600-μl scale, 500 nM apo-TycA and 500 nM apo-ProCAT-LeuCATTe were incubated with 500 μM acetyl-CoA (or CoASH as positive control), 10 mM MgCl2, and 25 nM Sfp in assay buffer (pH 7.0) at 37°C; and 5 mM ATP, 1 mM Phe, 1 mM Pro, 85 μM Leu, 17.5 μM [1-14C]-Leu (57 mCi/mmol), and 0 or 500 nM TEIIsrf were added after 10 min. All further steps were performed as described above.
5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB) Assay.
In a 200-μl cuvette, 1.3 μM TEIIsrf and 100 μM-3.75 mM acetyl-CoA were incubated in the presence of DTNB in assay buffer (10 mM MgCl2, pH 7.0) at 37°C. Malonyl-CoA, palmitoyl-CoA, and butyryl-CoA were added at a concentration of 1 mM. The formation of 5-thio-2-nitrobenzoate (TNB) that resulted from the reaction of DTNB with free thiols was monitored during the assay (λmax = 412 nm, ɛ = 13,600 M−1⋅cm−1).
Enzymatic Preparation of Acetyl-S-4′PP-PCP.
On a 5-ml scale, 85 μM apo-PCPtycC3 was incubated with 1 mM acetyl-CoA and 850 nM Sfp in assay buffer (10 mM MgCl2, pH 6.7) at 37°C for 45 min. To remove excess acetyl-CoA and Sfp the reaction mixture was applied to a Sephadex G75 gel filtration column followed by adjustment of pH to 7.0 by a desalting step using a HiTrap Desalting column (Amersham Pharmacia). Finally, the concentration was increased by using Vivaspin concentrators Membrane 5,000 MWCO (Satorius, Goettingen, Germany) to a final concentration of 1.25 mM of pure acetyl-S-4′PP-PCP in a total volume of 320 μl that was stored at −80°C until use.
Quantitative Determination of TEIIsrf-Catalyzed Acetyl-S-4′PP-PCP Hydrolysis.
TEIIsrf (29 nM) was incubated with various concentrations of acetyl-S-4′PP-PCP (1–30 μM) in the presence of DTNB (3.5 μl of a saturated solution) in assay buffer (10 mM MgCl2, pH 7.0) in a total volume of 350 μl. At defined time points, 50-μl samples were collected and the reaction was stopped by adding 100 μl methanol/trifluoroacetic acid (500:1, vol/vol). The ratio of acetyl-S-4′PP-PCP to TNB-S-4′PP-PCP was analyzed by the HPLC/MS method described below.
HPLC Separation and Online Electrospray Ionization (ESI)-MS Analysis of Acetyl-S-4′PP-PCP, TNB-S-4′PP-PCP, and Apo-PCP.
Separation and quantification of the PCPs was achieved by using an Agilent 1100 HPLC-MS system with a 250/3-Nucleosil-C18 reversed-phase column (pore size 120 Å, particle size 3 μm) from Macherey & Nagel. The following gradient was applied at a flow rate of 0.9 ml/min and a column temperature of 45°C [buffer A: 0.1% trifluoroacetic acid/water (vol/vol); buffer B: 0.1% trifluoroacetic acid/acetonitrile (vol/vol)]: linear gradient from 40% buffer B up to 57.3% in 15 min, followed by a linear gradient up to 95% in 1 min and then holding 95% buffer B for 4 min. The UV detection was carried out at 220 nm. The masses detected by ESI-MS were in the range of 100 to 2,500 atomic mass units. The deconvolution of the mass spectra was performed with the chemstation software package of Agilent. The parameters of the ESI-mass analyzer were as follows: fragmentor 70, drying gas flow 13 liter/min (gas temperature 350°C), nebulizer pressure 50, and capillary voltage 4,500 V.
Results
Overexpression and Purification of Recombinant TEIIs.
Fig. 1 shows the location of the genes encoding TEIIs within the tyrocidine, surfactin, and bacitracin synthetases gene clusters as well as the corresponding cyclic peptide products (6, 7, 22). The genes srfA-TE, bacT, and tycF, were cloned into expression plasmids pTEIIsrf, pTEIIbac, and pTEIItyc as described in Materials and Methods for overproduction in E. coli of the encoded TEIIs as C-terminal His-6-tag fusion proteins. TEIIsrf and TEIIbac could be overproduced and purified to apparent homogeneity by single-step Ni2+-affinity chromatography. TEIItyc remained essentially insoluble under all growth conditions tested (data not shown). Yields of TEIIbac were very poor and thus it was used for qualitative studies only.
Fig 1.
(a) Structures of the peptide antibiotics surfactin, tyrocidine, and bacitracin. (b) Organization of the corresponding gene clusters. Genes encoding the TEIIs are highlighted in gray.
Cloning and overproduction of all of the other proteins used in this study, namely TeItyc, PCPtycC3, TycA, ProCAT, ProCAT-LeuCAT, and ProCAT-LeuCTTe, have been reported (12, 23, 24).
TEII Catalyzed Cleavage of NRPS-Bound Amino Acids.
To test the cleaning enzyme model, we asked the question whether TEIIs can catalyze hydrolysis of a 4′PP-bound amino acyl moiety. For this purpose, the NRPS module holo-ProCAT (14) was incubated with ATP and [14C]-proline. The radiolabeled amino acid was activated by the adenylation domain, and subsequently transferred to the 4′PP cofactor of the PCP. After complete iminoacylation of ProCAT an ≈250-fold excess of nonlabeled proline was added. If the enzyme-bound [14C]-proline was hydrolyzed by the TEII, ProCAT would rapidly recharge itself with nonlabeled proline, which should result in a time-dependent decrease of trichloroacetic acid -precipitable radioactivity. Indeed, as shown in Fig. 2a, a TEIIsrf or TEIIbac-dependent decrease over time of precipitable radioactivity could be observed. These results show that TEIIs are capable of hydrolyzing aminoacyl moieties from NRPSs that cannot be further processed by the enzyme template.
Fig 2.
Overview of thioesterase assays. (a) Proline quench assay. (b) Acetyl-S-4′PP-NRPS hydrolysis assay. (c) Acetyl-S-4′PP-ACP hydrolysis assay. (Left) A schematic drawing of the corresponding assays. Compounds shown in red carry a radioactive label. In all cases, a NRPS/thioesterase ratio of 1:0.5 was used.
No such cleavage of the NRPS-bound proline was observed when the TEIIs were replaced by the TeItyc domain (Fig. 2a).
Are Cognate NRPS Elongation Intermediates also Cleaved by TEIIs?
To establish whether only aminoacyl or also peptidyl PCPs are hydrolyzed by TEIIs, the trimodular system TycA/ProCAT-LeuCAT was used to generate a peptidyl-S-4′PP-PCP substrate (24). This NRPS system consists of the first, second, and 10th module of the tyrocidine biosynthetic system. The first module incorporating d-Phe represents a separate enzyme, TycA. The second and 10th modules are fused in the hybrid enzyme ProCAT-LeuCAT and incorporate l-Pro and l-Leu, respectively. The system was shown to synthesize the tripeptide DPhe-Pro-Leu (dFPL), which remains covalently attached to the last PCP domain because of the lack of a terminal (TeI) domain (24). Thus we assumed that TEII might hydrolyze the stuck tripeptide and assayed this reaction by measuring the release and turnover of this product. In the developed assay, TycA and ProCAT-LeuCAT were incubated with the substrates Phe, Pro, and [14C]-Leu in the presence of either TEIIsrf or TEIIbac, and the time-dependent release of the linear tripeptide dFP-[14C]-L was determined. Reaction products were separated by TLC and quantified by autoradiography. Indeed, both TEIIs were competent to release the tripeptide dFP-[14C]-L from the enzyme. Hydrolytic release of dFPL was not observed in the absence of a TEII (data not shown). Table 1 summarizes the effect of different concentrations of TEIIsrf on the rate of product formation. At a molar ratio between NRPS and TEIIsrf of 1:1, the linear tripeptide dFPL was formed with a slow rate of 0.04 min−1. This TEII-catalyzed hydrolysis rate is ≈50-fold lower than that observed for the system TycA/ProCAT-LeuCATTe, featuring a natural termination (TeI) domain at its C-terminal end (24). Product release could be accelerated by a factor of 7 to 0.28 min−1 by adding TEIIsrf at a 10-fold molar excess.
Table 1.
TEIIsrf-dependent dFPL release
| NRPS/TEIIsrf ratio | Formation rate of the tripeptide DPhe-Pro-Leu, min−1 |
|---|---|
| 1:0 | 0 |
| 1:0.5 | 0.02 |
| 1:1 | 0.04 |
| 1:3 | 0.14 |
| 1:5 | 0.19 |
| 1:10 | 0.28 |
TycA/ProCAT-LeuCAT, 0.5 μM; TEIIsrf, 0–5 μM.
TEII Catalyzed Cleavage of Acetyl-S-4′PP-NRPS.
After showing TEII catalyzed cleavage of stuck aminoacyl and peptidyl PCP substrates, the proposed basic functions of the cleaning after misaminoacylation model, we aimed to test the validity of the deblocking after mispriming model. For this purpose, we first developed an assay with a misprimed NRPS module serving as the substrate for TEII. The module ProCAT from the tyrocidine synthetase was overproduced in E. coli in the apo form (14). The PCP domain of apo-ProCAT was then artificially misprimed in vitro with the promiscuous 4′PP transferase Sfp and [14C]-acetyl-CoA, to obtain holo-ProCAT with a 14C-labeled acetyl moiety attached to its 4′PP cofactor ([14C]-acetyl-S-4′PP-ProCAT). After complete mispriming, TEIIsrf and TEIIbac were added in a molar ratio NRPS/TEII of 1:0.5. As illustrated in Fig. 2b, both enzymes efficiently hydrolyzed the ProCAT-bound acetyl groups, resulting in a complete removal of precipitable radioactivity in less than 1 min. This effect could not be detected in the absence of a TEII (see Fig. 2b). Furthermore, no hydrolysis of [14C]-acetyl-S-4′PP-ProCAT was observed when TEII was replaced with the excised TeI domain of the tyrocidine NRPS (12) (see Fig. 2b).
We then tested whether also a single excised acetylated PCP domain is recognized by TEIIs. The TycC3 apo-PCP domain was misprimed with [14C]-acetyl-CoA as described above and used as a substrate for TEIIsrf and TEIIbac. The observed time courses of TEII-catalyzed hydrolysis were identical to those obtained by using [14C]-acetyl-S-4′PP-ProCAT (data not shown), indicating that PCP domains contain all necessary recognition elements for TEIIs. Therefore, further kinetic characterization of TEIIs was carried out by using only the ac(et)ylated PCP domain as substrate.
Kinetic Parameters of TEII-Catalyzed Acetyl-S-4′PP-PCP Hydrolysis.
To determine kinetic parameters for the TEIIsrf-catalyzed acetyl-S-4′PP-PCP hydrolysis, a quantitative HPLC assay was established based on the separation of acetyl-S-4′PP-PCP and its hydrolysis product HS-4′PP-PCP.
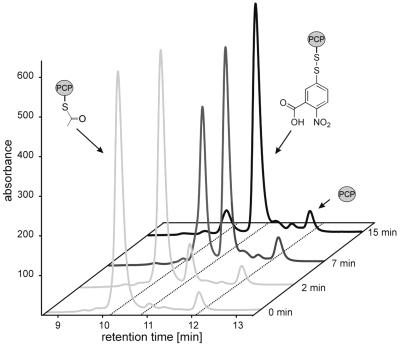
To quantify the amounts of the acetylated and free forms of holo-PCP, the mixture was applied to a reversed-phase C18 HPLC column. Because a sufficient separation could not be achieved, DTNB was used to selectively modify the free thiol moiety of the reaction product, namely of the free cysteamine group of the PCP-bound cofactor 4′PP. DTNB reacts with free thiol groups by transfer of the TNB group (25). The TycC3 PCP does not contain any cysteine residues. Thus, the deacetylated thiol group of the 4′PP cofactor is the only position to be modified in this assay. As illustrated in Fig. 3, acetyl-S-4′PP-PCP and TNB-S-4′PP-PCP eluted at retention times of tR,ac = 10.2 min and tR,TNB = 11.0 min, respectively, giving rise to a sufficient separation factor α = 1.07. Apo-PCP eluted at tR,apo = 12.3 min. The identity of all three PCP derivatives was confirmed by online electrospray ionization-MS, with observed masses of m/z = 10,358 (calculated 10,357.4) for acetyl-S-4′PP-PCP, m/z = 10,512 (calculated 10,512.4) for TNB-S-4′PP-PCP, and m/z = 9,974 (calculated 9,973.4) for apo-PCP.
Fig 3.
HPLC analysis of the TEIIsrf-catalyzed acetyl-4′PP-PCP hydrolysis. The identities of the compounds acetyl-S-4′PP-PCP, TNB-S-4′PP-PCP, and apo-PCP were confirmed by MS.
Pure acetyl-S-4′PP-PCP was prepared on a large scale as described in Materials and Methods, yielding a 1.25 mM stock solution of acetyl-S-4′PP-PCP. The integrity of this protein was verified by HPLC-MS as described above. For the kinetic analysis of TEIIsrf, the time-dependent hydrolysis of acetyl-S-4′PP-PCP was measured by using the HPLC assay for substrate concentrations ranging from 1 to 30 μM (constant concentration of TEIIsrf of 29 nM). The velocity of the time-dependent decrease of acetyl-S-4′PP-PCP was plotted against the starting concentration of acetyl-S-4′PP-PCP to determine the kinetic constants under substrate saturation. The Michaelis constants KM = 0.9 ± 0.4 μM and kcat = 95 ± 5 min−1, with a resulting catalytic efficiency of 1.75 × 106 M−1⋅s−1 were calculated.
Furthermore, no formation of apo-PCP could be observed during this assay, indicating that no hydrolysis of the phosphodiester bond between the cofactor and PCP occurred (Fig. 3).
Mispriming Simulation.
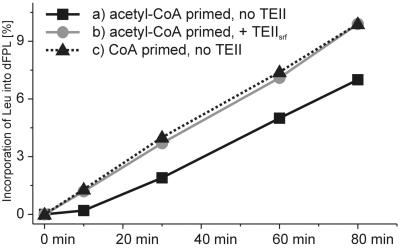
Finally, we were interested in a complete in vitro simulation of the proposed mispriming and deblocking process. To this end, apo-TycA and apo-ProCAT-LeuCATTe (24) (note: this is the fully functional NRPS system including the C-terminal TeI domain for product release) were misprimed in vitro with acetyl-CoA and Sfp. Subsequently, the enzymes were incubated with Phe, Pro, and [14C]-Leu, and with or without TEIIsrf in a 1:1 stoichiometric ratio. At various time points, the incorporation of [14C]-Leu into the tripeptide was measured. For comparison, the same reaction was performed with functional holo-NRPSs (primed with CoASH instead of acetyl-CoA). As shown in Fig. 4, an about 10-min delay in product formation of the misprimed NRPSs could be observed in absence of TEIIsrf (Fig. 4a). After this delay, formation of the tripeptide dFPL started slowly, presumably promoted by the slow noncatalyzed hydrolysis of the NRPS-bound acetyl moieties. Interestingly, however, no such delay could be observed for the reaction performed with the misprimed NRPS in the presence of TEIIsrf, which showed an essentially identical product formation pattern as the control reaction with functional holo–NRPS (see Fig. 4 b and c). This outcome strongly supports the model that the role of TEIIs is to deblock NRPSs after mispriming to achieve a high-level production of the products.
Fig 4.
In vitro simulation of the deblocking after mispriming model by a tripeptide formation assay using correctly primed, acetyl-CoA misprimed, and acetyl-CoA misprimed NRPSs in the presence of TEIIsrf.
TEIIs Do Not Interact with the ACP of Fatty Acid Synthesis.
The ACPs of the fatty acid synthases are also equipped with a 4′PP cofactor. In this case, the acetylated carrier domain, acetyl-S-4′PP-ACP, represents an actual intermediate in fatty acid synthesis. Therefore, hydrolysis of acetyl-S-4′PP-ACP by a TEII would certainly be harmful for the organism. We thus tested whether TEIIs associated with NRPSs could interfere with the ACP of primary metabolism. For this purpose, radiolabeled acetyl-S-4′PP-ACP was generated by incubating apo-ACP with [14C]-acetyl-CoA and Sfp. This product was used as a substrate for TEIIsrf and TEIIbac. Fig. 2c shows that no hydrolysis of [14C]-acetate could be observed, clearly indicating that TEIIs are able to distinguish between PCP substrates from NRPSs and ACP substrates from fatty acid synthases.
Interaction with CoA Derivatives.
Finally, we tested whether CoA derivatives themselves are substrates for the TEIIs. For this purpose, we used an established photometric assay based on the reaction of free thiol moieties with DTNB (10, 26, 27). As illustrated in Table 2, malonyl-CoA and the long chain palmitoyl-CoA were not hydrolyzed by TEIIsrf. In contrast, a slow hydrolysis of acetyl-CoA and butyryl-CoA could be detected. Measurement of turnover rates at different acetyl-CoA concentrations allowed determination of the catalytic efficiency for TEIIsrf-catalyzed acetyl-CoA cleavage. A kcat/KM of 43 M−1⋅s−1 was calculated from the linear slope of the received plot, indicating that this reaction was >40,000-fold less efficient than the cleavage of the acetyl-S-4′PP-PCP. Thus the hydrolysis of acyl-CoA derivatives by TEIIs seems to be of no physiological relevance.
Table 2.
TEIIsrf-catalyzed acyl-CoA hydrolysis
| CoA-derivative, 1 mM | Measured hydrolysis rate, min−1 |
|---|---|
| Acetyl-CoA | 2.2 |
| Propionyl-CoA | 0.6 |
| Malonyl-CoA | Not detected |
| Palmitoyl-CoA | Not detected |
Discussion
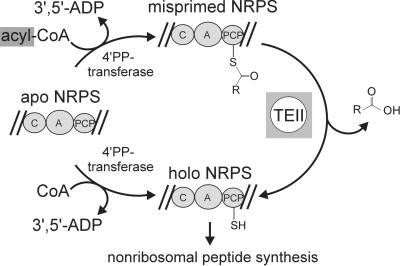
In this study, we have demonstrated that TEIIsrf and TEIIbac, which represent typical members of TEIIs associated with nonribosomal peptide synthesis, functionally interact with the PCP domains of NRPSs by hydrolyzing 4′PP cofactor-bound acetyl groups. The collected data support the deblocking after mispriming model with the TEIIs playing a critical part of the priming process of NRPSs. The model is illustrated in Fig. 5. The apo to holo conversion of NRPSs is performed by promiscuous 4′PP transferases like Sfp, which use both CoA and acyl-CoA derivatives as 4′PP donor (17). However, transfer of an acyl-4′PP would lead to misprimed NRPSs that are inactive because their 4′PP cofactors are blocked by acyl groups. At this point a TEII can recover activity of the misprimed NRPSs by hydrolyzing these acyl groups. The requirement for such a “recovery function” is obvious when the concentrations of CoA and its derivatives in the cell are considered. In the case of E. coli growing on glucose as a carbon source the total CoA concentration was measured to be 406 μM. Acetyl-CoA at 324 μM was the most common CoA derivative followed by free CoA at only 56 μM, succinyl-CoA at 24 μM, and malonyl-CoA at 2 μM. Growth on different carbon sources leads to different compositions of the cellular CoA pool with a maximum of 50% of free CoASH (18). Because one acyl-S-4′PP-cofactor transferred onto a single PCP domain would totally abolish the activity of a complete multimodular NRPS assembly line, the necessity for a hydrolytic activity that we have shown here for TEIIs becomes obvious.
Fig 5.
The deblocking after mispriming model for the role of TEIIs in nonribosomal peptide assembly. Apo to holo conversion of NRPSs is catalyzed by dedicated 4′PP transferases that accept CoA and acyl-CoA as substrates. If acyl-4′PP is the transferred cofactor, a TEII then hydrolyzes the acyl group and thereby regenerates a misprimed NRPS to the active free holo form.
Importantly, we have also shown that hydrolysis of acyl-ACP (ACP of fatty acid synthesis) or acyl-CoA substrates is not significantly catalyzed by the TEIIs. These enzymes seem to have specialized on acyl-PCP substrates.
In addition, we detected TEII-catalyzed hydrolysis of PCP-bound amino acids and peptides. These data are in agreement with another role of TEIIs according to the cleaning after aminomisacylation model. However, we have used only NRPS substrates that produced stuck biosynthesis intermediates because of the lack of downstream domains for further processivity. It cannot be ruled out that NRPS templates that are stuck in the peptide synthesis because of an aminomisacylation are better substrates for TEIIs. Biochemical studies showed that the adenylation domains of NRPSs, which catalyze the aminoacylation of the 4′PP-PCP domains, are in general the most specific units of the multifunctional enzymes (6, 22). Condensation and termination domains, for example, are capable of processing a comparatively broad range of unnatural substrates (12, 16), so that a slightly different substrate activated by an adenylation domain should result only in a product of different composition but not in blocking of the entire synthesis machinery. Furthermore, cleavage of aminoacyl or peptidyl intermediates would not be expected to be the main reaction of TEIIs because it would represent a futile cycle. Such an effect was not observed in our product formation studies described in the mispriming simulation section where the presence or absence of a TEII had no effect on the product formation rates. This finding is in accordance with studies by Miller et al. (28), who also observed no disturbing effect of the YbtT TEII in a reconstituted Yersiniabactin NRPS assembly line, even when YbtT was added at 10-fold molar excess. For these reasons we conclude that TEII-catalyzed hydrolysis of aminoacyl or peptidyl substrates does not play a significant physiological role.
Interestingly, PKSs, which have a similar modular architecture as NRPSs, also comprise TEIIs in their gene clusters (19, 20, 29). During polyketide synthesis, PKSs condense acetate and propionate units in a decarboxylative reaction from the building blocks malonate and methylmalonate that are loaded onto the 4′PP cofactors of ACPs from their CoA esters. Heathcote et al. (27) reported that these building blocks can be decarboxylated without elongation leading to acetyl- or propionyl-4′PP cofactors that cannot be further processed. According to their model, TEIIs of PKSs are therefore responsible to hydrolyze these cofactor-bound acyl groups. This model is similar to the cleaning model for the NRPSs in terms of biosynthetic logic. Interestingly, however, it is identical to our deblocking after mispriming model in terms of chemistry carried out by the TEIIs because misdecarboxylation and mispriming can lead to identical substrates for the TEIIs, like acetyl- or propionyl-S-4′PP-ACPs. Due to the fact that ACPs of PKSs are also primed by 4′PP transferases, deblocking of misprimed ACPs should also be a task for TEIIs in polyketide assembly.
Disruption of the TEII-encoding genes in NRPS- and PKS-producing strains is always accompanied by severe reduction in yields of the corresponding products (8, 19, 20). As reported, the phenotype of such a TEII disruption could be complemented by a heterologous TEII gene (30), suggesting a common specificity for these gene products from different biosynthetic clusters. We have simulated the situation in such a TEII-deficient mutant in a product assay using misprimed NRPSs and a TEII for the deblocking reaction. A lag phase was observed only when a TEII was omitted. In this case the enzyme regeneration totally depends on noncatalyzed hydrolysis of the acetyl groups. The explanation for the loss of efficient productivity would be that in a steady-state process of NRPS enzyme synthesis and degradation the pool of active enzymes is much lower when no TEII is present. This effect would be even more drastic if a 4′PP turnover on PCPs exists. Such a 4′PP turnover was demonstrated for the ACPs of fatty acid synthesis in E. coli where a phosphodiesterase was reported to cleave the 4′PP cofactor of ACP, which is then recharged by the 4′PP transferase (31, 32). Up to date, however, no such 4′PP turnover has been reported for NRPSs but could be conceivable because of the homology of ACPs and PCPs.
In summary, we clearly demonstrated that the TEIIs involved in nonribosomal peptide assembly can efficiently reactivate NRPSs that are blocked by nonelongatable substrates attached to their cofactors. We propose that their main physiological role is to deblock acylated 4′PP cofactors that result from mispriming by the 4′PP transferases and thereby regenerate functionality of NRPSs.
Acknowledgments
We thank laboratory students Anke Morbitzer, Simone Arnold, and Jo Mailliet for their helping hands, Robert Finking for providing apo-ACP, Mohammad Reza Mofid for providing Sfp, and Torsten Stachelhaus for comments on the manuscript. Work in M.A.M.'s laboratory was supported by Deutsche Forschungsgemeinschaft and Verband der Chemischen Industrie.
Abbreviations
ACP, acyl carrier protein
DTNB, 5,5′-dithiobis(2-nitrobenzoic acid)
NRPS, nonribosomal peptide synthetase
PCP, peptidyl carrier protein
PKS, polyketide synthase
4′PP, 4′-phosphopantetheine
TeI, type I thioesterase
TEII, type II thioesterase
TNB, 5-thio-2-nitrobenzoic acid
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Marahiel M. A., Stachelhaus, T. & Mootz, H. D. (1997) Chem. Rev. 97, 2651-2674. [DOI] [PubMed] [Google Scholar]
- 2.von Döhren H., Keller, U., Vater, J. & Zocher, R. (1997) Chem. Rev. 97, 2675-2706. [DOI] [PubMed] [Google Scholar]
- 3.Keating T. A. & Walsh, C. T. (1999) Curr. Opin. Chem. Biol. 3, 598-606. [DOI] [PubMed] [Google Scholar]
- 4.Lambalot R. H., Gehring, A. M., Flugel, R. S., Zuber, P., LaCelle, M., Marahiel, M. A., Reid, R., Khosla, C. & Walsh, C. T. (1996) Chem. Biol. 3, 923-936. [DOI] [PubMed] [Google Scholar]
- 5.Bearden S. W., Fetherston, J. D. & Perry, R. D. (1997) Infect. Immun. 65, 1659-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mootz H. D. & Marahiel, M. A. (1997) J. Bacteriol. 179, 6843-6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosmina P., Rodriguez, F., de Ferra, F., Grandi, G., Perego, M., Venema, G. & van Sinderen, D. (1993) Mol. Microbiol. 8, 821-831. [DOI] [PubMed] [Google Scholar]
- 8.Schneider A. & Marahiel, M. A. (1998) Arch. Microbiol. 169, 404-410. [DOI] [PubMed] [Google Scholar]
- 9.Geoffroy V. A., Fetherston, J. D. & Perry, R. D. (2000) Infect. Immun. 68, 4452-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naggert J., Williams, B., Cashman, D. P. & Smith, S. (1987) Biochem. J. 243, 597-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wertheimer A. M., Verweij, W., Chen, Q., Crosa, L. M., Nagasawa, M., Tolmasky, M. E., Actis, L. A. & Crosa, J. H. (1999) Infect. Immun. 67, 6496-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trauger J. W., Kohli, R. M., Mootz, H. D., Marahiel, M. A. & Walsh, C. T. (2000) Nature 407, 215-218. [DOI] [PubMed] [Google Scholar]
- 13.Kohli R. M., Trauger, J. W., Schwarzer, D., Marahiel, M. A. & Walsh, C. T. (2001) Biochemistry 40, 7099-7108. [DOI] [PubMed] [Google Scholar]
- 14.Schwarzer D., Mootz, H. D. & Marahiel, M. A. (2001) Chem. Biol. 8, 997-1010. [DOI] [PubMed] [Google Scholar]
- 15.Bruner S. D., Weber, T., Kohli, R. M., Schwarzer, D., Marahiel, M. A., Walsh, C. T. & Stubbs, M. T. (2002) Structure (London) 10, 301-310. [DOI] [PubMed] [Google Scholar]
- 16.Belshaw P. J., Walsh, C. T. & Stachelhaus, T. (1999) Science 284, 486-489. [DOI] [PubMed] [Google Scholar]
- 17.Quadri L. E., Weinreb, P. H., Lei, M., Nakano, M. M., Zuber, P. & Walsh, C. T. (1998) Biochemistry 37, 1585-1595. [DOI] [PubMed] [Google Scholar]
- 18.Vallari D. S., Jackowski, S. & Rock, C. O. (1987) J. Biol. Chem. 262, 2468-2471. [PubMed] [Google Scholar]
- 19.Butler A. R., Bate, N. & Cundliffe, E. (1999) Chem. Biol. 6, 287-292. [DOI] [PubMed] [Google Scholar]
- 20.Xue Y., Zhao, L., Liu, H. W. & Sherman, D. H. (1998) Proc. Natl. Acad. Sci. USA 95, 12111-12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stachelhaus T., Mootz, H. D., Bergendahl, V. & Marahiel, M. A. (1998) J. Biol. Chem. 273, 22773-22781. [DOI] [PubMed] [Google Scholar]
- 22.Konz D., Klens, A., Schörgendorfer, K. & Marahiel, M. A. (1997) Chem. Biol. 4, 927-937. [DOI] [PubMed] [Google Scholar]
- 23.Weber T., Baumgartner, R., Renner, C., Marahiel, M. A. & Holak, T. A. (2000) Structure Fold. Des. 8, 407-418. [DOI] [PubMed] [Google Scholar]
- 24.Mootz H. D., Schwarzer, D. & Marahiel, M. A. (2000) Proc. Natl. Acad. Sci. USA 97, 5848-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riddles P. W., Blakeley, R. L. & Zerner, B. (1983) Methods Enzymol. 91, 49-60. [DOI] [PubMed] [Google Scholar]
- 26.Gokhale R. S., Hunziker, D., Cane, D. E. & Khosla, C. (1999) Chem. Biol. 6, 117-125. [DOI] [PubMed] [Google Scholar]
- 27.Heathcote M. L., Staunton, J. & Leadlay, P. F. (2001) Chem. Biol. 8, 207-220. [DOI] [PubMed] [Google Scholar]
- 28.Miller D. A., Luo, L., Hillson, N., Keating, T. A. & Walsh, C. T. (2002) Chem. Biol. 9, 333-344. [DOI] [PubMed] [Google Scholar]
- 29.Haydock S. F., Dowson, J. A., Dhillon, N., Roberts, G. A., Cortes, J. & Leadlay, P. F. (1991) Mol. Gen. Genet. 230, 120-128. [DOI] [PubMed] [Google Scholar]
- 30.Kotowska M., Pawlik, K., Butler, A. R., Cundliffe, E., Takano, E. & Kuczek, K. (2002) Microbiology 148, 1777-1783. [DOI] [PubMed] [Google Scholar]
- 31.Jackowski S. & Rock, C. O. (1984) J. Bacteriol. 158, 115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischl A. S. & Kennedy, E. P. (1990) J. Bacteriol. 172, 5445-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]