Abstract
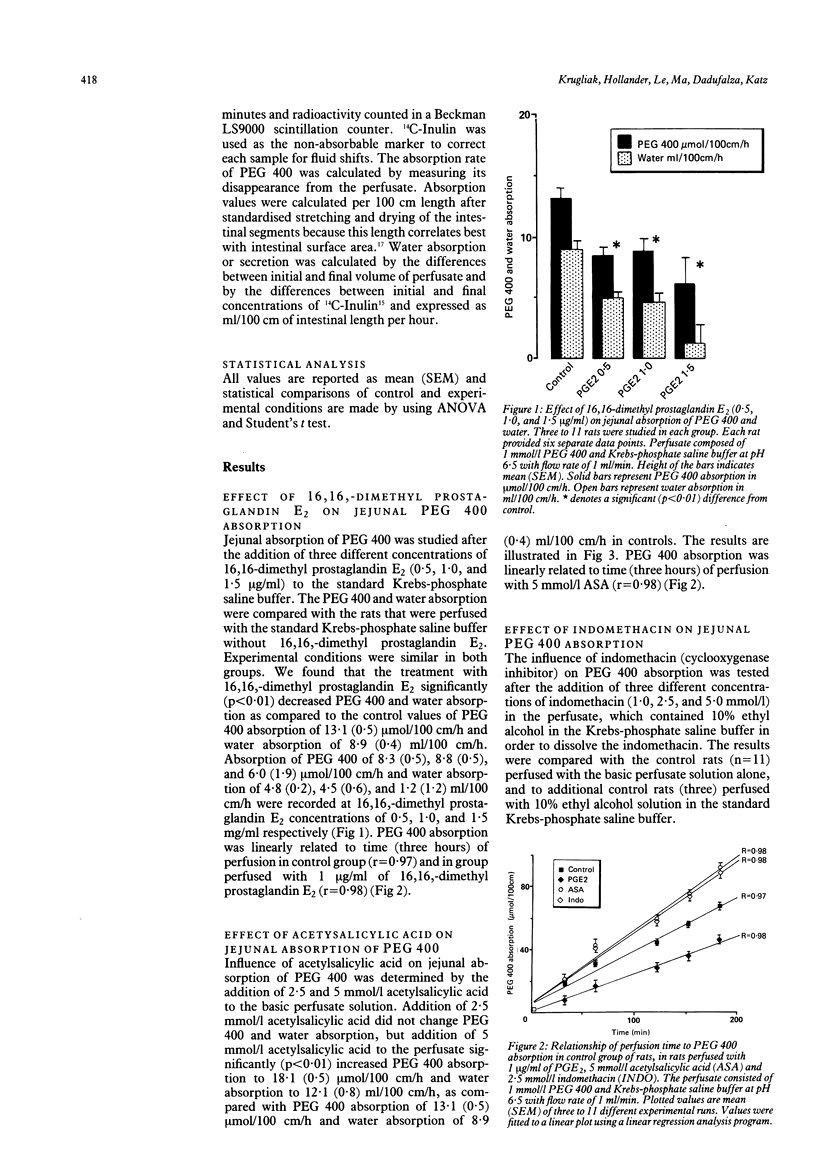
Polyethylene glycol 400 (PEG 400) is a clinically useful intestinal permeability probe whose rate of intestinal permeation is influenced in part by solvent drag. As mucosal prostanoids are increased in inflammatory bowel disease and affect water transport we examined the possible relationship between prostaglandin E2 (PGE2) and the inhibitors of endogenous prostaglandins--the non-steroidal anti-inflammatory drugs (NSAIDS)--on PEG 400 absorption in vivo using segmental perfusion of rat small intestine. We found that the addition of exogenous PGE2 in concentrations of 0.5, 1.0, and 1.5 micrograms/ml significantly (p less than 0.01) decreased PEG 400 and water absorption. Addition of 5 mmol/l of the cyclooxygenase inhibitors acetylsalicylic acid (ASA) or indomethacin in concentrations 2.5 or 5.0 mmol/l to the perfusate significantly (p less than 0.01) increased PEG 400 and water absorption. The simultaneous addition of 1.0 micrograms/ml of exogenous PGE2 to the perfusate with 5 mmol/l of ASA or with 2.5 mmol/l of indomethacin reversed the increase of PEG 400 and water transport (p less than 0.01). There were no differences in PEG 400 and water absorption when PGE2 was given alone or in combination with ASA or indomethacin. This study suggests that endogenous or exogenous prostanoids play an important role in the regulation of PEG 400 permeation. PGE2 and NSAIDS modify PEG 400 permeation in parallel with changes in water transport indicating that their effect on permeability is through changes in solvent drag. These findings provide a mechanism which might explain the increase in PEG 400 intestinal permeability in Crohn's disease patients and the increase in intestinal permeability found in patients receiving NSAIDS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjarnason I., Peters T. J., Veall N. A persistent defect in intestinal permeability in coeliac disease demonstrated by a 51Cr-labelled EDTA absorption test. Lancet. 1983 Feb 12;1(8320):323–325. doi: 10.1016/s0140-6736(83)91628-8. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Williams P., Smethurst P., Peters T. J., Levi A. J. Effect of non-steroidal anti-inflammatory drugs and prostaglandins on the permeability of the human small intestine. Gut. 1986 Nov;27(11):1292–1297. doi: 10.1136/gut.27.11.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Smith T., Prouse P., Williams P., Smethurst P., Delacey G., Gumpel M. J., Levi A. J. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology. 1987 Sep;93(3):480–489. doi: 10.1016/0016-5085(87)90909-7. [DOI] [PubMed] [Google Scholar]
- Brunsson I., Sjöqvist A., Jodal M., Lundgren O. Mechanisms underlying the small intestinal fluid secretion caused by arachidonic acid, prostaglandin E1 and prostaglandin E2 in the rat in vivo. Acta Physiol Scand. 1987 Aug;130(4):633–642. doi: 10.1111/j.1748-1716.1987.tb08186.x. [DOI] [PubMed] [Google Scholar]
- Chadwick V. S., Phillips S. F., Hofmann A. F. Measurements of intestinal permeability using low molecular weight polyethylene glycols (PEG 400). I. Chemical analysis and biological properties of PEG 400. Gastroenterology. 1977 Aug;73(2):241–246. [PubMed] [Google Scholar]
- Duffey M. E., Hainau B., Ho S., Bentzel C. J. Regulation of epithelial tight junction permeability by cyclic AMP. Nature. 1981 Dec 3;294(5840):451–453. doi: 10.1038/294451a0. [DOI] [PubMed] [Google Scholar]
- Hamilton I., Cobden I., Rothwell J., Axon A. T. Intestinal permeability in coeliac disease: the response to gluten withdrawal and single-dose gluten challenge. Gut. 1982 Mar;23(3):202–210. doi: 10.1136/gut.23.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkey C. J., Karmeli F., Rachmilewitz D. Imbalance of prostacyclin and thromboxane synthesis in Crohn's disease. Gut. 1983 Oct;24(10):881–885. doi: 10.1136/gut.24.10.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander D. Crohn's disease--a permeability disorder of the tight junction? Gut. 1988 Dec;29(12):1621–1624. doi: 10.1136/gut.29.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander D. Intestinal absorption of vitamins A, E, D, and K. J Lab Clin Med. 1981 Apr;97(4):449–462. [PubMed] [Google Scholar]
- Hollander D., Koyama S., Dadufalza V., Tran D. Q., Krugliak P., Ma T., Ling K. Y. Polyethylene glycol 900 permeability of rat intestinal and colonic segments in vivo and brush border membrane vesicles in vitro. J Lab Clin Med. 1989 Apr;113(4):505–515. [PubMed] [Google Scholar]
- Hollander D., Vadheim C. M., Brettholz E., Petersen G. M., Delahunty T., Rotter J. I. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986 Dec;105(6):883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- Jenkins R. T., Goodacre R. L., Rooney P. J., Bienenstock J., Sivakumaran T., Walker W. H. Studies of intestinal permeability in inflammatory diseases using polyethylene glycol 400. Clin Biochem. 1986 Oct;19(5):298–302. doi: 10.1016/s0009-9120(86)80045-5. [DOI] [PubMed] [Google Scholar]
- Jenkins R. T., Rooney P. J., Jones D. B., Bienenstock J., Goodacre R. L. Increased intestinal permeability in patients with rheumatoid arthritis: a side-effect of oral nonsteroidal anti-inflammatory drug therapy? Br J Rheumatol. 1987 Apr;26(2):103–107. doi: 10.1093/rheumatology/26.2.103. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugliak P., Hollander D., Ma T. Y., Tran D., Dadufalza V. D., Katz K. D., Le K. Mechanisms of polyethylene glycol 400 permeability of perfused rat intestine. Gastroenterology. 1989 Nov;97(5):1164–1170. doi: 10.1016/0016-5085(89)91686-7. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Pappenheimer J. R. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100(2):149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- Madara J. L. Tight junction dynamics: is paracellular transport regulated? Cell. 1988 May 20;53(4):497–498. doi: 10.1016/0092-8674(88)90562-4. [DOI] [PubMed] [Google Scholar]
- Matuchansky C., Bernier J. J. Effect of prostaglandin E 1 on glucose, water, and electrolyte absorption in the human jejunum. Gastroenterology. 1973 Jun;64(6):1111–1118. [PubMed] [Google Scholar]
- Meshkinpour H., Smith M., Hollander D. Influence of aging on the surface area of the small intestine in the rat. Exp Gerontol. 1981;16(5):399–404. doi: 10.1016/0531-5565(81)90061-9. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Schedl H. P. Total recovery studies of nonabsorbable indicators in the rat small intestine. Gastroenterology. 1970 Jan;58(1):40–46. [PubMed] [Google Scholar]
- Moriarty K. J., O'Grady J., Rolston D. D., Kelly M. J., Clark M. L. Effect of prostacyclin (PGI2) on water and solute transport in the human jejunum. Gut. 1986 Feb;27(2):158–163. doi: 10.1136/gut.27.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer J. R. Physiological regulation of transepithelial impedance in the intestinal mucosa of rats and hamsters. J Membr Biol. 1987;100(2):137–148. doi: 10.1007/BF02209146. [DOI] [PubMed] [Google Scholar]
- Pappenheimer J. R., Reiss K. Z. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol. 1987;100(2):123–136. doi: 10.1007/BF02209145. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Carpenter C. C., Jr, Elliott H. L., Greenough W. B., 3rd Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology. 1971 Jan;60(1):22–32. [PubMed] [Google Scholar]
- Rampton D. S., Sladen G. E. Relationship between rectal mucosal prostaglandin production and water and electrolyte transport in ulcerative colitis. Digestion. 1984;30(1):13–22. doi: 10.1159/000199086. [DOI] [PubMed] [Google Scholar]
- Sernka T. J., Rood R. P., Mah M. Y., Tseng C. H. Antiabsorptive effects of 16,16 dimethyl prostaglandin E2 in isolated rat colon. Prostaglandins. 1982 Mar;23(3):411–426. doi: 10.1016/0090-6980(82)90086-7. [DOI] [PubMed] [Google Scholar]


