Abstract
What are the mechanisms of ligand-induced allosteric transitions in proteins? A powerful method to characterize pathways and transition states of reactions is φ value analysis. A φ value is the ratio between the changes on a perturbation (e.g., mutation) in the activation and equilibrium free energies of a reaction. Here, φ value analysis is used to characterize the ATP-induced allosteric transitions of GroEL by using changes in ATP concentration as perturbations. GroEL consists of two stacked back-to-back heptameric rings that bind ATP with positive cooperativity within rings and negative cooperativity between rings. Evidence is presented for the existence of parallel pathways for the allosteric transition of each ring. In both allosteric transitions, there is an abrupt ATP-dependent switch from a pathway with ATP-binding sites in the transition state that are very similar to those of the initial T state (φ = 0) to a pathway with a φ value of ≈0.3. The φ value procedure outlined here should be useful in mapping the energy landscape of allosteric transitions of other proteins.
Keywords: nested allostery, cooperativity, chaperonins
Regulation of protein function is often achieved by ligand-induced changes in protein conformation. There is a wealth of structural data regarding different stable allosteric states of various proteins (for review, see ref. 1), but very little is known about pathways of allosteric transitions. A striking example of an allosteric system is the chaperonin GroEL (for reviews, see refs. 2–4), consisting of two seven-membered rings of identical subunits, stacked back-to-back, with a cavity at each end (5). GroEL undergoes ATP-induced conformational changes (6, 7) that are reflected in the binding of ATP with positive cooperativity within rings and negative cooperativity between rings (8). On the cooperative binding of ATP, GroEL rings switch from a T state with low affinity for ATP and high affinity for nonfolded proteins to an R state with high affinity for ATP and low affinity for nonfolded proteins (9). The structures of some stable allosteric states of GroEL have been determined by using x-ray crystallography (5, 10) and electron cryomicroscopy (6, 7) but, as is the case for most other allosteric systems, there is little experimental information about the pathways by which the different allosteric states interconvert. A powerful approach to characterize pathways and transition states of reactions is to invoke linear free energy relations (LFER) (11) such as φ value analysis (12). φ values may be expressed as follows: φ = ΔΔG‡/ΔΔGeq, where ΔΔG‡ and ΔΔGeq are the changes on perturbation (e.g., mutation) in the activation and equilibrium free energies, respectively. Values of φ = 0 and φ = 1 indicate no bond breaking and complete bond breaking in the transition state relative to the initial state, respectively. Mutational φ value analysis has been used extensively to characterize transition and intermediate states in enzyme catalysis (13) and protein folding (12). The transition states of ligand-induced protein conformational changes have also been probed by using LFER (14, 15), such as, more recently, mutational φ value analysis (16, 17). For example, it was found that the Arg-197–Glu-386 intersubunit interaction that breaks during the T→R allosteric transition of GroEL (7, 18) is already disrupted in the transition state (16).
Ambiguities in φ value analysis exist with regard to fractional φ values between 0 and 1 and nonclassical values φ > 1 and φ < 0 (19). For example, do fractional φ values reflect interactions that are present but weakened or a heterogeneous population of molecules some with fully intact and others with fully broken interactions? It is possible to distinguish between these two possibilities by measuring φ values for a series of increasingly destabilizing mutations at a given site (20). A linear dependence between ΔΔG‡ and ΔΔGeq for the series of mutants is evidence for a nonheterogeneous population (i.e., for the presence of a single pathway). Recently, an approach similar to mutational φ value analysis was described in which addition of a metal ligand that can bind to an engineered site in the protein serves as the perturbation (21). In this case, a linear dependence between ΔΔG‡ and ΔΔGeq at different ligand concentrations is evidence for the existence of a single pathway. This type of procedure is ideally suited for studying pathways of conformational changes of allosteric proteins because they already contain natural ligand-binding sites. In addition, the initial and final states of allosteric transitions are well defined, whereas the unfolded state in protein folding reactions is an ensemble of many different conformations. Here, we describe the use of such an approach to study the effects of changes in ATP concentration on the transition state of the T to R allosteric switch of GroEL. There are two important differences, however, between the model in our study and the one described by Krantz and Sosnick (21). First, allosteric proteins such as GroEL contain multiple interacting ligand-binding sites and not just one site. Second, it is assumed that both the initial (T) and final (R) states in an allosteric transition bind the ligand (although with different affinities), whereas in folding studies, it may often be assumed that the unfolded state does not have any measurable affinity for the ligand. These differences lead to a different mathematical treatment than before (21), as described next.
The Model
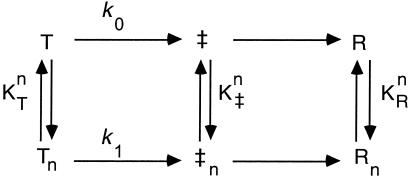
For simplicity, we consider a scheme (Fig. 1) where it is assumed (as in the original formulation of the Hill equation) that n molecules of ATP bind to the T and R states of a GroEL ring in an all-or-none manner with intrinsic association constants KT and KR, respectively. In the absence of ATP, each ring of GroEL is in equilibrium between the T and R states (L0 = [R]/[T]), as described previously (8, 9). Binding of ATP induces a concerted T→R conformational change in the protein. In the presence of ATP, an apparent equilibrium constant, L, between the T and R states is, therefore, given by:
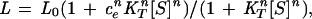 |
where S designates the substrate, ATP, and ce stands for KR/KT [this definition of L corresponds to the reciprocal of that in the original Monod–Wyman–Changeux model (22)]. Following Hammes and Schimmel (23), it is assumed that ligand (ATP) binding occurs much faster than the ligand-induced conformational changes. It is also assumed that the transitions from the R to T states may be neglected on rapid mixing of ATP and unliganded GroEL, so that we can, therefore, express the observed rate constant for the conformational changes, kobs, as follows:
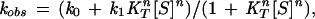 |
where k0 and k1 are the forward rate constants for the respective conformational changes T→R and Tn→Rn of the unliganded and fully liganded rings, respectively.
Fig 1.
Scheme showing the different states of a GroEL ring considered in the analysis here. Binding of n molecules of ATP to the T, ‡ (transition state), and R states of GroEL takes place in an all-or-none fashion with apparent association constants KT, K‡, and KR, respectively. Binding of ATP induces a T→R conformational change in the GroEL ring. Tn and Rn designate the T and R states of GroEL rings with n bound molecules of ATP. The forward rate constants of the conformational changes T→R and Tn→Rn are designated by k0 and k1, respectively.
According to transition-state theory, the forward rate constant, k0, of an unbound ring can be written as AK0, where A is a constant and K0 is the equilibrium constant between the unbound transition state, ‡, and T state (K0 = [‡]/[T]). Because of free energy conservation in the thermodynamic cycles shown in Fig. 1, it follows that: k1 = k0c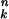 , where ck stands for K‡/KT and K‡ is the association constant of the transition state for ATP. In the case of the kinetic scheme in Fig. 1, the observed forward rate constant for the conformational change in GroEL, kobs, can, therefore, be expressed, as follows:
, where ck stands for K‡/KT and K‡ is the association constant of the transition state for ATP. In the case of the kinetic scheme in Fig. 1, the observed forward rate constant for the conformational change in GroEL, kobs, can, therefore, be expressed, as follows:
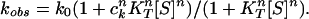 |
In principle, one could express kobs as follows: k0(1 + ckKT[S])n/(1 + KT[S])n. This expression has the advantage that it takes into account all possible ligation states, but it is based on an assumption that we find here to be wrong (see below) that the transition state does not change during ligation. Thus, data of kobs as a function of [ATP] could not be fitted to this type of expression (not shown). Both kobs and L were, therefore, expressed by using Hill-type expressions (Eqs. 1 and 3), in which n stands for the Hill coefficient and not the number of sites. The φ value is defined, as follows:
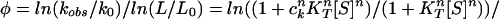 |
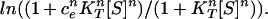 |
The values of the Hill coefficients determined by fitting steady-state and transient kinetic data to the Hill equation and Eq. 2, respectively, are found to be the same (16, 24). Hence, inspection of Eq. 4 shows that φ = 0 when ck = 1 (i.e., when K‡ = KT), thus indicating that the structure of the ATP-binding sites in the transition state is T-like. A value of φ = 1 occurs when ck = ce (i.e., when K‡ = KR), thus indicating that the structure of the ATP-binding sites in the transition state is R-like. Nonclassical values of φ > 1 will arise in the unlikely situation that the affinity for ATP of the transition state is greater than that of the R state (K‡ > KR). Nonclassical values of φ < 0 will arise when the affinity for ATP of the transition state is lower than that of the T state (K‡ < KT).
Results and Discussion
The mutation Phe-44→Trp was previously introduced into GroEL (which has no tryptophan residues) to facilitate the following of ATP-induced conformational changes by monitoring time-resolved changes in fluorescence. The allosteric properties of the Phe-44→Trp mutant are similar to those of wild-type GroEL (25). Values of ln(kobs/k0) at different concentrations of ATP were calculated from the published transient kinetic data for the Phe-44→Trp GroEL mutant (25). Values of ln(L/L0) at different concentrations of ATP were calculated from ((1 + KR[S])/(1 + KT[S]))7. The values of the parameters KT, KR, and L0 were determined by refitting the steady-state ATPase data for the first transition of the Phe-44→Trp GroEL mutant (25) to an equation based on the Monod–Wyman–Changeux model (22) (i) without assuming exclusive binding to the R state and (ii) taking into account the observation that kcat of ATP hydrolysis of the T state is about 4-fold larger than that of the R state (4, 9). The values of KT, KR, ce (= KR/KT), and L0 were found to be 0.0017 (±0.0013) μM−1, 0.148 (±0.02) μM−1, 89 (±71), and 0.0023 (±0.0018), respectively. The values of KR and L0 are in good agreement with those reported previously when exclusive binding to the R state was assumed (25). It is assumed that the values of KR and KT for ATP binding to the first ring are similar to the respective values for ATP binding to the second ring (25).
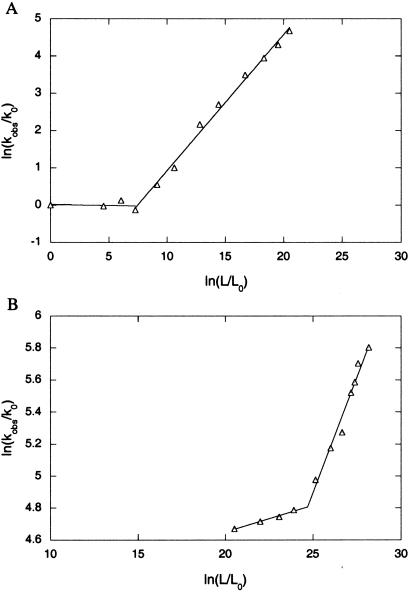
Values of ln(kobs/k0) at different concentrations of ATP were plotted against the corresponding values of ln(L/L0). GroEL undergoes two ATP-dependent allosteric transitions: one at relatively low ATP concentrations (<100 μM) and the second at higher concentrations of ATP. In the case of the first transition (Fig. 2A), the φ value is 0.0 (±0.1) until ≈12.5 μM ATP, when an abrupt switch occurs to a different pathway with a φ value of 0.37 (±0.01). In the case of the second transition that begins at about 150 μM ATP (Fig. 2B), the φ value is 0.033 (±0.003) until ≈350 μM ATP, when an abrupt switch occurs to a different pathway with a φ value of 0.29 (±0.02). The excellent fit of the data for each allosteric transition to two linear phases indicates that two parallel pathways exist for each transition. The φ values report on the structure of the ATP-binding sites in the transition state. They indicate that the structure of the ATP-binding sites in the transition state of the pathway that dominates at the low ATP concentrations is T-like in the case of both allosteric transitions. The structure of the ATP-binding sites in the transition state of the pathway that dominates at the higher ATP concentrations is also T-like but less so in the case of both allosteric transitions. The linear relationship between ln(kobs/k0) and ln(L/L0) at the higher ATP concentrations indicates that the fractional φ values here reflect a transition-state structure that is less T-like and not the presence of a mixed population.
Fig 2.
Plots of ln(kobs/k0) against the corresponding values of ln(L/L0) at different low (A) and high (B) concentrations of ATP corresponding to the allosteric transitions of the first and second GroEL rings, respectively. The data are from Yifrach and Horovitz (25) and were reanalyzed, as described in the text. The errors in kobs are <2%.
The transition-state structure of the equatorial domain was previously also probed by mutational φ value analysis of Arg-501 (16). This residue makes an intradomain salt bridge with Glu-409 that becomes weaker on the T to R transition (26). The φ value for this residue was found to be 0.17, indicating that the structure of this region in the equatorial domain is also T-like in the transition state. In contrast, mutational φ value analysis of Arg-197 in the apical domain indicates that the salt bridge it makes with Glu-386 in the intermediate domain is significantly perturbed in the transition state (16). It is clear from the analysis here that our previous mutational φ value analysis corresponded to the transition state that dominates at the low ATP concentrations. Hence, larger effects may be observed if mutational φ value analysis of the transition state that dominates at the higher ATP concentrations is carried out for these residues.
The finding that the φ values of the allosteric transition of the first ring are similar to those of the allosteric transition of the second ring suggests that the allosteric switch of the two rings involves the same pathways. Hence, inter-ring negative cooperativity in GroEL does not appear to be linked to a change in the pathway of the conformational changes. The ATP concentration where the switch between pathways occurs differs, however, between the allosteric transitions of the first and second rings. In the case of the allosteric transition of the first ring, the pathway with φ = 0 dominates in the presence of ATP in the concentration range from 0 to 12.5 μM. In the case of the allosteric transition of the second ring, the pathway with φ = 0 dominates in the presence of ATP in the concentration range from 150 to 350 μM. Negative cooperativity between the two GroEL rings is, therefore, reflected in the larger concentration of ATP required to effect the switch from the pathway with φ = 0 to the pathway with a φ value of about 0.3 in the second ring relative to the first ring. In other words, it is likely that a larger number of occupied sites in a ring is required to effect the switch between pathways in the case of the allosteric transition of the second ring.
In conclusion, it is shown here that φ value analysis in which changes in ATP concentration serve as perturbations can be used to map the pathways of allosteric transitions in GroEL. φ value analysis in which changes in ligand concentration serve as perturbations should be useful in mapping the pathways of allosteric transitions of other proteins. It will be of interest to determine in the future how various allosteric effectors and mutations alter the energy landscape of allosteric transitions of different proteins.
Acknowledgments
A.H. is an incumbent of the Carl and Dorothy Bennett Professorial Chair in Biochemistry. This work was supported by the Israel Science Foundation administered by the Israel Academy of Sciences and Humanities.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Perutz M. F. (1989) Q. Rev. Biophys. 22, 139-237. [DOI] [PubMed] [Google Scholar]
- 2.Sigler P. B., Xu, Z., Rye, H. S., Burston, S. G., Fenton, W. A. & Horwich, A. L. (1998) Annu. Rev. Biochem. 67, 581-608. [DOI] [PubMed] [Google Scholar]
- 3.Thirumalai D. & Lorimer, G. H. (2001) Annu. Rev. Biophys. Biomol. Struct. 30, 245-269. [DOI] [PubMed] [Google Scholar]
- 4.Horovitz A., Fridmann, Y., Kafri, G. & Yifrach, O. (2001) J. Struct. Biol. 135, 104-114. [DOI] [PubMed] [Google Scholar]
- 5.Braig K., Otwinowski, Z., Hegde, R., Boisvert, D. C., Joachimiak, A., Horwich, A. L. & Sigler, P. B. (1994) Nature 371, 578-586. [DOI] [PubMed] [Google Scholar]
- 6.Roseman A. M., Chen, S., White, H., Braig, K. & Saibil, H. R. (1996) Cell 87, 241-251. [DOI] [PubMed] [Google Scholar]
- 7.Ranson N. A., Farr, G. W., Roseman, A. M., Gowen, B., Fenton, W. A., Horwich, A. L. & Saibil, H. R. (2001) Cell 107, 869-879. [DOI] [PubMed] [Google Scholar]
- 8.Yifrach O. & Horovitz, A. (1995) Biochemistry 34, 5303-5308. [DOI] [PubMed] [Google Scholar]
- 9.Yifrach O. & Horovitz, A. (1996) J. Mol. Biol. 255, 356-361. [DOI] [PubMed] [Google Scholar]
- 10.Xu Z., Horwich, A. L. & Sigler, P. B. (1997) Nature 388, 741-750. [DOI] [PubMed] [Google Scholar]
- 11.Leffler J. E. (1953) Science 117, 340-341. [DOI] [PubMed] [Google Scholar]
- 12.Fersht A. R., Matouschek, A. & Serrano, L. (1992) J. Mol. Biol. 224, 771-782. [DOI] [PubMed] [Google Scholar]
- 13.Fersht A. R., Leatherbarrow, R. J. & Wells, T. N. C. (1986) Nature 322, 284-286. [Google Scholar]
- 14.Eaton W. A., Henry, E. R. & Hofrichter, J. (1991) Proc. Natl. Acad. Sci. USA 88, 4472-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edelstein S. J., Schaad, O., Henry, E., Bertrand, D. & Changeux, J.-P. (1996) Biol. Cybern. 75, 361-379. [DOI] [PubMed] [Google Scholar]
- 16.Yifrach O. & Horovitz, A. (1998) J. Am. Chem. Soc. 120, 13262-13263. [Google Scholar]
- 17.Grosman C., Zhou, M. & Auerbach, A. (2000) Nature 403, 773-776. [DOI] [PubMed] [Google Scholar]
- 18.Ma J., Sigler, P. B., Xu, Z. & Karplus, M. (2000) J. Mol. Biol. 302, 303-313. [DOI] [PubMed] [Google Scholar]
- 19.Ozkan S. B., Bahar, I. & Dill, K. A. (2001) Nat. Struct. Biol. 8, 765-769. [DOI] [PubMed] [Google Scholar]
- 20.Fersht A. R., Itzhaki, L. S., Elmasry, N. F., Matthews, J. M. & Otzen, D. E. (1994) Proc. Natl. Acad. Sci. USA 91, 10426-10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krantz B. A. & Sosnick, T. R. (2001) Nat. Struct. Biol. 8, 1042-1047. [DOI] [PubMed] [Google Scholar]
- 22.Monod J., Wyman, J. & Changeux, J.-P. (1965) J. Mol. Biol. 12, 88-118. [DOI] [PubMed] [Google Scholar]
- 23.Hammes G. G. & Schimmel, P. R. (1966) J. Phys. Chem. 70, 2319-2324. [Google Scholar]
- 24.Horovitz A. & Yifrach, O. (2000) Bull. Math. Biol. 62, 241-246. [DOI] [PubMed] [Google Scholar]
- 25.Yifrach O. & Horovitz, A. (1998) Biochemistry 37, 7083-7088. [DOI] [PubMed] [Google Scholar]
- 26.Aharoni A. & Horovitz, A. (1997) Proc. Natl. Acad. Sci. USA 94, 1698-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]