Abstract
Previously we demonstrated that specific chromatographically resolvable isoforms of rabbit brain GAPDH catalyze either glycolytic flux or membrane fusion activity (but not both). Moreover, GAPDH membrane fusion activity was latent until it was separated from an endogenous cytosolic inhibitor by anion-exchange chromatography. Herein we demonstrate that the cytosolic inhibitor is nondialyzable, heat-labile, and trypsin-sensitive, thereby identifying it as a cytosolic protein constituent. Chromatographic purification of the rabbit-brain cytosolic protein inhibitor of GAPDH isoform-catalyzed membrane fusion identified a predominant 55-kDa doublet that contained an internal 15-aa peptide identical to a sequence present in α-tubulin (residues 65–79). The identity of the 55-kDa doublet as tubulin was substantiated through Western blot analysis and inhibition of GAPDH-catalyzed membrane fusion by authentic tubulin. Stopped-flow kinetic analysis demonstrated the high-affinity, rapid, and direct modulation of GAPDH-catalyzed fusion activity by tubulin. Because GTP-activated Rab 2 recruits GAPDH to membranes about to undergo fusion [Tisdale, E. J. (2001) J. Biol. Chem. 276, 2480–2486] and protein kinase Cι/λ phosphorylates GAPDH modulating its interactions with tubulin [Tisdale, E. J. (2001) J. Biol. Chem. 277, 3334–3341] , the present study suggests a coordinated mechanism through which membrane trafficking and cellular signaling can be integrated with glycolytic flux.
One motif commonly used in intra- and intercellular communication is the coordinated packaging of chemical signals into intracellular vesicles, the intracellular trafficking of vesicles along microtubules to predesignated fusion sites, docking, and the subsequent fusion of spatially opposed vesicles to their target membranes during cellular stimulation (1–8). The biochemical mechanisms regulating these processes control, in large part, the ability of a cell to either regulate its own metabolism and function or propagate the flow of biologic information to selected target cells (9–12). The mechanisms that modulate cellular fusion processes include vesicle budding, the translocation of vesicles along microtubules to target membrane fusion sites, docking mediated by specific protein–protein interactions (e.g., vesicular SNAP receptor and target SNAP receptor), and the protein-catalyzed fusion of docked vesicles with their target membranes (13–17). Although substantial progress has been made in identifying the protein–protein interactions that facilitate the translocation and targeting of vesicles to their appropriate membrane fusion sites, there has been considerable debate on the biochemical mechanisms that actually catalyze and regulate the fusion process itself.
Previously, we reported the identification, characterization, and purification of a novel isoform of GAPDH that catalyzes membrane fusion (18–20) and satisfies the kinetic constraints imposed by the extraordinary rates of membrane fusion manifest in in vivo systems (11). One of the most remarkable features of this fusion activity is that it is latent (i.e., unmeasurable) in crude rabbit-brain cytosol and is demonstrable only after chromatographic separation of the GADPH isoform-catalyzing fusion activity from an endogenous inhibitor. Herein we purify and characterize the endogenous inhibitor of GAPDH isoform-catalyzed membrane fusion and demonstrate its identity as tubulin. Because microtubules are prominent constituents comprising critical portions of the cytoskeletal architecture at (or near) membrane sites destined for fusion and many types of signaling vesicles are transported along microtubules, these results suggest a role for tubulin–GAPDH isoform interactions in modulating the kinetics of membrane trafficking and cellular signaling processes through regulating the kinetics of membrane fusion.
Experimental Procedures
Materials.
Bovine-brain ethanolamine glycerophospholipids, 1-hexadecanoyl-2-octadec-9′-enyl-sn-glycero-3-phosphoserine (POPS), and 1-hexadecanoyl-2-octadec-9′-enyl-sn-glycero-3-phosphocholine (POPC) were purchased from Avanti Polar Lipids and purified by HPLC as described (21). Plasmenylethanolamine was synthesized and purified as described (22). Cholesterol was purchased from Nu Chek Prep (Elysian, MN). Octadecyl rhodamine (R18) was obtained from Molecular Probes and purified as described (22). DE-52 anion-exchange resin was purchased from Whatman. CNBr-Sepharose and electrophoresis supplies were from Amersham Pharmacia. Most other chemicals were obtained from Sigma.
Membrane Fusion Assays and Inhibition of Fusion Activity by an Endogenous Inhibitor.
Small unilamellar vesicles (SUVs) were prepared by sonication, and fusion assays were performed by using a stopped-flow apparatus by quantifying the intensity of R18 fluorescence as described (18). Assays for inhibition of membrane fusion were performed by mixing aliquots of column fractions with a partially purified fraction of the GAPDH isoform-catalyzing membrane fusion (i.e., DE void volume) for 30 s at 37°C before loading of the stopped-flow apparatus. Inhibitory potency of column eluents was expressed in terms of the inhibition dilution factor representing the amount each column fraction could be diluted to produce 50% inhibition of the initial fusion rate.
Purification of the Inhibitor of GAPDH Isoform-Catalyzed Membrane Fusion.
New Zealand rabbit brains were harvested, and homogenization of the brains was performed as described (18). Brain cytosol was prepared by sequential ultracentrifugation from the homogenate, dialyzed, and loaded onto a DE-52 column as described (18). Adsorbed proteins were eluted by using a 0–400 mM NaCl gradient over 400 ml. The inhibitory potency of column eluents was assessed after appropriate dilution (as indicated in the figure legends) by quantifying the differences in the percentage of GAPDH isoform-catalyzed membrane fusion in the presence relative to the absence of diluted aliquots of column eluents. Fractions containing the highest levels of inhibitory activity were pooled, dialyzed against 100 volumes of buffer A [20 mM Tris⋅Cl/0.1 mM EGTA/0.1 mM EDTA/1 mM DTT (pH 7.0) at 4°C], and loaded onto a Mono Q column previously equilibrated with buffer A. Adsorbed proteins were eluted by using a nonlinear NaCl gradient (0–600 mM NaCl in buffer A) as described in the figure legends. To exploit the resolving power of Mono Q chromatography further, the most potent inhibitory fractions were pooled, dialyzed against buffer A, and rechromatographed on the Mono Q column by using a shallow gradient of NaCl in buffer A.
Purification of Tubulin by Assembly/Disassembly Cycling.
Tubulin was purified by assembly/disassembly of microtubules by using the method of Shelanski et al. (23). Briefly, rabbit-brain cytosol was prepared as described above and diluted (1:1, vol/vol) with buffer comprised of 8 M glycerol, 2 mM GTP, 200 mM NaMES, 1 mM MgCl2, and 2 mM EGTA (pH 6.4). After incubation at 37°C for 20 min (during which time microtubule assembly occurred), microtubules were pelleted by centrifugation at 100,000 × g for 60 min at 25°C. The pellet was resuspended in ice-cold (4°C) buffer [100 mM NaMES/0.5 mM MgCl2/1 mM EGTA (pH 6.4)], dispersed by using a Potter Elvehjem homogenizer, and incubated at 4°C for 20 min. After microtubule disassembly, the sample was centrifuged at 100,000 × g, and the supernatant was collected. To the supernatant, an equal volume of buffer [8 M glycerol/2 mM GTP/200 mM NaMES/1 mM MgCl2/2 mM EGTA (pH 6.4)] was added. Microtubule formation was accomplished again by incubation at 37°C for 20 min, and the above sequence of centrifugation and disassembly was repeated. Tubulin was purified further from microtubule-associated proteins by Mono Q chromatography. The supernatant was filtered, dialyzed against buffer B [100 mM NaMES/1 mM EGTA/0.5 mM MgCl2 (pH 6.6)], and loaded onto a Mono Q column previously equilibrated with buffer B. Bound tubulin was eluted by using a nonlinear NaCl gradient in buffer B as indicated in the figure legends. Tubulin-containing column eluents were dialyzed twice against a 100-fold excess of buffer C [10 mM potassium phosphate (pH 7.0) containing 0.3 mM CaCl2] before loading onto an BioGel high-pressure, high performance hydroxylapatite column (Bio-Rad) (1 × 5 cm) previously equilibrated with buffer C. The column was developed by using a linear gradient of 10 mM potassium phosphate, 0.3 mM CaCl2 to 350 mM potassium phosphate, and 0.01 mM CaCl2 over a 30-ml volume.
Preparation of Tubulin-Sepharose Affinity Resin and Affinity Chromatography.
Homogeneous tubulin (purified by the assembly/disassembly method followed by sequential Mono Q and hydroxylapatite chromatographies) was dialyzed twice against 100 volumes of buffer D [0.1 M NaHCO3 with 0.5 M NaCl (pH 8.0)] for 15 h and added in equal volumes to a solution of activated CNBr-Sepharose (1 g/5 ml). After incubation for 24 h at 4°C, unreacted groups were blocked with 0.2 M glycine (pH 8.0) for 2 h, and the tubulin-Sepharose matrix was washed with repetitive alternating washes of buffer D and buffer E [0.1 M acetate/0.5 M NaCl (pH 4.5)]. After equilibration of the tubulin-Sepharose column (0.9 × 5 cm) with buffer F [50 mM Tris⋅Cl/0.1 mM EGTA/0.1 mM EDTA/1 mM DTT (pH 7.0) at 4°C], protein was loaded onto the column and washed with 10 column volumes of buffer F, and bound proteins were eluted in a step gradient with 0.5 M NaCl in buffer F.
Results
Identification of the Endogenous Inhibitor of GAPDH Isoform-Mediated Membrane Fusion as a Cytosolic Protein Constituent and Purification of the Inhibitor.
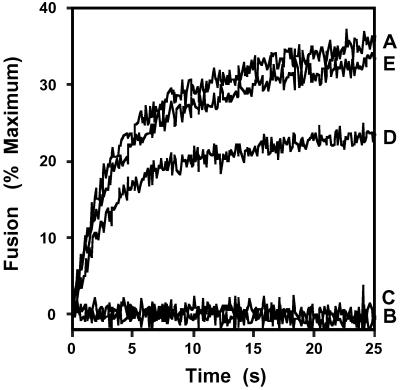
Previously we identified and purified a GAPDH isoform from rabbit-brain cytosol that rapidly catalyzed the fusion of vesicles comprised of physiologic mole fractions of phospholipids and cholesterol but did not possess intrinsic dehydrogenase activity (18–20). This isoform of GAPDH did not possess demonstrable fusion activity until endogenous inhibitors in the cytosolic fraction were removed by anion-exchange chromatography. Accordingly, in initial experiments we reconstituted inhibition of GAPDH-catalyzed membrane fusion by mixing the DE-52 void volume with crude cytosol (Fig. 1). Characterization of the cytosolic factor inhibiting membrane fusion demonstrated that it was nondialyzable, heat-labile (65°C for 1 h), and trypsin-sensitive (1%, wt/vol) (Fig. 1). Thus, a cytosolic protein constituent is the endogenous inhibitor that masks GAPDH isoform-catalyzed membrane fusion activity in the cytosolic fraction of rabbit-brain homogenates.
Fig 1.
Characterization of the inhibitor of GAPDH isoform-catalyzed membrane fusion in dialyzed rabbit-brain cytosol. The cytosolic inhibitor of GAPDH isoform-catalyzed membrane fusion was characterized by using physiologically modeled SUVs as substrate in the R18 fusion assay as described in Experimental Procedures. Vesicles were mixed with protein samples from the DE-52 void volume alone (A), the DE-52 void volume preincubated for 30 s at 37°C with a 1:10 dilution of untreated cytosol (B), the DE-52 void volume preincubated for 30 s at 37°C with a 1:10 dilution of heat-treated cytosol (i.e., previously heated at 37°C for 2 h) (C), the DE-52 void volume preincubated for 30 s at 37°C with a 1:10 dilution of heat-denatured cytosol (i.e., previously heated at 65°C for 1 h) (D), or the DE-52 void volume preincubated for 30 s at 37°C with 1:10 dilution of proteolyzed cytosol [previously treated with trypsin (1%, wt/wt) for 2 h at 37°C] (E). Membrane fusion was expressed as a percentage of maximum fusion as described in Experimental Procedures.
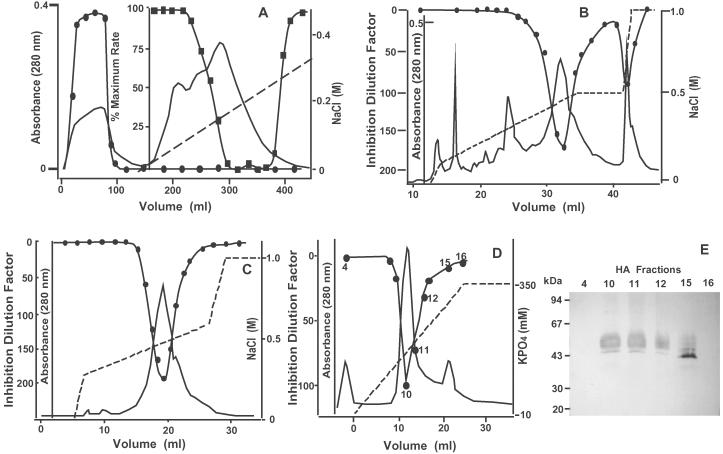
Because robust membrane fusion activity was present in the void volume after anion-exchange chromatography but latent in dialyzed cytosol, a DE-52 column was used first to separate the fusion activity from the fusion inhibitor. The adsorbed cytosolic proteins were eluted by using a linear NaCl gradient. The protein inhibitor of GAPDH isoform-mediated membrane fusion eluted as a single broad peak (at ≈200 mM NaCl) (Fig. 2A). Inhibition of membrane fusion activity by column eluates was not due to alterations in ionic strength, because column fractions corresponding to much higher concentrations of sodium chloride were not inhibitory. As anticipated, treatment of anion-exchange column chromatographic eluates containing inhibitory activity with trypsin (1%, wt/wt) or heat (65°C for 2 h) completely ablated their inhibitory activity (data not shown).
Fig 2.
Purification of the inhibitor of GAPDH isoform-catalyzed membrane fusion by multiple column chromatographies. (A) DE-52 chromatography of dialyzed rabbit-brain cytosol. Dialyzed rabbit-brain cytosol was loaded onto a previously equilibrated DE-52 column, adsorbed proteins were eluted by using a NaCl gradient (0–400 mM NaCl), and aliquots of column fractions were tested for their ability to catalyze fusion activity as described in Experimental Procedures ( ). Inhibition was assessed by briefly incubating column eluates [in a 1:1 (vol/vol) ratio] with the DE-52 void volume and evaluating the resultant activity by using the R18 fusion assay (
). Inhibition was assessed by briefly incubating column eluates [in a 1:1 (vol/vol) ratio] with the DE-52 void volume and evaluating the resultant activity by using the R18 fusion assay ( ) as described in Experimental Procedures. The data for reconstitution assays are expressed as a percent maximal rate, with 100% representing the fusion rate observed when using the DE-52 void volume fractions alone. UV absorbance at 280 nm, ——; NaCl gradient, — —. (B) Mono Q chromatography of the protein inhibitor of GAPDH isoform-catalyzed membrane fusion. Column eluates from anion-exchange chromatography containing the largest amounts of inhibitory activity were pooled, dialyzed, and loaded onto a Mono Q column as described in Experimental Procedures. Adsorbed proteins were eluted with a nonlinear NaCl gradient (0–1 M NaCl) and aliquots of column eluents were evaluated for their ability to inhibit membrane fusion as described in Experimental Procedures. The inhibition dilution factor represents the amount the column eluate could be diluted to produce 50% inhibition of membrane fusion (
) as described in Experimental Procedures. The data for reconstitution assays are expressed as a percent maximal rate, with 100% representing the fusion rate observed when using the DE-52 void volume fractions alone. UV absorbance at 280 nm, ——; NaCl gradient, — —. (B) Mono Q chromatography of the protein inhibitor of GAPDH isoform-catalyzed membrane fusion. Column eluates from anion-exchange chromatography containing the largest amounts of inhibitory activity were pooled, dialyzed, and loaded onto a Mono Q column as described in Experimental Procedures. Adsorbed proteins were eluted with a nonlinear NaCl gradient (0–1 M NaCl) and aliquots of column eluents were evaluated for their ability to inhibit membrane fusion as described in Experimental Procedures. The inhibition dilution factor represents the amount the column eluate could be diluted to produce 50% inhibition of membrane fusion ( ) (C) Iterative Mono Q chromatography of the protein inhibitor of GAPDH isoform-catalyzed membrane fusion. Active fractions from the first Mono Q column were pooled, dialyzed against buffer, and again loaded onto a previously equilibrated Mono Q column. The column was developed with a nonlinear NaCl gradient (0–1 M NaCl) and aliquots of column eluents were assessed for their ability to inhibit GAPDH isoform-catalyzed membrane fusion as described in Experimental Procedures. (D) Hydroxylapatite chromatography of the protein inhibitor of GAPDH isoform-catalyzed membrane fusion. Inhibitory fractions from the second Mono Q column were dialyzed twice against a 100-fold excess of buffer [10 mM potassium phosphate (pH 7.0) containing 0.3 mM CaCl2] and loaded onto a previously equilibrated BioGel high-pressure, high-performance hydroxylapatite column. The column was developed by using a phosphate gradient, and aliquots of column eluates were screened for their ability to inhibit GAPDH-catalyzed membrane fusion (
) (C) Iterative Mono Q chromatography of the protein inhibitor of GAPDH isoform-catalyzed membrane fusion. Active fractions from the first Mono Q column were pooled, dialyzed against buffer, and again loaded onto a previously equilibrated Mono Q column. The column was developed with a nonlinear NaCl gradient (0–1 M NaCl) and aliquots of column eluents were assessed for their ability to inhibit GAPDH isoform-catalyzed membrane fusion as described in Experimental Procedures. (D) Hydroxylapatite chromatography of the protein inhibitor of GAPDH isoform-catalyzed membrane fusion. Inhibitory fractions from the second Mono Q column were dialyzed twice against a 100-fold excess of buffer [10 mM potassium phosphate (pH 7.0) containing 0.3 mM CaCl2] and loaded onto a previously equilibrated BioGel high-pressure, high-performance hydroxylapatite column. The column was developed by using a phosphate gradient, and aliquots of column eluates were screened for their ability to inhibit GAPDH-catalyzed membrane fusion ( ), as described in Experimental Procedures. UV absorbance at 280 nm, ——; potassium phosphate gradient, — —. (E) Protein constituents in column eluates from D were resolved by SDS/PAGE (10–15% gradient PhastGel) and visualized by silver staining. HA, hydroxylapatite.
), as described in Experimental Procedures. UV absorbance at 280 nm, ——; potassium phosphate gradient, — —. (E) Protein constituents in column eluates from D were resolved by SDS/PAGE (10–15% gradient PhastGel) and visualized by silver staining. HA, hydroxylapatite.
Column eluates containing inhibitory activity after DE-52 chromatography were loaded onto a Mono Q column as described in Experimental Procedures. Application of a nonlinear salt gradient resulted in the elution of two well defined peaks of inhibitory activity, a major peak at 400 mM NaCl and a minor peak at 750 mM NaCl (Fig. 2B). Fractions corresponding to the major peak of inhibitory activity were pooled, dialyzed, and subsequently reapplied to a second previously equilibrated Mono Q column (Fig. 2C). Final purification was effected by high-performance hydroxylapatite chromatography using a phosphate gradient (Fig. 2D). Analysis of inhibitory fractions by SDS/PAGE and subsequent silver staining demonstrated that the major peak of inhibition corresponded to a 55-kDa protein band, the silver staining intensity of which precisely “waxed and waned” with inhibitory activity (Fig. 2E).
To determine the chemical identity of the 55-kDa protein, the fractions containing inhibitory activity were concentrated, subjected to SDS/PAGE, and stained with Coomassie blue dye, and the 55-kDa protein constituent was excised. The 55-kDa protein was subsequently subjected to in situ trypsinolysis, and the resultant peptides were separated by reverse-phase chromatography. A symmetrically eluting peptide was selected, and automated Edman degradation demonstrated a 15-aa sequence (Ala-Val-Phe-Val-Asp-Leu-Glu-Pro-Thr-Val-Ile-Asp-Glu-Val-Arg) that possessed complete identity to human and mouse α-tubulin (residues 65–79) as well as chicken-brain α-tubulin (residues 24–40). Western blotting of fractions from Mono Q chromatography with commercially available antibodies directed against β-tubulin confirmed the identity of the 55-kDa protein doublet as a tubulin heterodimer (data not shown).
To substantiate further the ability of tubulin to serve as a regulator of membrane fusion, an independent method of purification (i.e., assembly of tubulin to form microtubules and then disassembly of isolated microtubules as described in ref. 23) was used to prepare homogeneous preparations of tubulin from rabbit-brain cytosol. Assembly/disassembly cycling led to a preparation of tubulin that was ≈90% pure. Next, Mono Q anion-exchange chromatography was used to purify tubulin to homogeneity, and elution of tubulin precisely cochromatographed with the ability to inhibit GAPDH isoform-catalyzed membrane fusion. The direct application of active fractions from Mono Q chromatography onto a high-performance hydroxylapatite column and subsequent application of a linear phosphate gradient resulted in the elution of a single UV-absorbing peak of protein that again precisely cochromatographed with inhibitory activity and the 55-kDa band of tubulin (data not shown). Analysis of column eluents from Mono Q and hydroxylapatite chromatography by SDS/PAGE and silver staining demonstrated a single intense 55-kDa protein band that was identified as tubulin by Western blotting (data not shown). Collectively, these results demonstrate that tubulin is the endogenous inhibitor that masks GAPDH isoform-catalyzed membrane fusion activity in the cytosolic compartment until its removal by anion-exchange chromatography.
Identification of the Mechanism of Inhibition of GAPDH-Mediated Membrane Fusion Activity by Tubulin.
Although the preceding experiments establish that tubulin is the endogenous inhibitor masking GAPDH isoform-mediated membrane fusion in rabbit-brain cytosol (18), they do not identify the mechanism of tubulin-mediated inhibition of membrane fusion [i.e., they do not discriminate between tubulin interacting with the GAPDH isoform directly (protein–protein interactions) or by tubulin interacting with membrane vesicles to inhibit protein–lipid interactions by membrane surface depletion]. To characterize the mechanism through which tubulin inhibits GAPDH isoform-mediated membrane fusion, three independent methods were used.
First, the direct interaction of the GAPDH isoform and tubulin was demonstrated by affinity chromatography. Purified tubulin was linked covalently to cyanogen bromide-activated Sepharose and highly purified preparations of the GAPDH isoform-catalyzing membrane fusion (i.e., the eluate from GTP-agarose affinity chromatography) were applied to the affinity column as described in Experimental Procedures. Both fusion activity and GAPDH isoform protein mass (assessed by silver staining after SDS/PAGE) quantitatively adsorbed to the tubulin-Sepharose affinity resin. Elution with 500 mM NaCl resulted in the concomitant elution of both GAPDH isoform mass and fusion activity. To determine the specificity of these interactions, crude anion-exchange column eluents were loaded onto an independent tubulin-Sepharose affinity column and washed extensively, and bound proteins were eluted with 500 mM NaCl. The tubulin-Sepharose affinity matrix selectively bound GAPDH, whereas other proteins eluted in the void volume (data not shown), demonstrating the specificity of the interaction of tubulin with GAPDH. Once again, elution with 500 mM NaCl yielded both concentrated fusion activity and a 38-kDa protein band.
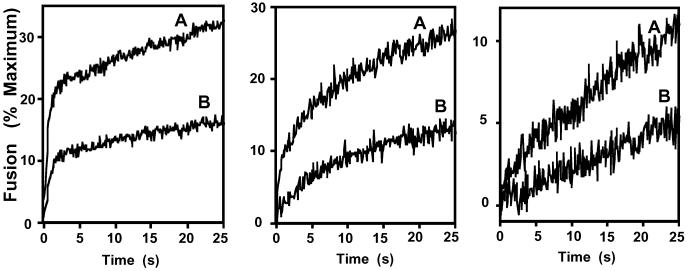
Second, experiments were performed to examine the stoichiometry of inhibition and the effects of altering the residence times of tubulin with the vesicles before the initiation of membrane fusion. Tubulin and the GAPDH fusion protein were mixed at selected molar ratios, and the resultant effects on the rate of membrane fusion were quantified. Tubulin-mediated inhibition reflected the molar ratio of tubulin to GAPDH present and, at the concentrations used, was largely independent of the molar concentration of tubulin present (data not shown). Furthermore, the inhibition of the initial rates of membrane fusion by tubulin was similar over a 16-fold difference in vesicle concentration (Fig. 3). To demonstrate that the inhibition was independent of vesicle curvature, purified tubulin was shown to inhibit GAPDH isoform-catalyzed fusion of SUVs and large unilamellar vesicles similarly (data not shown).
Fig 3.
Independence of vesicle concentration on the initial rate of tubulin inhibition of membrane fusion catalyzed by the GAPDH isoform. Physiologically modeled SUVs were prepared as described in Experimental Procedures and used at concentrations of 800 (Left), 200 (Center), or 50 μM (Right). After mixing the GAPDH isoform-catalyzing membrane fusion (GTP-agarose column eluate, 1 μg/ml) with purified tubulin at a concentrations of 0 (A) or 0.2 μg/ml (B) for 30 s at 37°C, membrane fusion was quantified as described in Experimental Procedures. The data represent the mean of eight determinations from two separate preparations.
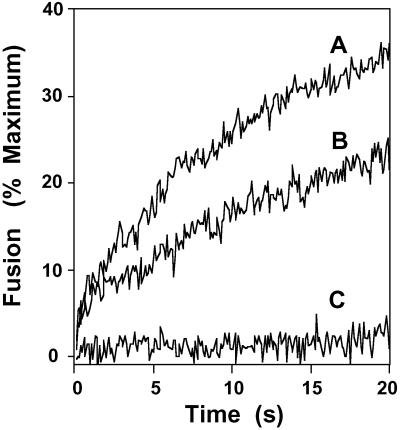
Third, the effects of contact time between tubulin and the GAPDH isoform-catalyzing membrane fusion were examined by using a stopped-flow system (Fig. 4). Preincubation of tubulin with GAPDH fusion protein (for ≈15 s) resulted in the complete ablation of membrane fusion activity even at the earliest time points examined. In contrast, loading of tubulin and the GAPDH isoform-mediating membrane fusion in separate chambers of the stopped-flow apparatus (i.e., preventing their interaction until rapidly mixed in the stopped-flow chamber) demonstrated an “uninhibited” initial rate of membrane fusion followed by the subsequent attenuation of the rate of membrane fusion at times >3 s. Collectively, these results demonstrate the highly specific interaction between tubulin and the GAPDH isoform-catalyzing membrane fusion, the high-affinity of tubulin for GAPDH, and the rapidity with which tubulin-mediated inhibition of GAPDH-catalyzed fusion can occur after rapid mixing (i.e., the rapid on-rate of their interaction).
Fig 4.
Contact time between the GAPDH isoform-catalyzing membrane fusion and tubulin as a determinant of fusion velocity. Physiologically modeled SUVs were prepared and loaded into one chamber of a stopped-flow apparatus as described in Experimental Procedures. The other chamber was loaded with the membrane fusion-catalyzing protein from the GTP-agarose column diluted to a concentration of 1 μg/ml (A). Purified tubulin (5 μg/ml) was preincubated with SUVs for 30 s at 37°C before mixing in the stopped-flow apparatus with the GAPDH-containing solution (1 μg/ml) (B). Tubulin (5 μg/ml) and the GAPDH isoform-catalyzing membrane fusion (1 μg/ml) were placed in one chamber, and vesicles were placed in the other chamber; fusion was initiated by rapid mixing of chambers and quantified by the increase in R18 fluorescence as described in Experimental Procedures (C).
Discussion
The results of the present study unambiguously demonstrate that the inhibitor of GAPDH-catalyzed membrane fusion activity in rabbit-brain cytosol (18, 19) is tubulin. The protein inhibitor was purified to near homogeneity by two independent techniques and identified as tubulin through chemical, chromatographic, biophysical, and immunologic criteria. The affinity and specificity of the protein–protein interactions underlying the interaction of tubulin with the GAPDH isoform-catalyzing membrane fusion were demonstrated by multiple independent criteria. First, only a single protein in rabbit-brain cytosol was capable of inhibiting GAPDH-catalyzed membrane fusion (i.e., a single peak of inhibitory activity during the NaCl gradient of anion-exchange chromatography was present). Moreover, the affinity and potency of tubulin as an inhibitor of membrane fusion was underscored by the demonstration that (i) cytosol could be diluted markedly (1 part in 100) while retaining significant inhibition of membrane fusion activity, (ii) tubulin-mediated inhibition of membrane fusion activity was manifest at physiologic ionic strength (150 mM KCl), and (iii) the apparent KD for tubulin-mediated inhibition of GAPDH-mediated membrane fusion was ≤200 nM. Collectively, these results underscore the selectivity, specificity, affinity, and mode of the interaction between tubulin and the GAPDH isoform-catalyzing membrane fusion.
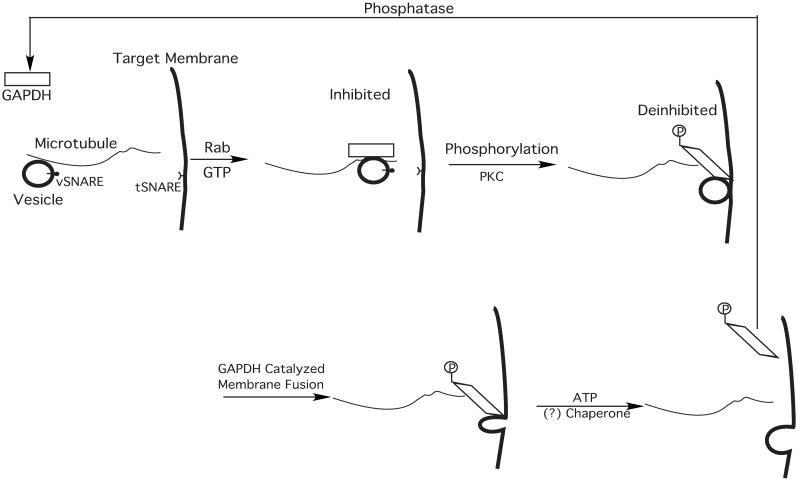
Multiple studies from different groups employing independent methods have now demonstrated the participation of one or more GAPDH isoforms in membrane fusion and trafficking in biological systems. For example, Robbins et al. (24) demonstrated that a heterozygous mutation of GAPDH in Chinese hamster ovary cells altered membrane trafficking. Consistent with the regulatory functions of tubulin on GAPDH-mediated membrane fusion proposed herein, the main biochemical defect discovered in those cells was that mutant GAPDH bound more tightly to microtubules than native GAPDH, and the mutant GAPDH could not be released by physiologic amounts of ATP, which rapidly released wild-type GAPDH (24). Recently, Tisdale demonstrated that GAPDH is required for vesicular transport cycling and that GAPDH associated with vesicles is required for fusion (25, 26). In Scheme we illustrate a proposed mechanism that is consistent with a role for tubulin-modulated GAPDH-catalyzed membrane fusion in systems that use microtubule-directed vesicular trafficking (e.g., GLUT 4 vesicle trafficking, endoplasmic reticulum to Golgi/vesicular tubular cluster membrane trafficking). In this proposed mechanism, specialized GAPDH isoforms are recruited to tubulin-associated vesicles in a Rab- and GTP-dependent manner, and subsequent protein kinase C (PKC) phosphorylation results in a conformational change of GAPDH altering its interactions with tubulin and facilitating membrane fusion by deinhibition of GAPDH. Subsequently, GAPDH can be released from the target membrane in an ATP-dependent fashion (which may require additional ATP-dependent chaperones), and the catalytic cycle is complete by the dephosphorylation of P-GAPDH catalyzed by an as-yet-unknown phosphatase. We believe it is likely that multiple other proteins participate in this process that facilitate and regulate appropriate interactions between fusion partners. This scheme contains several testable hypotheses including the release of tubulin-mediated inhibition of GAPDH by PKCι-mediated phosphorylation of GAPDH. Finally, the release of sequestered GAPDH from the fusion complex is an important area of study, and it seems likely that additional insights into this process will be gleaned from identification of the proteins facilitating the ATP-dependent release of GAPDH from tubulin. Finally, we point out that in many cases, membrane fusion occurs in the absence of identifiable microtubular networks. Membrane fusion in these cases may still be mediated by a GAPDH isoform-catalyzing fusion, but that regulation likely is mediated by other accessory proteins in those specialized fusion complexes. For example, in the case of calcium-mediated exocytosis, synaptotagmin (or other regulatory proteins) may confer calcium sensitivity to GAPDH-catalyzed fusion, which in purified reconstituted systems is in itself a calcium-independent process. Additional roles for tubulin monomers or the possibility of additional proteins that catalyze the fusion process cannot be ruled out.
Scheme 1.
A proposed mechanism for GAPDH isoform-catalyzed membrane fusion regulated by GTP, Rab, PKC, ATP, and tubulin. First, GTP-activated Rab recruits specialized isoforms of GAPDH to vesicles on microtubule tracks. Subsequent phosphorylation of GAPDH by PKC results in altered tubulin–GAPDH interactions, leading to the release of tubulin inhibition of GAPDH-catalyzed membrane fusion. After membrane fusion is complete, GAPDH can be released from the microtubules in an ATP-dependent process (24), potentially requiring chaperone proteins. The catalytic cycle is completed by dephosphorylation of GAPDH by an unknown phosphatase. Multiple accessory proteins likely participate in these processes, some of which may confer calcium sensitivity (e.g., synaptotagmin) in some systems through modulating interactions of GAPDH (phosphorylated and nonphosphorylated forms) with tubulin or other specialized regulatory proteins. In addition, the possibility that some of the sequential steps shown here occur through a concerted mechanism cannot be excluded by the experimental evidence available at present. vSNARE, vesicular SNAP receptor; tSNARE, target SNAP receptor.
We have demonstrated that GAPDH can catalyze membrane fusion of purified pancreatic β cell secretory granules with plasma membranes (19). Because glycolytically derived ATP is an important modulator of GAPDH and GAPDH isoforms catalyze membrane fusion, these results integrate cellular glycolytic flux and energy metabolism with membrane trafficking. Moreover, several glycolytic metabolites bind to and modulate GAPDH tertiary structure, thereby providing an additional mechanism through which glycolytic flux and membrane trafficking can be regulated coordinately. The integration of separate biochemical processes (i.e., glycolysis and membrane fusion) may have important consequences for regulating cell growth or alternatively in processes such as facilitating the trafficking of insulin secretory granules in pancreatic β cells to regulate energy supply and demand.
Collectively, prior work and the results of this study demonstrate that (i) specific GAPDH isoforms can catalyze fusion of vesicles comprised of physiologic mole % of phospholipids and cholesterol (18), (ii) Rab2 mediates the recruitment of GAPDH to vesicles about to undergo membrane fusion (25), (iii) GAPDH-catalyzed membrane fusion is modulated by direct interactions with tubulin (this study), and (iv) PKCι/λ phosphorylates GAPDH modulating its interaction with tubulin (26). Further research into the precise molecular mechanisms through which these moieties interact at critical stages in the fusion process should facilitate the identification of the chemical mechanisms that initiate and regulate membrane fusion in biologic systems.
Acknowledgments
This research was supported jointly by National Institutes of Health Grants 2P01HL5727806A1, 2R01H41250-10, 5R01AA1109405, and P60DK20579-22.
Abbreviations
R18, octadecyl rhodamine
SUV, small unilamellar vesicle
References
- 1.Hessler N. A., Shirke, A. M. & Malinow, R. (1993) Nature 366, 569-572. [DOI] [PubMed] [Google Scholar]
- 2.Pelham H. R. B. (1999) Exp. Cell Res. 247, 1-8. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda R., McNew, J. A., Weber, T., Parlati, F., Engel, T., Nickel, W., Rothman, W. & Söllner, T. H. (2000) Nature 407, 198-202. [DOI] [PubMed] [Google Scholar]
- 4.Parlati F., McNew, J. A., Fukuda, R., Miller, R., Söllner, T. H. & Rothman, J. E. (2000) Nature 407, 194-198. [DOI] [PubMed] [Google Scholar]
- 5.Mayer A. (1999) Curr. Opin. Cell Biol. 11, 447-452. [DOI] [PubMed] [Google Scholar]
- 6.Gerst J. E. (1999) Cell. Mol. Life Sci. 55, 707-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahn R. & Sudhof, T. C. (1999) Annu. Rev. Biochem. 68, 863-911. [DOI] [PubMed] [Google Scholar]
- 8.Sirover M. A. (1999) Biochim. Biophys. Acta 1432, 159-184. [DOI] [PubMed] [Google Scholar]
- 9.Turk J., Gross, R. W. & Ramanadham, S. (1993) Diabetes 42, 367-374. [DOI] [PubMed] [Google Scholar]
- 10.Oliet S. H. & Bourque, C. W. (1993) Nature 364, 341-343. [DOI] [PubMed] [Google Scholar]
- 11.Kelly R. B. (1993) Cell 72, 43-53. [DOI] [PubMed] [Google Scholar]
- 12.Weber T., Zemelman, B. V., McNew, J. A., Westermann, B., Gmachi, M., Parlati, F., Söllner, T. H. & Rothman, J. E. (1998) Cell 92, 759-772. [DOI] [PubMed] [Google Scholar]
- 13.Schekman R. (1992) Curr. Opin. Cell Biol. 4, 587-592. [DOI] [PubMed] [Google Scholar]
- 14.Söllner T. & Rothman, J. E. (1994) Trends Neurosci. 17, 344-348. [DOI] [PubMed] [Google Scholar]
- 15.DeBello W. M., O'Connor, V., Dresbach, T., Whiteheart, S. W., Wang, S. S. M., Schweizer, F. E., Betz, M., Rothman, J. E. & Augustine, G. J. (1995) Nature 373, 626-630. [DOI] [PubMed] [Google Scholar]
- 16.Avery J., Jahn, R. & Edwardson, J. M. (1999) Annu. Rev. Physiol. 61, 777-807. [DOI] [PubMed] [Google Scholar]
- 17.McNew J. A., Parlati, F., Fukuda, R., Johnston, R. J., Paz, K., Paumet, F., Söllner, T. H. & Rothman, J. E. (2000) Nature 407, 153-159. [DOI] [PubMed] [Google Scholar]
- 18.Glaser P. E. & Gross, R. W. (1995) Biochemistry 34, 12193-12203. [DOI] [PubMed] [Google Scholar]
- 19.Han X., Ramanadham, S., Turk, J. & Gross, R. W. (1998) Biochim. Biophys. Acta 1414, 95-107. [DOI] [PubMed] [Google Scholar]
- 20.Ramanadham S., Hsu, F., Bohrer, A., Nowatzke, W., Ma, Z. & Turk, J. (1998) Biochemistry 37, 4553-4567. [DOI] [PubMed] [Google Scholar]
- 21.Han X., Abendschein, D. R., Kelley, J. G. & Gross, R. W. (2000) Biochem. J. 352, 79-89. [PMC free article] [PubMed] [Google Scholar]
- 22.Glaser P. E. & Gross, R. W. (1994) Biochemistry 33, 5805-5812. [DOI] [PubMed] [Google Scholar]
- 23.Shelanski M. L., Gaskin, F. & Cantor, C. R. (1973) Proc. Natl. Acad. Sci. USA 70, 765-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbins A. R., Ward, R. D. & Oliver, C. (1995) J. Cell Biol. 130, 1093-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tisdale E. J. (2001) J. Biol. Chem. 276, 2480-2486. [DOI] [PubMed] [Google Scholar]
- 26.Tisdale E. J. (2002) J. Biol. Chem. 277, 3334-3341. [DOI] [PubMed] [Google Scholar]