Abstract
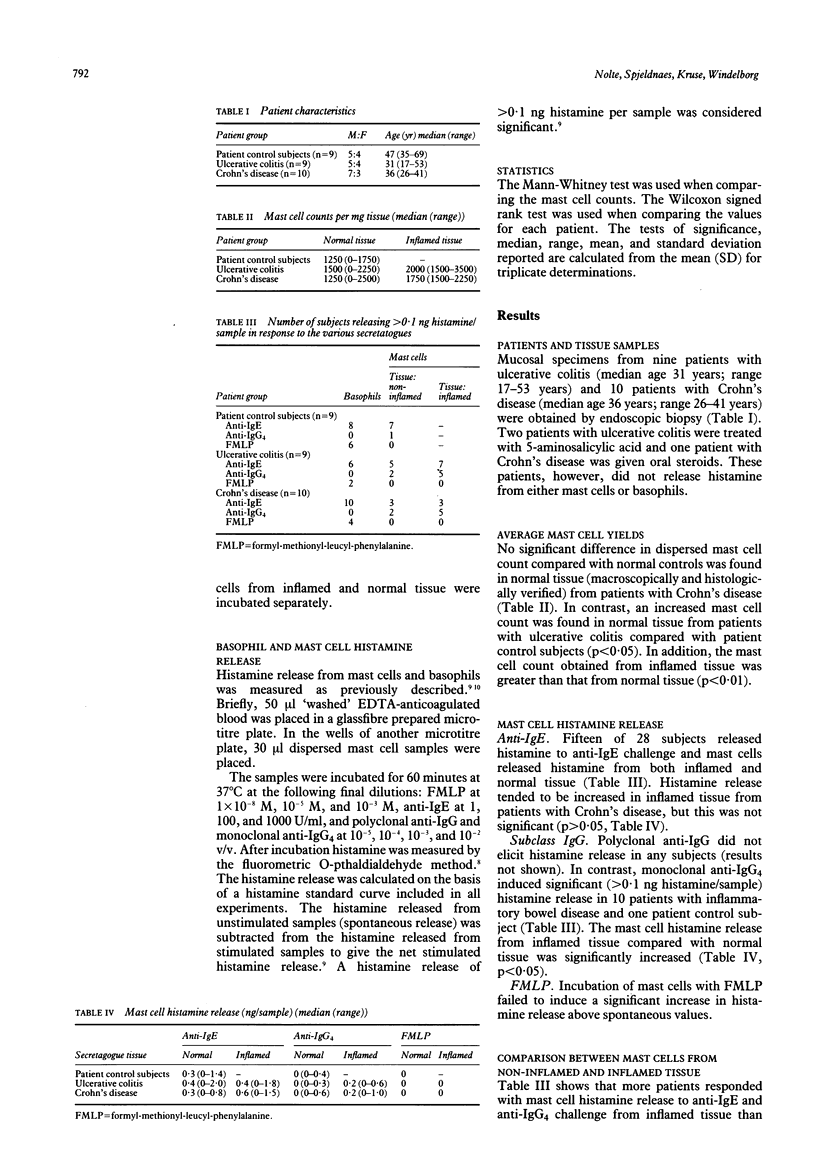
Inflammatory mediators from intestinal mast cells may serve as initiators of acute and delayed inflammation. Mast cell histamine release was measured in 19 patients with inflammatory bowel diseases using gut mast cells from enzymatically dispersed endoscopic forceps biopsy specimens of macroscopically inflamed and normal tissue. Mast cells and corresponding basophils were challenged with anti-IgE, anti-IgG, subclass anti-IgG4, and formyl-methionyl-leucyl-phenylalanine (FMLP) and results were compared with those from nine patient control subjects. The mast cell count in patients with ulcerative colitis was increased compared with that in control subjects and patients with Crohn's disease, and the mast cell count obtained from inflamed tissue was greater than that of normal tissue. The study also shows the heterogeneity of the responsiveness of the histamine releasing cells to various secretagogues. Thus, mast cells released 0.4 (0.0-2.0) (median (range)) ng histamine per sample at anti-IgE challenge, and basophils were also anti-IgE responsive. In contrast, mast cells did not respond to FMLP but the corresponding basophils did. Gut mast cells released 0.3 (0.0-1.0) (median (range)) ng histamine per sample at anti-IgG4 challenge; however, the corresponding basophils did not respond to anti-IgG4. In addition, the anti-IgG4 mediated histamine release was primarily confined to patients with inflammatory bowel disease. This study substantiates previous histopathological findings that mast cells may play a functional role in the inflammatory process of inflammatory bowel diseases and provides evidence for a possible role of subclass IgG4 as a reaginic antibody.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett K. E., Metcalfe D. D. The mucosal mast cell and its role in gastrointestinal allergic diseases. Clin Rev Allergy. 1984 Feb;2(1):39–53. doi: 10.1007/BF02991210. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Monahan R. A., Osage J. E., Dickersin G. R. Crohn's disease: transmission electron microscopic studies. II. Immunologic inflammatory response. Alterations of mast cells, basophils, eosinophils, and the microvasculature. Hum Pathol. 1980 Nov;11(6):606–619. doi: 10.1016/s0046-8177(80)80072-4. [DOI] [PubMed] [Google Scholar]
- Fox C. C., Kagey-Sobotka A., Schleimer R. P., Peters S. P., MacGlashan D. W., Jr, Lichtenstein L. M. Mediator release from human basophils and mast cells from lung and intestinal mucosa. Int Arch Allergy Appl Immunol. 1985;77(1-2):130–136. doi: 10.1159/000233767. [DOI] [PubMed] [Google Scholar]
- Fox C. C., Wolf E. J., Kagey-Sobotka A., Lichtenstein L. M. Comparison of human lung and intestinal mast cells. J Allergy Clin Immunol. 1988 Jan;81(1):89–94. doi: 10.1016/0091-6749(88)90225-4. [DOI] [PubMed] [Google Scholar]
- Kett K., Rognum T. O., Brandtzaeg P. Mucosal subclass distribution of immunoglobulin G-producing cells is different in ulcerative colitis and Crohn's disease of the colon. Gastroenterology. 1987 Nov;93(5):919–924. doi: 10.1016/0016-5085(87)90552-x. [DOI] [PubMed] [Google Scholar]
- Lemanske R. F., Jr, Atkins F. M., Metcalfe D. D. Gastrointestinal mast cells in health and disease. Part I. J Pediatr. 1983 Aug;103(2):177–184. doi: 10.1016/s0022-3476(83)80341-2. [DOI] [PubMed] [Google Scholar]
- Lloyd G., Green F. H., Fox H., Mani V., Turnberg L. A. Mast cells and immunoglobulin E in inflammatory bowel disease. Gut. 1975 Nov;16(11):861–865. doi: 10.1136/gut.16.11.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman M. A., Rees P. H., Benyon R. C., Church M. K. Human mast cell heterogeneity: histamine release from mast cells dispersed from skin, lung, adenoids, tonsils, and colon in response to IgE-dependent and nonimmunologic stimuli. J Allergy Clin Immunol. 1988 Mar;81(3):590–597. [PubMed] [Google Scholar]
- Nolte H., Kruse A., Stahl Skov P., Schiøtz P. O. Passive sensitization of human intestinal mast cells. Agents Actions. 1989 Apr;27(1-2):93–96. doi: 10.1007/BF02222208. [DOI] [PubMed] [Google Scholar]
- Nolte H., Schiøtz O., Skov P. S. A new glass microfibre-based histamine analysis for allergy testing in children. Results compared with conventional leukocyte histamine release assay, skin prick test, bronchial provocation test and RAST. Allergy. 1987 Jul;42(5):366–373. doi: 10.1111/j.1398-9995.1987.tb02223.x. [DOI] [PubMed] [Google Scholar]
- Nolte H., Schiøtz P. O., Kruse A., Stahl Skov P. Comparison of intestinal mast cell and basophil histamine release in children with food allergic reactions. Allergy. 1989 Nov;44(8):554–565. doi: 10.1111/j.1398-9995.1989.tb04200.x. [DOI] [PubMed] [Google Scholar]
- Nolte H., Stahl Skov P., Kruse A., Schiøtz P. O. Histamine release from dispersed human intestinal mast cells. A method using biopsies from children and adults. Allergy. 1989 Nov;44(8):543–553. doi: 10.1111/j.1398-9995.1989.tb04199.x. [DOI] [PubMed] [Google Scholar]
- O'Donoghue D. P., Kumar P. Rectal IgE cells in inflammatory bowel disease. Gut. 1979 Feb;20(2):149–153. doi: 10.1136/gut.20.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen L. K., Stahl Skov P., Mosbech H., Weeke B. Role of IgG4 in histamine release from human basophil leucocytes. I. Sensitization of cells from normal donors. Int Arch Allergy Appl Immunol. 1988;86(4):383–390. doi: 10.1159/000234623. [DOI] [PubMed] [Google Scholar]
- Stahl Skov P., Norn S., Weeke B. A new method for detecting histamine release. Agents Actions. 1984 Apr;14(3-4):414–416. doi: 10.1007/BF01973840. [DOI] [PubMed] [Google Scholar]
- Tharp M. D., Kagey-Sobotka A., Fox C. C., Marone G., Lichtenstein L. M., Sullivan T. J. Functional heterogeneity of human mast cells from different anatomic sites: in vitro responses to morphine sulfate. J Allergy Clin Immunol. 1987 Apr;79(4):646–653. doi: 10.1016/s0091-6749(87)80162-8. [DOI] [PubMed] [Google Scholar]


