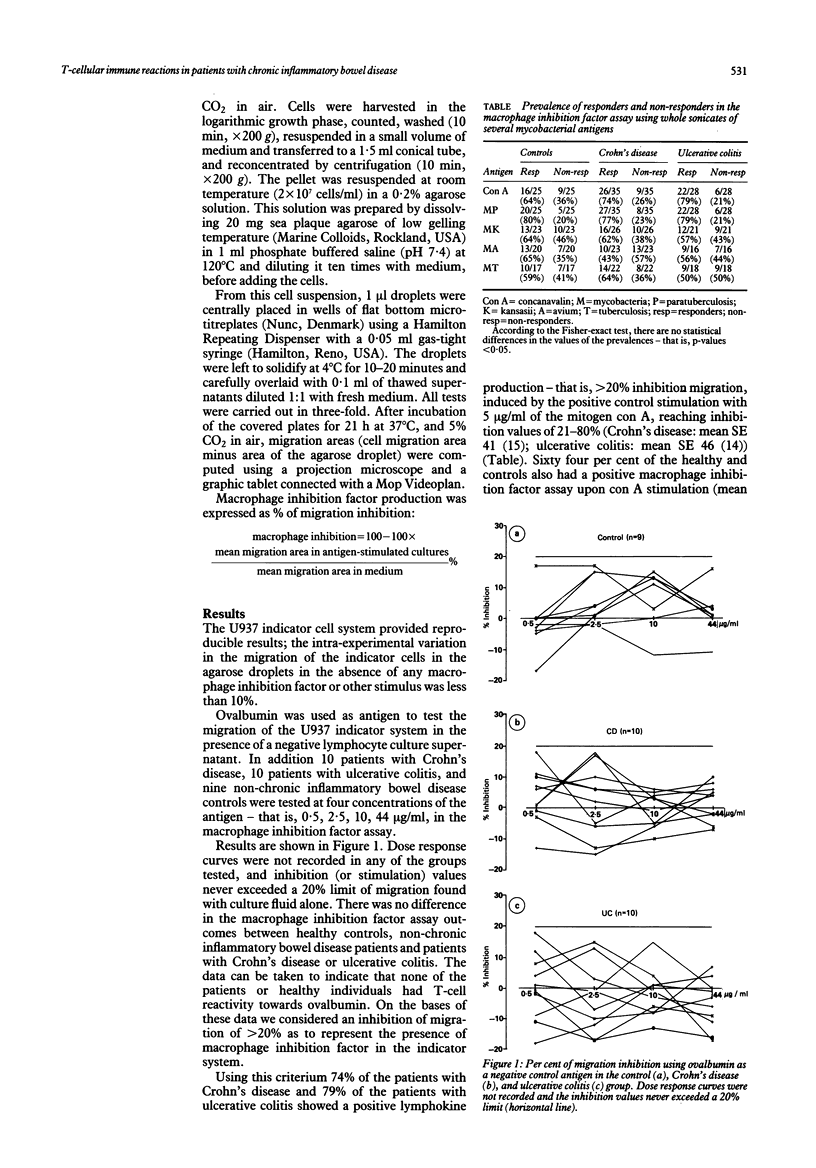
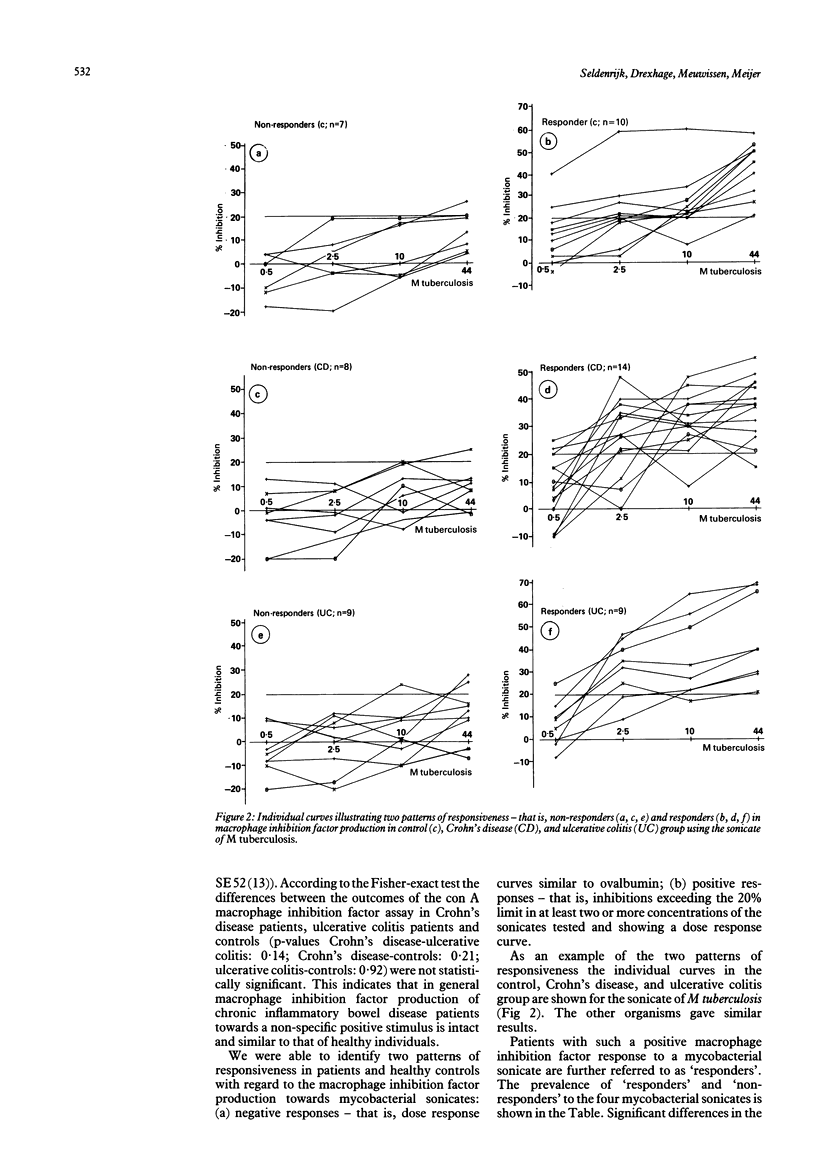
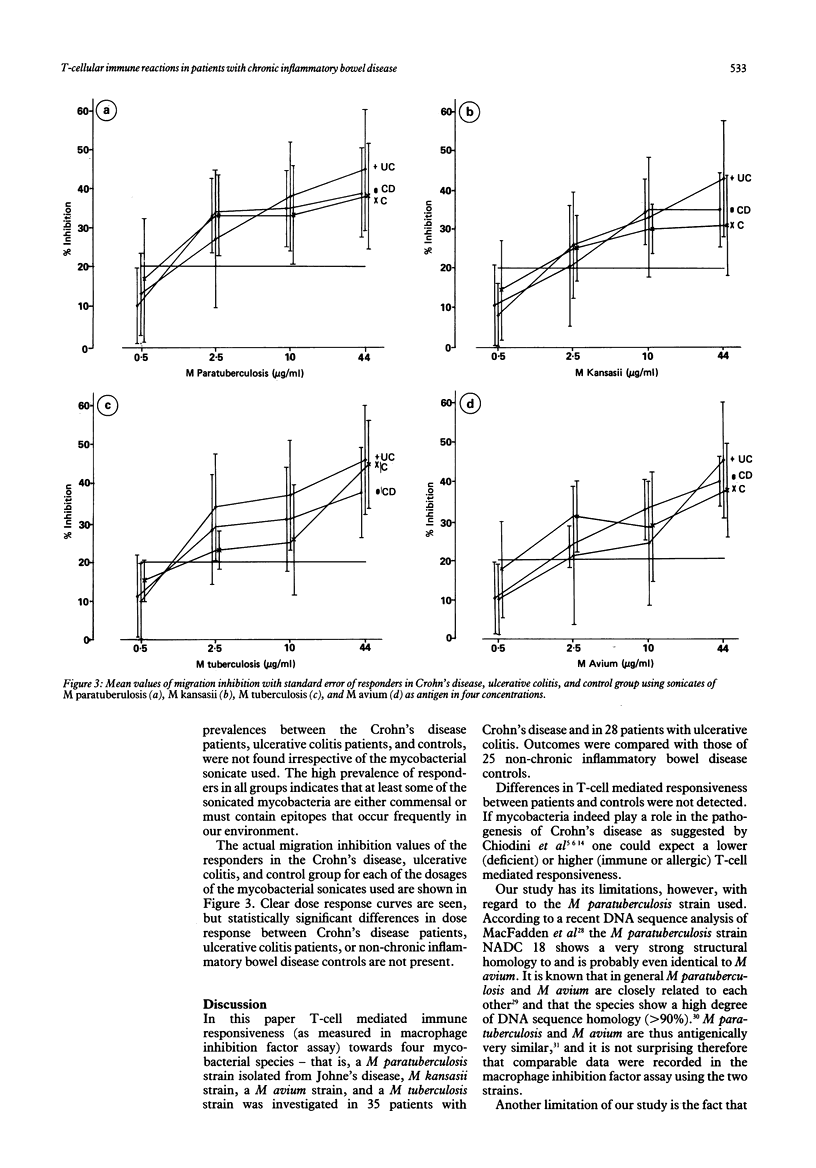
Abstract
A mycobacterial aetiology has been suggested for Crohn's disease. A slow growing mycobacterium, biochemically and genetically identical to M paratuberculosis, the causative agent of enteritis in ruminants (Johne's disease), has been isolated from gut specimens of patients affected by Crohn's disease. If M paratuberculosis or other mycobacteria play a role in the pathogenesis of Crohn's disease, then patients may have been sensitised to these mycobacteria or show an anergy immune reaction. We therefore investigated the T-cell mediated immune response to sonicates of M paratuberculosis, M kansasii, M avium, and M tuberculosis in 35 patients with Crohn's disease, 28 with ulcerative colitis, and 25 controls using a macrophage inhibition factor assay on peripheral blood lymphocytes. Two types of reaction patterns were identified--that is, 'responders' (subjects with a macrophage inhibition factor assay in which a dose response relation was present and a percentage of inhibition exceeding 20%), and 'non-responders'. There was no significant difference in the prevalence of responders (59%-80%) and non-responders (20%-41%) to these mycobacteria between the group of Crohn's disease, ulcerative colitis, and control group. We found also that a large proportion of controls showed T-cell immunisation to the mycobacteria which supports the contention that the antigens are practically commensal. Our results do not support the proposed involvement of mycobacteria in the pathogenesis of Crohn's disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baess I. Deoxyribonucleic acid relatedness among species of slowly-growing mycobacteria. Acta Pathol Microbiol Scand B. 1979 Aug;87(4):221–226. doi: 10.1111/j.1699-0463.1979.tb02430.x. [DOI] [PubMed] [Google Scholar]
- Belsheim M. R., Darwish R. Z., Watson W. C., Schieven B. Bacterial L-form isolation from inflammatory bowel disease patients. Gastroenterology. 1983 Aug;85(2):364–369. [PubMed] [Google Scholar]
- Burnham W. R., Lennard-Jones J. E., Stanford J. L., Bird R. G. Mycobacteria as a possible cause of inflammatory bowel disease. Lancet. 1978 Sep 30;2(8092 Pt 1):693–696. doi: 10.1016/s0140-6736(78)92699-5. [DOI] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Merkal R. S., Thayer W. R., Jr, Coutu J. A. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn's disease. J Clin Microbiol. 1984 Nov;20(5):966–971. doi: 10.1128/jcm.20.5.966-971.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Thayer W. R., Merkal R. S., Coutu J. A. Possible role of mycobacteria in inflammatory bowel disease. I. An unclassified Mycobacterium species isolated from patients with Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1073–1079. doi: 10.1007/BF01317078. [DOI] [PubMed] [Google Scholar]
- EDWARDS L. B., PALMER C. E. Isolation of "atypical" mycobacteria from healthy persons. Am Rev Respir Dis. 1959 Nov;80:747–749. doi: 10.1164/arrd.1959.80.5.747. [DOI] [PubMed] [Google Scholar]
- Elliott P. R., Lennard-Jones J. E., Burnham W. R., White S., Stanford J. L. Further data on skin testing with mycobacterial antigens in inflammatory bowel disease. Lancet. 1980 Aug 30;2(8192):483–484. doi: 10.1016/s0140-6736(80)91922-4. [DOI] [PubMed] [Google Scholar]
- Gitnick G. Is Crohn's disease a mycobacterial disease after all? Dig Dis Sci. 1984 Dec;29(12):1086–1088. doi: 10.1007/BF01317080. [DOI] [PubMed] [Google Scholar]
- Golde D. W. Aetiology of regional enteritis. Lancet. 1968 May 25;1(7552):1144–1145. doi: 10.1016/s0140-6736(68)90202-x. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Markesich D. C., Yoshimura H. H. Mycobacteria and inflammatory bowel disease. Results of culture. Gastroenterology. 1987 Feb;92(2):436–442. doi: 10.1016/0016-5085(87)90139-9. [DOI] [PubMed] [Google Scholar]
- Graybill J. R., Alford R. H. Variability of sequential studies of lymphocyte blastogenesis in normal adults. Clin Exp Immunol. 1976 Jul;25(1):28–35. [PMC free article] [PubMed] [Google Scholar]
- Hampson S. J., McFadden J. J., Hermon-Taylor J. Mycobacteria and Crohn's disease. Gut. 1988 Aug;29(8):1017–1019. doi: 10.1136/gut.29.8.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson S. J., Portaels F., Thompson J., Green E. P., Moss M. T., Hermon-Taylor J., McFadden J. J. DNA probes demonstrate a single highly conserved strain of Mycobacterium avium infecting AIDS patients. Lancet. 1989 Jan 14;1(8629):65–68. doi: 10.1016/s0140-6736(89)91427-x. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Klajman A., Drucker I., Lapidot Z., Yaretzky A., Frenkel A., van Eden W., Cohen I. R. T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet. 1986 Aug 9;2(8502):305–309. doi: 10.1016/s0140-6736(86)90003-6. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Mason U. G., 3rd, Farhi D. C., Iseman M. D. Disseminated infection with Mycobacterium avium-intracellulare. A report of 13 cases and a review of the literature. Medicine (Baltimore) 1985 Jan;64(1):36–48. doi: 10.1097/00005792-198501000-00003. [DOI] [PubMed] [Google Scholar]
- Klatser P. R., van Rens M. M., Eggelte T. A. Immunochemical characterization of Mycobacterium leprae antigens by the SDS-polyacrylamide gel electrophoresis immunoperoxidase technique (SGIP) using patients' sera. Clin Exp Immunol. 1984 Jun;56(3):537–544. [PMC free article] [PubMed] [Google Scholar]
- McFadden J. J., Butcher P. D., Chiodini R., Hermon-Taylor J. Crohn's disease-isolated mycobacteria are identical to Mycobacterium paratuberculosis, as determined by DNA probes that distinguish between mycobacterial species. J Clin Microbiol. 1987 May;25(5):796–801. doi: 10.1128/jcm.25.5.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden J. J., Butcher P. D., Thompson J., Chiodini R., Hermon-Taylor J. The use of DNA probes identifying restriction-fragment-length polymorphisms to examine the Mycobacterium avium complex. Mol Microbiol. 1987 Nov;1(3):283–291. doi: 10.1111/j.1365-2958.1987.tb01934.x. [DOI] [PubMed] [Google Scholar]
- McIntyre G., Stanford J. L. Immunodiffusion analysis shows that Mycobacterium paratuberculosis and other mycobactin-dependent mycobacteria are variants of Mycobacterium avium. J Appl Bacteriol. 1986 Oct;61(4):295–298. doi: 10.1111/j.1365-2672.1986.tb04290.x. [DOI] [PubMed] [Google Scholar]
- Merkal R. S., Kopecky K. E., Larsen A. B. Immunologic mechanisms in bovine paratuberculosis. Am J Vet Res. 1970 Mar;31(3):475–485. [PubMed] [Google Scholar]
- Mills C. C. Occurrence of Mycobacterium other than Mycobacterium tuberculosis in the oral cavity and in sputum. Appl Microbiol. 1972 Sep;24(3):307–310. doi: 10.1128/am.24.3.307-310.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K. L. Johne's and Crohn's. Chronic inflammatory bowel diseases of infectious aetiology? Lancet. 1987 May 2;1(8540):1017–1019. doi: 10.1016/s0140-6736(87)92280-x. [DOI] [PubMed] [Google Scholar]
- Parent K., Mitchell P. Cell wall-defective variants of pseudomonas-like (group Va) bacteria in Crohn's disease. Gastroenterology. 1978 Sep;75(3):368–372. [PubMed] [Google Scholar]
- Patterson D. S., Allen W. M. Chronic mycobacterial enteritis in ruminants as a model of Crohn's disease. Proc R Soc Med. 1972 Nov;65(11):998–1001. [PMC free article] [PubMed] [Google Scholar]
- Portaels F., Larsson L., Smeets P. Isolation of mycobacteria from healthy persons' stools. Int J Lepr Other Mycobact Dis. 1988 Sep;56(3):468–471. [PubMed] [Google Scholar]
- Thayer W. R., Jr, Coutu J. A., Chiodini R. J., Van Kruiningen H. J., Merkal R. S. Possible role of mycobacteria in inflammatory bowel disease. II. Mycobacterial antibodies in Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1080–1085. doi: 10.1007/BF01317079. [DOI] [PubMed] [Google Scholar]
- Thurman G. B., Stull H. B., Miller P. J., Stevenson H. C., Oldham R. K. Utilization of purified human monocytes in the agarose droplet assay for measuring migration inhibitory factors. J Immunol Methods. 1983 Dec 16;65(1-2):41–53. doi: 10.1016/0022-1759(83)90302-2. [DOI] [PubMed] [Google Scholar]
- Whorwell P. J., Davidson I. W., Beeken W. L., Wright R. Search by immunofluorescence for antigens of Rotavirus, Pseudomonas maltophilia, and Mycobacterium kansasii in Crohn's disease. Lancet. 1978 Sep 30;2(8092 Pt 1):697–698. doi: 10.1016/s0140-6736(78)92700-9. [DOI] [PubMed] [Google Scholar]
- Yoshimura H. H., Graham D. Y., Estes M. K., Merkal R. S. Investigation of association of mycobacteria with inflammatory bowel disease by nucleic acid hybridization. J Clin Microbiol. 1987 Jan;25(1):45–51. doi: 10.1128/jcm.25.1.45-51.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Plassche-Boers E. M., Drexhage H. A., Kokjé-Kleingeld M., Leezenberg H. A. Parameters of T cell mediated immunity to commensal micro-organisms in patients with chronic purulent rhinosinusitis: a comparison between delayed type hypersensitivity skin test, lymphocyte transformation test and macrophage migration inhibition factor assay. Clin Exp Immunol. 1986 Dec;66(3):516–524. [PMC free article] [PubMed] [Google Scholar]


