Abstract
A case of severe acute hepatitis caused by cyproterone acetate in a 71 year old man with prostatic carcinoma is reported with a review of the literature on hepatic reactions to this drug. The association between the use of cyproterone acetate and liver abnormalities is poorly documented. This is the fourth published report of adverse hepatic reaction to cyproterone acetate and it substantiates other evidence that cyproterone acetate is potentially hepatotoxic. Monitoring of liver function tests should be mandatory in patients receiving high doses of cyproterone acetate; the drug should be withdrawn immediately if abnormal liver function tests are found.
Full text
PDF
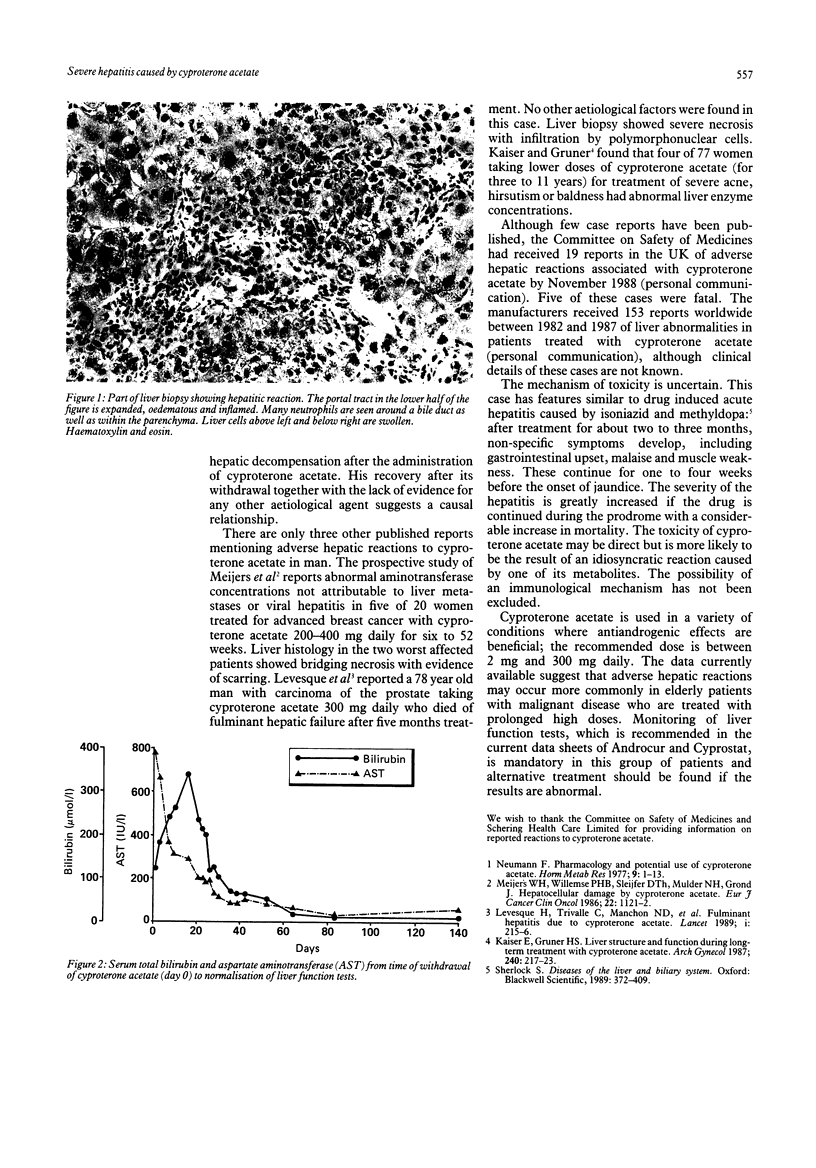
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kaiser E., Gruner H. S. Liver structure and function during long-term treatment with cyproterone acetate. Arch Gynecol. 1987;240(4):217–223. doi: 10.1007/BF02134071. [DOI] [PubMed] [Google Scholar]
- Lévesque H., Trivalle C., Manchon N. D., Vinel J. P., Moore N., Hémet J., Courtois H., Bercoff E., Bourreille J. Fulminant hepatitis due to cyproterone acetate. Lancet. 1989 Jan 28;1(8631):215–216. doi: 10.1016/s0140-6736(89)91225-7. [DOI] [PubMed] [Google Scholar]
- Meijers W. H., Willemse P. H., Sleijfer D. T., Mulder N. H., Grond J. Hepatocellular damage by cyproterone acetate. Eur J Cancer Clin Oncol. 1986 Sep;22(9):1121–1122. doi: 10.1016/0277-5379(86)90017-9. [DOI] [PubMed] [Google Scholar]
- Neumann F. Pharmacology and potential use of cyproterone acetate. Horm Metab Res. 1977 Jan;9(1):1–13. doi: 10.1055/s-0028-1093574. [DOI] [PubMed] [Google Scholar]



