Abstract
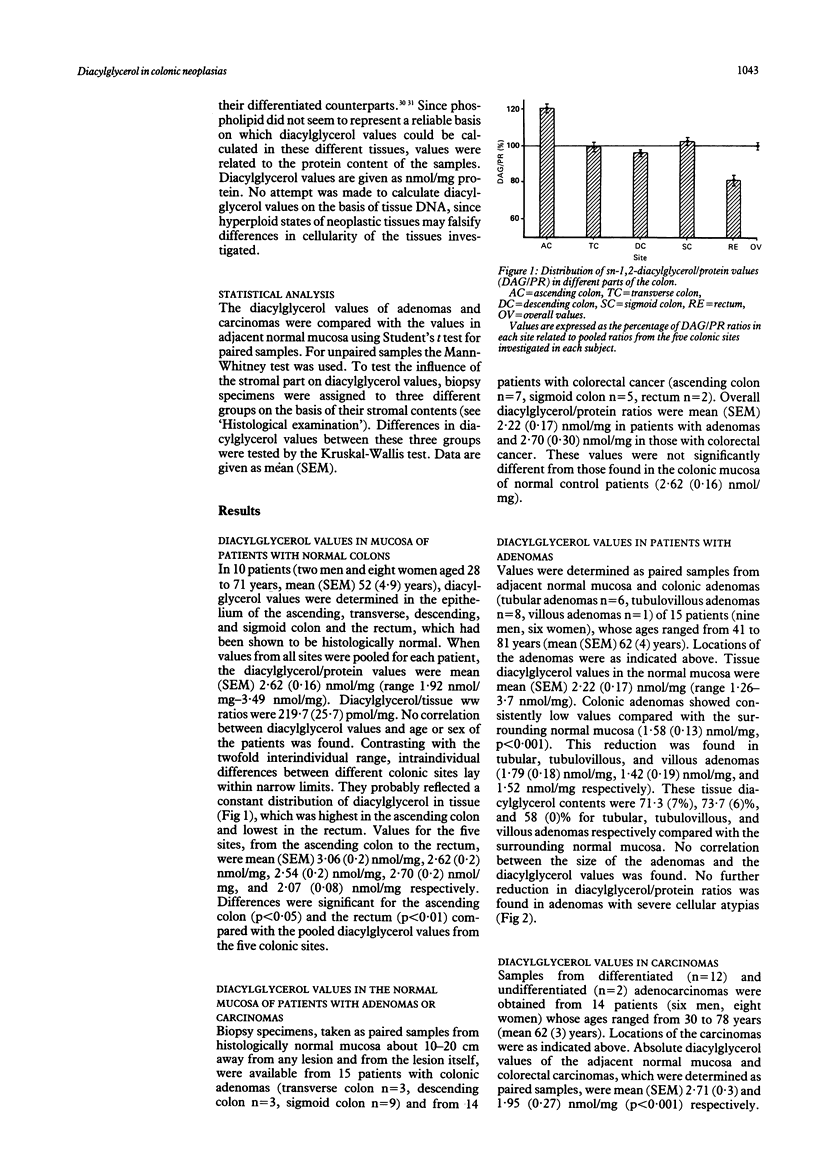
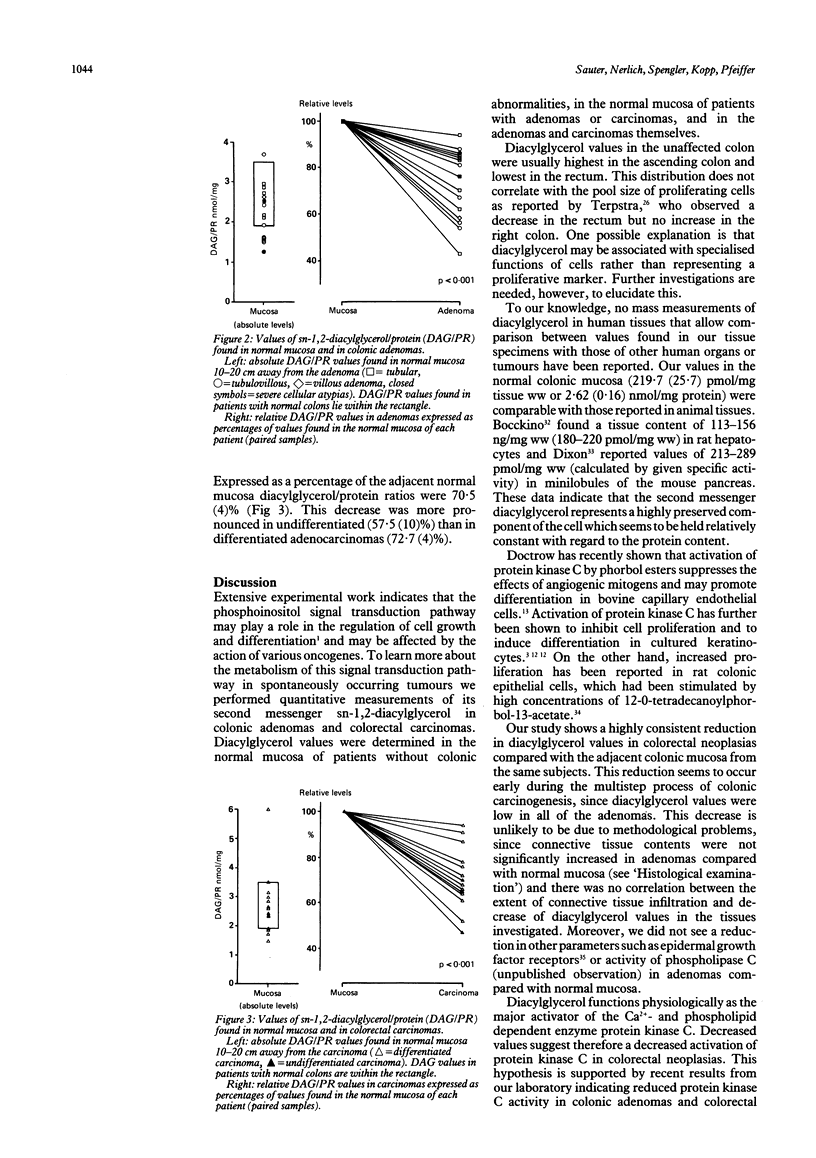
The biochemical events that make colonic epithelial cells proceed along the adenoma-carcinoma sequence are not well understood. The phosphoinositol signal transduction pathway is involved in the regulation of cell growth and differentiation. To determine its role in colonic neoplasias we performed mass measurements of its second messenger sn-1,2-diacylglycerol in biopsy specimens from normal mucosa and neoplasias of the colon. Normal colonic mucosa was also investigated in patients without colonic abnormalities (n = 10). Compared with pooled diacylglycerol values from five colonic sites (100%), values in patients with a normal colon were highest in the ascending colon (120 (5)%, p less than 0.05) and lowest in the rectum (81 (5)%, p less than 0.01). Absolute diacylglycerol values in patients with normal colons (2.62 (0.16) nmol/mg protein) were not significantly different from those found in the normal mucosa of patients with colorectal neoplasias (2.45 (0.17) nmol/mg protein). Both colonic adenomas (n = 15) and colorectal carcinomas (n = 14) showed significantly decreased diacylglycerol values compared with the adjacent normal mucosa of each patient (72 (4)%, p less than 0.001, and 71 (4)%, p less than 0.001 respectively). The appreciable decrease in mass diacylglycerol values clearly distinguishes adenomas and carcinomas of the colon from the surrounding normal mucosa. This finding suggests that profound metabolic changes of the phosphoinositol signal transduction pathway occur early in the adenoma-carcinoma sequence and may be important in colonic carcinogenesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bennett A., Civier A., Hensby C. N., Melhuish P. B., Stamford I. F. Measurement of arachidonate and its metabolites extracted from human normal and malignant gastrointestinal tissues. Gut. 1987 Mar;28(3):315–318. doi: 10.1136/gut.28.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Brown K. D., Irvine R. F., Heslop J. P. Phosphoinositides and cell proliferation. J Cell Sci Suppl. 1985;3:187–198. doi: 10.1242/jcs.1985.supplement_3.18. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M. Protein kinase C as the receptor for the phorbol ester tumor promoters: sixth Rhoads memorial award lecture. Cancer Res. 1988 Jan 1;48(1):1–8. [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Exton J. H. Stimulation of 1,2-diacylglycerol accumulation in hepatocytes by vasopressin, epinephrine, and angiotensin II. J Biol Chem. 1985 Nov 15;260(26):14201–14207. [PubMed] [Google Scholar]
- Chambard J. C., Paris S., L'Allemain G., Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987 Apr 23;326(6115):800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Subcellular distribution of protein kinase C in rat colonic epithelial cells with different proliferative activities. Cancer Res. 1987 Jul 1;47(13):3434–3438. [PubMed] [Google Scholar]
- Dixon J. F., Hokin L. E. Secretogogue-stimulated phosphatidylinositol breakdown in the exocrine pancreas liberates arachidonic acid, stearic acid, and glycerol by sequential actions of phospholipase C and diglyceride lipase. J Biol Chem. 1984 Dec 10;259(23):14418–14425. [PubMed] [Google Scholar]
- Doctrow S. R., Folkman J. Protein kinase C activators suppress stimulation of capillary endothelial cell growth by angiogenic endothelial mitogens. J Cell Biol. 1987 Mar;104(3):679–687. doi: 10.1083/jcb.104.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling J. G., Vandenbark G. R., Kuhn L. J., Ganong B. R., Bell R. M., Niedel J. E. Diacylglycerols mimic phorbol diester induction of leukemic cell differentiation. Proc Natl Acad Sci U S A. 1985 Feb;82(3):815–819. doi: 10.1073/pnas.82.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faletto D. L., Arrow A. S., Macara I. G. An early decrease in phosphatidylinositol turnover occurs on induction of Friend cell differentiation and precedes the decrease in c-myc expression. Cell. 1985 Nov;43(1):315–325. doi: 10.1016/0092-8674(85)90037-6. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Phorbol ester binding and activation of protein kinase C on triton X-100 mixed micelles containing phosphatidylserine. J Biol Chem. 1986 Jul 15;261(20):9341–9347. [PubMed] [Google Scholar]
- Hansson A., Serhan C. N., Haeggström J., Ingelman-Sundberg M., Samuelsson B. Activation of protein kinase C by lipoxin A and other eicosanoids. Intracellular action of oxygenation products of arachidonic acid. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1215–1222. doi: 10.1016/0006-291x(86)90380-3. [DOI] [PubMed] [Google Scholar]
- Huang C. L., Ives H. E. Growth inhibition by protein kinase C late in mitogenesis. 1987 Oct 29-Nov 4Nature. 329(6142):849–850. doi: 10.1038/329849a0. [DOI] [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Rettenmier C. W., Sherr C. J., Rock C. O. A guanine nucleotide-dependent phosphatidylinositol 4,5-diphosphate phospholipase C in cells transformed by the v-fms and v-fes oncogenes. J Biol Chem. 1986 Apr 15;261(11):4978–4985. [PubMed] [Google Scholar]
- Jaken S., Yuspa S. H. Early signals for keratinocyte differentiation: role of Ca2+-mediated inositol lipid metabolism in normal and neoplastic epidermal cells. Carcinogenesis. 1988 Jun;9(6):1033–1038. doi: 10.1093/carcin/9.6.1033. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Protein kinase C and calcium ion in mitogenic response of macrophage-depleted human peripheral lymphocytes. J Biol Chem. 1985 Feb 10;260(3):1366–1369. [PubMed] [Google Scholar]
- Krieg L., Kühlmann I., Marks F. Effect of tumor-promoting phorbol esters and of acetic acid on mechanisms controlling DNA synthesis and mitosis (Chalones) and on the biosynthesis of histidine-rich protein in mouse epidermis. Cancer Res. 1974 Nov;34(11):3135–3146. [PubMed] [Google Scholar]
- Luk G. D., Baylin S. B. Ornithine decarboxylase as a biologic marker in familial colonic polyposis. N Engl J Med. 1984 Jul 12;311(2):80–83. doi: 10.1056/NEJM198407123110202. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Deckmyn H., Ross T. S., Bross T. E., Ishii H., Bansal V. S., Wilson D. B. The metabolism of phosphoinositide-derived messenger molecules. Science. 1986 Dec 19;234(4783):1519–1526. doi: 10.1126/science.3024320. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Phorbol ester and diacylglycerol mimic growth factors in raising cytoplasmic pH. Nature. 1984 Nov 22;312(5992):371–374. doi: 10.1038/312371a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Rosoff P. M., Stein L. F., Cantley L. C. Phorbol esters induce differentiation in a pre-B-lymphocyte cell line by enhancing Na+/H+ exchange. J Biol Chem. 1984 Jun 10;259(11):7056–7060. [PubMed] [Google Scholar]
- Rothbauer E., Mann K., Wiebecke B., Borlinghaus P., Lamerz R., Pratschke E., Krämling H. J., Pfeiffer A. Epidermal growth factor receptors and epidermal growth factor-like activity in colorectal mucosa, adenomas and carcinomas. Klin Wochenschr. 1989 May 15;67(10):518–523. doi: 10.1007/BF01719776. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena A., Coombs M., Sinnett-Smith J. Diacylglycerol stimulates DNA synthesis and cell division in mouse 3T3 cells: role of Ca2+-sensitive phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5748–5752. doi: 10.1073/pnas.81.18.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra O. T., van Blankenstein M., Dees J., Eilers G. A. Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology. 1987 Mar;92(3):704–708. doi: 10.1016/0016-5085(87)90021-7. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Mannaerts G. P. Inorganic and organic phosphate measurements in the nanomolar range. Anal Biochem. 1987 Feb 15;161(1):45–48. doi: 10.1016/0003-2697(87)90649-x. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Sassone-Corsi P. Proto-oncogene fos: complex but versatile regulation. Cell. 1987 Nov 20;51(4):513–514. doi: 10.1016/0092-8674(87)90115-2. [DOI] [PubMed] [Google Scholar]
- Whitman M., Kaplan D. R., Schaffhausen B., Cantley L., Roberts T. M. Association of phosphatidylinositol kinase activity with polyoma middle-T competent for transformation. Nature. 1985 May 16;315(6016):239–242. doi: 10.1038/315239a0. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Ben T., Hennings H. The induction of epidermal transglutaminase and terminal differentiation by tumor promoters in cultured epidermal cells. Carcinogenesis. 1983 Nov;4(11):1413–1418. doi: 10.1093/carcin/4.11.1413. [DOI] [PubMed] [Google Scholar]
- Zwingelstein G., Tapiero H., Portoukalian J., Fourcade A. Changes in phospholipid and fatty acid composition in differentiated Friend leukaemic cells. Biochem Biophys Res Commun. 1981 Jan 30;98(2):349–358. doi: 10.1016/0006-291x(81)90847-0. [DOI] [PubMed] [Google Scholar]


