Abstract
The Hawaiian honeycreepers (Drepanidae) represent a superb illustration of evolutionary radiation, with a single colonization event giving rise to 19 extant and at least 10 extinct species [Curnutt, J. & Pimm, S. (2001) Stud. Avian Biol. 22, 15–30]. They also represent a dramatic example of anthropogenic extinction. Crop and pasture land has replaced their forest habitat, and human introductions of predators and diseases, particularly of mosquitoes and avian malaria, has eliminated them from the remaining low- and mid-elevation forests. Landscape analyses of three high-elevation forest refuges show that anthropogenic climate change is likely to combine with past land-use changes and biological invasions to drive several of the remaining species to extinction, especially on the islands of Kauai and Hawaii.
Fossil evidence shows that the Hawaiian Islands were once home to more than 100 endemic species and subspecies of land and water birds (1). The arrival of Polynesians and, subsequently, Europeans and other colonists ended the isolation that fostered the evolution of this diverse avifauna. Currently, 48 of the more than 100 original species are listed as extant; however, 11 of these species have not been seen in more than a decade and are probably extinct (2). To date, extinctions of the honeycreepers in particular have been driven largely by habitat loss, introduced predators, and diseases (3–6). Habitat loss began with Polynesian colonists, who cleared much of the low-elevation and seasonally dry forest for agricultural purposes (7). Europeans and other colonists introduced new agricultural technology and additional domesticated animals; the introduction of cattle and sheep in particular led to the development of pasturages in high-elevation forest that further reduced the amount of suitable habitat for native forest birds (8, 9). Today, the distribution of montane tropical rainforest is constrained by agriculture and urban development at low elevations, and pasture remains the major human land use at high elevations.
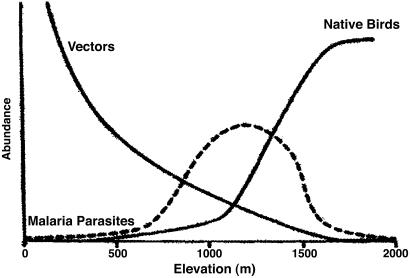
The Polynesians hunted some birds for food and feathers and also introduced rats and dogs that preyed on nesting birds. Later colonists introduced several additional predators, most notably mongooses, cats, and two additional species of rats. For forest-dwelling native birds, honeycreepers in particular, the accidental introduction of mosquitoes and avian pox, and more recently avian malaria, has had the greatest consequences. Field research in Hawaiian forests shows that native bird abundances, malarial parasite prevalence, and mosquito vector levels vary predictably along elevational gradients (ref. 5; Fig. 1). The introduced mosquito, Culex quinquefasciatus, is present in high numbers at lower elevations where many introduced birds and almost no native birds are found. Because the introduced birds are resistant to malaria, the prevalence of Plasmodium in avian populations from those elevations is low. Malaria prevalence increases significantly in mid-elevation forest, corresponding to the lowest elevations at which native birds are found currently, and here transmission of malaria to native birds is significant (10). At higher elevations, both Culex populations and prevalence of Plasmodium decline while native birds reach their peak abundance and diversity. These high-elevation forests are the last refuge for 10 species of endangered forest birds, 8 of which are honeycreepers: the Hawaii creeper (Oreomystis mana), palila (Loxioides bailleui), àkiapola àu (Hemignathus munroi), Hawaii àkepa (Loxops c. coccineus), and àlala (Corvus hawaiiensis) of the island of Hawaii, the àkohekohe (Palmeria dolei), parrotbill (Pseudonestor xanthophrys), and po òuli (Melamprosops phaeosoma) of Maui, and the puaiohi (Myadestes palmeri) and Kauai àkepa (Loxops c. caeruleirostris) of Kauai. Several other species remain federally listed as endangered, but there is little hope that any individuals will survive. Other native species remain abundant in these higher elevation forests, although some of them are declining as well.
Fig 1.
A generalized model of native bird abundances, malarial parasite incidence, and mosquito vector levels along an elevation gradient in Hawaii [Reproduced with permission from ref. 5 (Copyright 1986, Ecol. Soc. Am.)].
There is substantial evidence from studies of human as well as avian malaria that the development of Plasmodium parasites within mosquitoes is temperature-dependent and that there is a threshold temperature below which Plasmodium cannot develop to its infective stage (11, 12). In Hawaii, the threshold temperature for transmission of Plasmodium relictum has been estimated to be 13°C, whereas peak Plasmodium prevalence in wild mosquitoes occurs in mid-elevation forests where the mean ambient summer temperature is 17°C (10).
Could anthropogenic climate change exacerbate the effects of an introduced temperature-dependent disease, driving many of the remaining native birds over the brink to extinction? The key climatic features of Hawaiian montane forests are the northeast trade winds and the associated trade-wind inversion. The altitude of this inversion averages 1,900 m; above that altitude, humidity and precipitation decline rapidly, setting an upper limit to forest vegetation (13). Although the Hawaiian Islands are maritime, and so relatively well buffered climatically, palynological studies show that both local temperatures and the elevation of the trade-wind inversion have responded substantially to past climate changes (14–16). How will global climate change affect the regional and local climate in Hawaiian montane rainforests?
Although detailed predictions of regional climate remain uncertain, particularly in the tropics, recent studies that focused on montane forests of the Neotropics provide some evidence for a common climatic response under a warming scenario. Based on global climate simulations under elevated CO2 conditions, Still et al. (17) suggest that sea-surface warming in the Pacific will cause an intensification of the tropical hydrologic cycle, which in turn will cause the release of latent heat after condensation, warming the atmosphere. From these results, Still et al. (17) and Pounds et al. (18) suggest an increase in the lifting condensation level and the height of orographic clouds. These changes, termed the lifting cloud-base hypothesis, would increase both the lower and upper altitudinal limits of montane cloud forests (19). Changes consistent with this prediction have been observed in Central American montane forests, although an alternative mechanism, upwind deforestation of lowland forests, also could increase convective and orographic cloud bases (20).
In Hawaii, the upper treeline on Haleakala, Maui increased in elevation during the Holocene climatic optimum when postglacial radiative forcing was at its maximum (21). This observation is consistent with the lifting cloud-base hypothesis. Alternatively, Loope and Giambelluca (22) suggest that the El Niño phase of El Niño/Southern Oscillation could provide an analog for the local consequences of a warmer world. Under these conditions, higher temperatures are associated with high-elevation drought, which ultimately would depress the limits of cloud forest. In our analysis, we assumed that lifting cloud bases and increases in upper-elevation treeline will predominate. The alternative of upper-elevation drought and a depressed treeline would be far more damaging to the Hawaiian forests and to the honeycreepers that they support.
Methods
We evaluated the probable effects of climate change on the extent of forests with low risk for avian malaria for three Hawaiian Islands: Hawaii, Maui, and Kauai. Within each island we chose a specific area representative of the most intact forest and highest abundance of native birds: the Hakalau National Wildlife Refuge on the island of Hawaii, the Hanawi Forest on Maui, and the Alakai Swamp region on Kauai. Federal, state, and/or private owners manage all these areas as parks and/or preserves for conservation; all support substantial research on the endangered Hawaiian honeycreepers. We assumed that a 2°C increase in regional temperatures would occur by some time late in the next century, in keeping with climate-model predictions for the region. We further assumed that the trade-wind inversion would increase in altitude, bringing increased precipitation to high-elevation forests. Finally, we assumed that the rate of temperature change and of expansion in the transmission of malaria will be substantially more rapid than the rate of forest expansion above the current treeline.
Our analyses entailed projecting spatially the isotherms for the critical temperatures for Plasmodium development under both current conditions and a 2°C warming scenario. Using a 30-m U.S. Geological Survey digital elevation model for each island and a lapse rate of ≈6.5°C/km (23), we mapped the location of the 17 and 13°C isotherms as a vector data layer. The area below the 17°C isotherm (increasing temperature) is the region where malarial infection is certain, and above the 13°C isotherm (decreasing temperature) development of the parasite does not occur. Next, we overlaid the vector coverages of the refuge boundaries and temperature isotherms onto a false-color composite Satellite Pour l'Observation de la Terre (SPOT) satellite image collected in January of 1995. We then calculated the change in forest reserve area under the warmer climate scenario for the following three temperature zones: above 17°C, between 17 and 13°C, and below 13°C. These zones correspond to a high-risk zone for malaria infection (above 17°C), a transition zone where some transmission is possible but limited (between 17 and 13°C), and a low-risk zone (high-elevation forest at or below 13°C), respectively. Some malarial infections are present in these low-risk zones, but they may reflect mobility of the birds. Transmission, if it occurs at all in the uppermost temperature zone, is likely brief, episodic, and limited to periods of warm weather (ref. 24 and C.T.A., unpublished data).
Results and Discussion
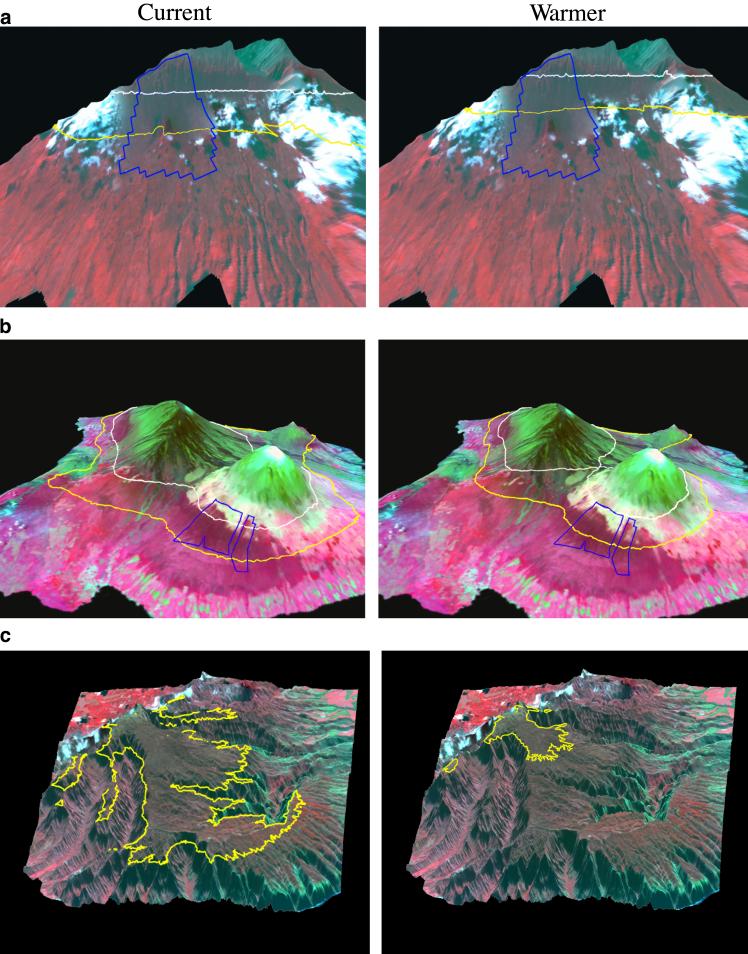
Results for the three areas are shown in Table 1 and Fig. 2. Hanawi Forest on the island of Maui (Fig. 2a) yields the most straightforward and hopeful result in response to the climate-change scenario. For this preserve, the area of forest with a low risk of malarial infection (below 13°C) is cut in half with 2°C of warming. Although a reduction of this magnitude is likely to affect endemic forest bird populations substantially, Hanawi represents the best-case example, because past land use practices did not include the clearing of high-elevation forests for pastures. In contrast, 2°C of warming nearly eliminates low-risk forest in Hakalau Wildlife Refuge (Table 1; Fig. 2b). The predominant land use above Hakalau Refuge is pasture land, which constrains the amount of forest available at higher elevations and could prevent migration of forests upslope. Restoration of high-elevation forests above the refuge is crucial to improving the chances for survival of the honeycreeper species, particularly the Hawaii àkepa, a cavity nester that requires large trees (25). Restoration efforts underway now at these higher elevations could significantly affect mature forest cover 100 years or so in the future.
Table 1.
Total forest area in hectares for each zone under current (before) and after 2°C warming scenario for each island refuge
| Zones
|
Hanawi (3,166 hectares) | Hakalau (12,999 hectares) | Alakai region (15,326 hectares) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Above 17°C | 1,266 | 1,995 | 650 | 5,200 | 0 | 12,937 |
| Between 17 and 13°C | 1,235 | 886 | 9,229 | 7,669 | 15,236 | 2,299 |
| Below 13°C | 665 | 285 | 3,120 | 130 | 0 | 0 |
Total refuge area is shown in parentheses; values are rounded to the nearest whole number.
Fig 2.
Projected changes in forest cover in relation to 17 (yellow) and 13°C (white) isotherms under current and 2°C warming conditions. Changes are shown for Hanawi Reserve (blue boundary) on the island of Maui (a), Hakalau Refuge (blue boundary) on Hawaii (b), and the Alakai swamp region on the island of Kauai (c). Images were created by using a 1995 Satellite Pour l'Observation de la Terre (SPOT) false-color composite image draped over 30-m digital elevation models for each island.
Finally, the island of Kauai offers the least hope for maintaining endemic honeycreepers in the face of malaria and climate change. The Alakai Swamp region on Kauai occupies the top of a plateau that has been eroding for millions of years as the island itself has been subsiding. It presently supports no forest above the 13°C isotherm, and no area is free of malaria. The area above the 17°C isotherm is indicative of transition forest, where transmission is limited and the prevalence of Plasmodium in mosquitoes is tied to episodic warming events (ref. 24 and C.T.A., unpublished data). Under the warming scenario, this isotherm shifts upward ≈300 m in elevation, corresponding to an 85% reduction in transition forest area (Table 1; Fig. 2c). On this island, prevention of the disease in the remaining populations must become the main conservation focus.
Conclusion
There have been a number of analyses of species range shifts in response to climate change, retrospectively using glacial and interglacial records of climate (26, 27), and currently and prospectively evaluating human-caused changes in climate (28–30). Some of the latter studies note that other human-caused changes could interact with climate change to make it more difficult for species to shift their ranges in response to climate perturbations (31). Our analysis clearly illustrates this point in that interactions of climate change with land-use change and biological invasions exacerbate the direct effects of climate change substantially.
Acknowledgments
We thank Hawaii Volcanoes National Park and the U.S. Fish and Wildlife Service for providing logistical and technical support. We thank Erin Bohensky and David Saah for assistance with the geographic information system analysis, and Mary Cadenasso, Gretchen Daily, Jack Ewel, and S. T. A. Pickett for providing comments on a previous version of this manuscript.
References
- 1.Olson S. L. & James, H. F. (1982) Science 217, 633-635. [DOI] [PubMed] [Google Scholar]
- 2.Jacobi J. D. & Atkinson, C. T. (1995) in Our Living Resources: A Report to the Nation on the Distribution, Abundance, and Health of U.S. Plants, Animals, and Ecosystems, eds. La Roe, E. T., Farris, G. S., Puckett, C. E., Doran, P. D. & Mac, M. J. (U.S. Dept. of Interior, Natl. Biol. Service, Washington, DC), pp. 376–381.
- 3.Warner R. E. (1968) Condor 70, 101-120. [Google Scholar]
- 4.Scott J. M., Mountainspring, S., Ramsey, F. L. & Kepler, C. B. (1986) Stud. Avian Biol. 9, 1-431. [Google Scholar]
- 5.van Riper C., van Riper, S. G., Goff, M. L. & Laird, M. (1986) Ecol. Monogr. 56, 327-344. [Google Scholar]
- 6.Atkinson C. T., Woods, K. L., Dusek, R. J., Sileo, L. S. & Iko, W. M. (1995) Parasitology 111, 559-569. [DOI] [PubMed] [Google Scholar]
- 7.Christensen C. C. & Kirch, P. V. (1986) Occas. Pap. B. P. Bishop Mus. 26, 52-80. [Google Scholar]
- 8.Cuddihy L. W. & Stone, C. P., (1990) Alteration of Native Hawaiian Vegetation: Effects of Humans, Their Activities, and Introductions (Coop. Natl. Park Res. Stud. Unit, Univ. of Hawaii, Honolulu), pp. 1–138.
- 9.van Riper C. & Scott, J. M. (2001) Stud. Avian Biol. 22, 221-233. [Google Scholar]
- 10.LaPointe D. A., (2000) Dissertation (Univ. of Hawaii, Manoa).
- 11.Lindsay S. W. & Martens, W. J. M. (1998) Bull. W. H. O. 76, 33-45. [PMC free article] [PubMed] [Google Scholar]
- 12.Patz J. A. & Reisen, W. K. (2001) Trends Immunol. 22, 171-172. [DOI] [PubMed] [Google Scholar]
- 13.Juvik S. P. & Juvik, J. O., (1998) Atlas of Hawaii (Univ. of Hawaii Press, Honolulu).
- 14.Gavenda R. T. (1992) Pac. Sci. 46, 295-307. [Google Scholar]
- 15.Hotchkiss S. C., (1998) Dissertation (Univ. of Minnesota, Minneapolis).
- 16.Hotchkiss S. C. & Juvik, J. O. (1999) Quat. Res. 52, 115-128. [Google Scholar]
- 17.Still C. J., Foster, P. N. & Schneider, S. (1999) Nature 398, 608-610. [Google Scholar]
- 18.Pounds A. J., Fogden, M. P. & Campbell, J. H. (1999) Nature 398, 611-614. [Google Scholar]
- 19.Pounds A. J., Fogden, M. P. & Campbell, J. H. (1997) in Meeting Report, Bird Life International/WWF Workshop on the Impacts of Climate Change on Flora and Fauna, ed. Briggs, B. (R. Soc. Prot. Birds, Bedfordshire, U.K.).
- 20.Lawton R. O., Nair, U. S., Pielke, R. A., Sr. & Welch, R. M. (2001) Science 294, 584-587. [DOI] [PubMed] [Google Scholar]
- 21.Burney D. A., DeCandido, R. V., Burney, L. P., Kostel-Hughes, F. N., Stafford, T. W. & James, H. F. (1995) J. Paleolimnol. 13, 209-217. [Google Scholar]
- 22.Loope L. L. & Giambelluca, T. W. (1998) Clim. Change 39, 503-517. [Google Scholar]
- 23.Juvik J. O. & Nullet, D. A (1994) J. Appl. Meteorol. 33, 1304-1312. [Google Scholar]
- 24.Feldman R. A., Freed, L. A. & Cann, R. L. (1995) Mol. Ecol. 4, 663-673. [DOI] [PubMed] [Google Scholar]
- 25.Freed L. A., Telecky, T. M., Tyler, W. A., III & Kjargaard, M. A. (1987) `Elepaio 47, 79-81. [Google Scholar]
- 26.Nowak C. L., Nowak, R. S., Tausch, R. J. & Wigand, P. E (1994) Am. J. Bot. 81, 265-277. [Google Scholar]
- 27.Davis M. B. & Shaw, R. G. (2001) Science 292, 673-679. [DOI] [PubMed] [Google Scholar]
- 28.Parmesan C. (1996) Nature 382, 765-766. [Google Scholar]
- 29.Wayne P. M., Reckie, E. G. & Bazazz, F. A. (1998) Oecologia 114, 335-342. [DOI] [PubMed] [Google Scholar]
- 30.Sagarin R. D., Barry, J. P., Gilman, S. E. & Baxter, C.H. (1999) Ecol. Monogr. 69, 465-490. [Google Scholar]
- 31.Gryj E. (1998) in Conservation Biology, eds. Fiedler, P. L. & Kareiva, P. M. (Chapman & Hall, New York), pp. 478–496.