Abstract
One of the enduring problems in the study of social evolution has been to understand how cooperation can be maintained in the presence of freeloaders, individuals that take advantage of the more cooperative members of groups they are eager to join. The freeloader problem has been particularly troublesome when groups consist of nonrelatives, and no inclusive fitness benefits accrue to individuals that contribute more heavily to communal activities. These theoretical difficulties, however, are not mirrored by the numerous examples of cooperative or even altruistic behaviors exhibited by groups of nonrelatives in nature (e.g., many human groups, communally nesting bees, multiple queen-founding ants, cellular slime molds, and social bacteria). Using a model in which cooperation and grouping tendencies are modeled as coevolving dynamical variables, I show that the freeloader problem can be addressed when group-size effects on fitness are considered explicitly. I show that freeloaders, whose presence is reflected in the development of linkage disequilibrium between grouping and cooperation, increase in frequency when rare, but are selected against when common due to the reduced productivity of the groups they overburden with their presence. Freeloader frequencies thus periodically rise and fall around an equilibrium shown here to be dynamic. These results highlight the importance of group-level effects in the origin and maintenance of sociality, illustrate the dynamic nature of equilibria when multiple levels of selection are involved, and provide a solution to the freeloaders paradox.
Keywords: group size, freerider, linkage disequilibrium, multilevel selection, complexity
Early reviews of the evolution of social behavior (1, 2) clearly distinguished between two distinct aspects of sociality: the formation of groups and the evolution of social behavior within groups. Although these traits must be linked inextricably in nature, evolutionary biologists and ecologists have largely treated them separately in their models. On the one hand, kin selection (e.g., refs. 3–7) and game-theory models (e.g., refs. 8–10) have considered the evolution of altruistic or mutualistic interactions among a fixed number of interactors (typically pairs or n individuals). Models for the evolution of group size such as those of Giraldeau and Caraco (11) and Higashi and Yamamura (12), on the other hand, have considered the formation of groups given fixed costs and benefits of group living, with some unspecified level of cooperation presumably being responsible for at least part of the benefits. There has been some crossfertilization between these two traditions in that game-theory models have considered the effects of group size on equilibrium levels of cooperation (e.g., ref. 9) and models for the formation of groups have incorporated inclusive fitness arguments to explore the effects of relatedness on equilibrium group sizes (e.g., ref. 11). There has been little exploration, however, of grouping and cooperation as coevolving dynamical variables.
Here I present results of a model in which grouping and cooperation, coded as separate polygenic traits, coevolve as groups of variable size emerge from the interaction of these traits with each other and with fixed ecological and demographic parameters. Elsewhere (13), I use this model to explore the effects of ecology and demography on equilibrium levels of cooperation, grouping tendencies, and group size. Here I describe the patterns of association among these traits and what these patterns tell us about the mechanisms that may allow costly cooperation to evolve in groups of nonrelatives. In particular, I demonstrate the development of linkage disequilibrium of periodically changing sign between grouping and cooperation and present a solution to the problem of how cooperation is maintained in the presence of freeloaders that does not depend on kinship, assortative associations (14), or mechanisms to enforce cooperation such as policing (e.g., ref. 15) or punishment (e.g., ref. 16). The computer model used in the simulations is described in detail in ref. 13. Here I present an abbreviated description of the model and expand on aspects of the genetic and breeding systems relevant to the results presented here.
The Model
Let us envision a simple social system in which individuals who may not be related to one another come together in one-generation breeding associations, as occurs in some colonial breeding birds (17), communally nesting bees (18), pleometrotic ants (19), certain male coalitions in birds (20), and mammals (21), tree-killing bark beetles (22), cellular slime molds (23, 24), or social bacteria (25). Because of behaviors individuals perform in such associations, group members may enjoy certain benefits such as increased feeding success, access to resources that are unavailable to solitary individuals, increased predator protection, ability to outcompete conspecifics, or ability to escape harsh environmental conditions (1, 2). These benefits are expected to be large enough to compensate, up to a point, for inevitable costs of group living such as competition for local resources or increased parasite loads.
In the model, individuals are endowed with genomes in which grouping and cooperation are coded as separate polygenic traits subject to mutation and recombination (see below). Individuals associate with each other as a function of their grouping tendencies but help one another once within groups as a function of their cooperative tendencies. Associations endure for one reproductive period, after which the offspring produced within the groups join a global pool from which they disperse to initiate a new cycle of group formation. Grouping and cooperation evolve in the simulations along with the average group size and group and global population dynamics.
In the version of the model considered here, relatedness plays no role in group formation. Groups form by accretion, with the probability that a new member will join a group being a function only of the grouping tendencies of the prospective joiner, gj, and of the current number of group members, n, and their average grouping tendencies, g, according to the function
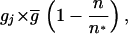 |
where n* is the expected stable group size at the time of group formation, i.e., the size at which additional members would cause the average fitness in the groups to drop below 1 due to overcrowding (derived by setting the function in Eq. 2, below, to 1, with γ, the cooperation parameter, corresponding to the global average; see refs. 13 and 26 for details). Whenever n ≥ n*, the group acceptance probability is zero. Note that because n* is a function of an evolving cooperation parameter, it also evolves in the simulations.
Once within groups, individuals help one another as a function of their cooperative tendencies. Helping behavior, which may range from mutualistic to altruistic (see below), causes some components of fitness to be an increasing function of group size. This is modeled by adding a positive density-dependent factor to a simple population growth model,
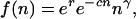 |
where n is the number of group members, r is an intrinsic rate of growth parameter, c is the inverse of a group carrying capacity parameter, and γ, taken to range between 0 and 1, represents the average cooperative tendencies of a group's members (see ref. 26 for a full justification and analysis of this function). The three factors in Eq. 2 represent, in order of appearance, (i) the reproductive output of individuals in the absence of cooperative or competitive interactions, (ii) the negative effects of crowding and competition due to limited resources available to a group, and (iii) the synergistic effects of cooperation. By “cooperation” is meant any behaviors (joint resource acquisition, information exchange, communal brood care, predator defense, etc.) that despite any individual costs have a net beneficial effect on group members (see ref. 10 for a similar definition of cooperation). Whenever γ > 0, the per capita rate of growth (or average individual fitness) is largest at intermediate group sizes. In the simulations, r and c are fixed parameters of a run while γ evolves.
I allow for the possibility that cooperators may suffer a relative fitness cost within their groups by calculating the reproductive output of particular individuals within a group with the function
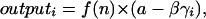 |
where f(n) is Eq. 2, and a − βγi is a within-group relative fitness factor, a linearly decreasing function of individual i's cooperative tendencies. The intercept of this factor, a, is calculated such that the average cooperator within a group will have a relative fitness of 1.0 (i.e., a = 1 + β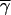 , where
, where 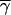 are the average cooperative tendencies of the group's members; for details, see ref. 13); β, the slope of the factor, determines the extent to which cooperation carries a relative fitness cost. When β = 0, cooperation does not carry a relative fitness cost, and the interactions are said to be mutualistic. As β increases in magnitude, the relative fitness of individuals with lower than average cooperative tendencies is more than 1.0, that of the average cooperator is 1.0, and that of those with greater than average cooperative tendencies is less than 1.0. In this case, the interactions are said to be altruistic, because individuals that help more give up a portion of their fitness to benefit others in their group (for similar definitions of altruism see refs. 27 and 28).
are the average cooperative tendencies of the group's members; for details, see ref. 13); β, the slope of the factor, determines the extent to which cooperation carries a relative fitness cost. When β = 0, cooperation does not carry a relative fitness cost, and the interactions are said to be mutualistic. As β increases in magnitude, the relative fitness of individuals with lower than average cooperative tendencies is more than 1.0, that of the average cooperator is 1.0, and that of those with greater than average cooperative tendencies is less than 1.0. In this case, the interactions are said to be altruistic, because individuals that help more give up a portion of their fitness to benefit others in their group (for similar definitions of altruism see refs. 27 and 28).
Individuals are diploid, with grouping and cooperative tendencies each coded by 15 loci with either a “1” or “0” allele at each locus and a mutation rate of 10−2 per locus per generation. Phenotypic values representing grouping or cooperative tendencies were calculated as the proportion of 1s in the diploid complement corresponding to each trait. In simulations with sexual reproduction, I implemented a meiosis-like procedure that allowed for recombination involving variable levels of random assortment of loci within and among traits. In models with outcrossing, individuals mated before group formation with randomly chosen individuals from the global population. For simplicity, no separate sexes were assumed. All offspring in a clutch shared the same two parents.
In the simulations reported here I assume that the global population size is limited by a maximum number of sites available for group formation. A parallel set of simulations in which there was no limit to the number of groups but the global population size was limited by a global negative density-dependent factor yielded qualitatively similar results (13).
Simulations and Analyses
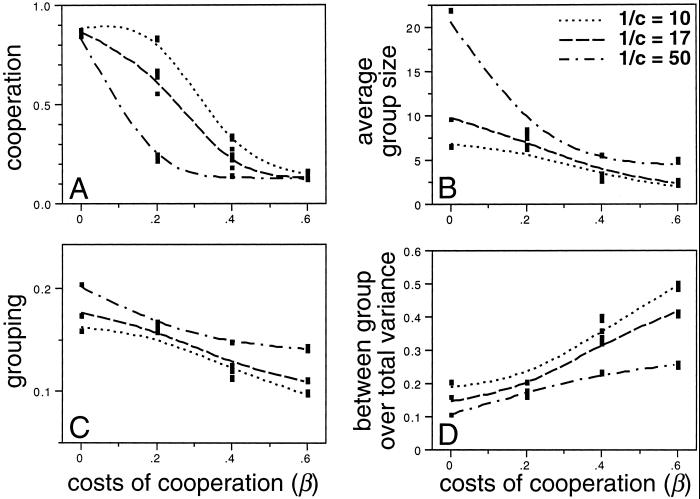
Elsewhere (13) I systematically explore the effects of the group carrying capacity (1/c, Eq. 2), intrinsic rate of growth (r, Eq. 2), and relative fitness costs of cooperation (β, Eq. 3) on equilibrium levels of cooperation, grouping tendencies, and group size. Here I briefly summarize these findings and consider a subset of parameter values to illustrate properties representative of equilibrium conditions. For this subset of parameter values, I systematically explored a range of breeding systems and linkage patterns, because they were relevant to the results reported here. These systems included parthenogenesis, selfing, partial outcrossing, and complete outcrossing. In the case of complete outcrossing, I considered patterns of full to no linkage of loci within and between traits. Results shown in Fig. 1 were obtained with complete outcrossing and no linkage; those shown in Figs. 2–4 were obtained with complete outcrossing and full linkage. Any significant differences among breeding systems and linkage patterns are discussed in the text.
Fig 1.
Equilibrium levels of cooperation (A), the average group size (B), grouping tendencies (C), and the between-group/total genetic variance (D) as a function of the relative fitness costs of cooperation (β = 0.0, 0.2, 0.4, or 0.6) and group carrying-capacity (1/c = 10, 17, or 50) parameters. The simulations were run for a maximum of 200 groups (limited nesting sites model; ref. 13) and an intrinsic rate of growth of r = 2.0. Nearly identical results were obtained for r = 0.5, 1.0, and 1.5 (13). The equilibrium values shown are averages over the last 500 generations of simulations run for 2,500 generations. Curves are cubic spline fits (λ = 0.0001 for cooperation and 0.001 for the others) across four replicates of each combination of parameter values.
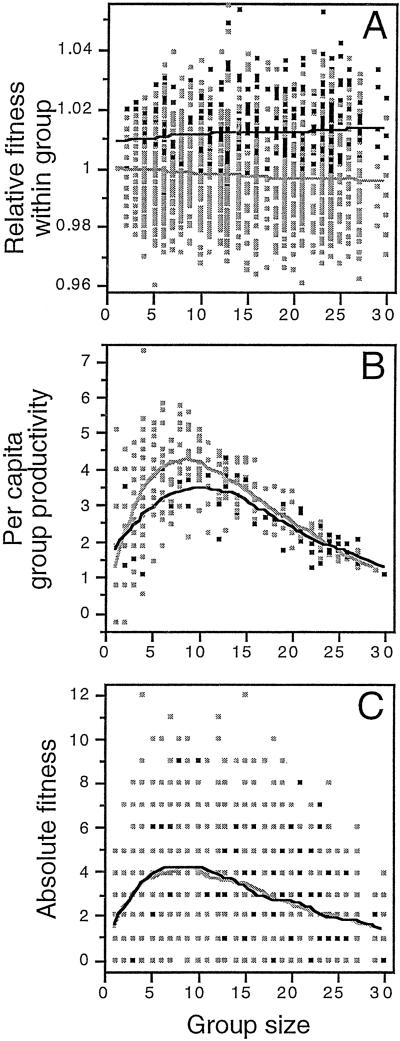
Fig 2.
A potential solution to the freeloaders paradox. (A) Relative fitness within groups for freeloaders (dark dots and line) and other group members [light dots and line; F(1,3439) = 304.9, P < 0.0001; whole-model R2 = 0.21, with freeloader, group size, and group size-freeloader interaction in the model]. (B) Difference in the per capita group productivity of groups with greater than average freeloader frequencies, termed “loaded” (dark dots and line) vs. other groups in the population (light dots and line) [F(1, 494) = 72.7, P < 0.0006; whole-model R2 = 0.42, with “loaded,” group size, squared group size, and the interactions of the latter two with “loaded” in the model]. (C) Absolute fitness of freeloaders and other group members after within- and between-group level effects are accounted for (ordinal logistic Wald χ2 = 0.92, P = 0.34; whole-model R2 = 0.04, with freeloader, group size, squared group size, and their interactions with freeloader in the model). Data obtained are from a simulation run for β = 0.2, r = 0.5, c = 0.1 and 500 groups. Curves are cubic spline fits of the data, with λ = 1,000 (A) or 100 (B and C).
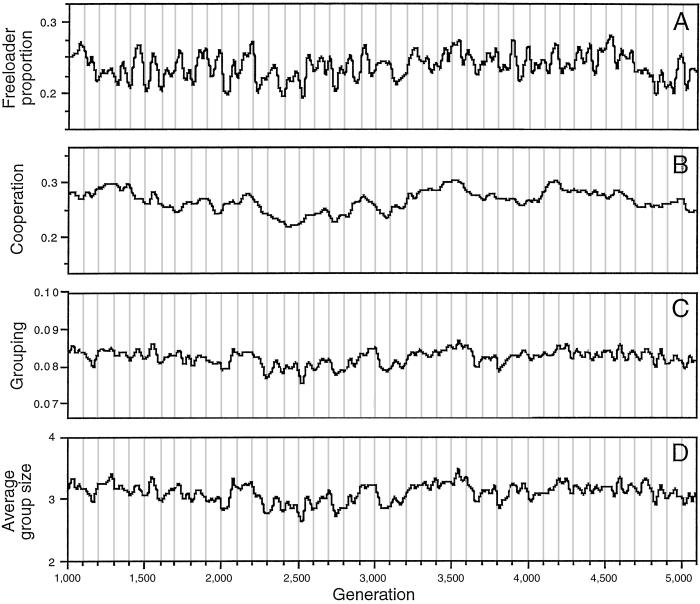
Fig 4.
Time series showing oscillations in the proportion of freeloaders, cooperative and grouping tendencies, and the average group size in a simulation run for β = 0.6, r = 2.0, c = 0.1 and 500 groups. The lines shown are cubic spline fits, with flexibility parameter λ = 10,000, of the original data. Fourier analyses show that the oscillations had intrinsic periodicity and were not the result of white noise [Fisher's κ was 100.0, P ≪ 0.00001 (A), 618.0, P ≪ 0.00001 (B), 254.3, P ≪ 0.00001 (C), and 182.4, P ≪ 0.00001 (D)].
Simulations were initiated with individuals who, except for new mutations, lacked grouping and cooperative tendencies. Equilibrium conditions, evidenced by stable values of the evolving variables, were usually reached by generation 1,000. The simulations were allowed to proceed for another 1,500 generations, the last 500 of which were used to calculate reported equilibrium values. Four replicates with different random-number seeds were run for each combination of parameter values. Additionally, a subset of simulations was run for 5,096 generations to obtain sufficiently long time series for Fourier analysis (29). Significant values of the Fisher's κ statistic allow us to reject the null hypothesis that fluctuations in the series are due to white noise (30).
I recorded average cooperative and grouping tendencies, group size, and the proportion of freeloaders in the global population for each cycle of group formation (for a definition of freeloaders, see below). To test for fitness differences between individuals or groups with different cooperative tendencies, I recorded the reproductive output of all individuals and groups in representative generations at equilibrium. An individual's relative fitness within a group was the ratio of its reproductive output to the output of the average cooperator in the group. I used the analysis of variance to test for within-group relative fitness differences between freeloaders and other group members or to test for productivity differences between groups with greater than average freeloader frequencies vs. other groups in the population. Because individuals produced a discrete number of offspring, I used ordinal logistic regression to test for differences in the absolute fitness between freeloaders and other group members. In all cases, I used group size (relative fitness analyses) or group size and group size square (group productivity and absolute fitness tests) as covariates.
Results and Discussion
I found that from an initially solitary state groups formed and cooperation evolved under a broad range of parameter values (Fig. 1; see also ref. 13) even when cooperators suffered a relative fitness cost within their groups and thus behaved altruistically. Because in the model (and by definition) cooperation enhances the per capita group productivity (Eq. 2), it is not surprising that groups, grouping, and cooperation readily developed when cooperation did not carry a relative fitness cost, i.e., the interactions were mutualistic. More interesting is the fact that, albeit to lower levels, groups, grouping, and cooperation still developed even in the presence of considerable relative fitness costs of cooperation. This result is of particular interest for three reasons. First, the groups consisted of nonrelatives, and thus no inclusive fitness benefits due to kinship accrued to cooperators. Second, because the decision to join or be accepted in a group was independent of an individual's cooperative tendencies, there were no direct mechanisms to ensure that cooperators were associated with each other (see ref. 14 for a model in which such a mechanism is present). Finally, there were no mechanisms such as policing (e.g., ref. 15) or punishment (e.g., ref. 16) that may have served to enforce cooperation. I examine below how, even under these circumstances, groups formed and costly altruistic behaviors within the groups evolved.
The first point to note is that genetic variance for cooperation was maintained in the simulations, and thus cheaters (individuals with lower cooperative tendencies than the global average) and freeloaders (individuals with lower cooperative and higher grouping tendencies than the global average) were present throughout the simulations, including under equilibrium conditions. The question is then: What prevented freeloaders and cheaters from taking over the populations, in particular when cooperators suffered a relative fitness cost within their groups? Fig. 2 presents a potential solution to this dilemma for the more interesting case of freeloaders (essentially, those cheaters with greater grouping tendencies than the global average, a justified subcategory given the development of genetic associations between grouping and cooperation discussed below). As expected, because of their lower cooperative tendencies, freeloaders enjoyed a fitness advantage within their groups whenever cooperation was costly (Fig. 2A). However, the within-group advantage of freeloaders was countered by the lower productivity of the groups they overburdened with their presence (Fig. 2B). At equilibrium, these within- and between-group level effects cancelled each other out, resulting in indistinguishable absolute fitnesses for freeloaders and other group members (Fig. 2C).
I found that the detrimental effect that freeloaders had on group productivity had two sources. First, freeloaders lowered the group productivity curve by cooperating to a lesser extent or failing to cooperate altogether (Fig. 2B). Second, because of their greater grouping tendencies, freeloaders increased the size of the groups they joined, causing them to grow beyond the optimum group size (Fig. 2B). Because of the nonlinear relationship between individual fitness and group size (Eq. 2 and Fig. 2B), therefore, differences in group productivity had an effect not only on cooperative tendencies but also on grouping tendencies.
A parallel argument applied to the more general category of cheaters (as defined above) except that, unlike freeloaders who were represented more frequently in larger groups because of their greater grouping tendencies (logistic regression χ2 = 86.4, P < 0.0001 on data set shown in Fig. 2), cheaters were represented equally in groups of all sizes (χ2 = 0.23, P = 0.63; same data set as shown in Fig. 2). Cheaters, therefore, only affected group productivity by lowering the per capita rate of growth curve (data not shown) but not by making groups too large.
Perhaps the most interesting result that emerged from the simulations was the development of linkage disequilibrium of periodically changing sign between grouping and cooperation, reflected in the progressive build-up of positive and then negative associations between the two variables at the level of group averages (Fig. 3) and individual genomes (correlation coefficients across individuals belonging to the three generations shown in Fig. 3 A–C, respectively, were as follows: −0.08, P = 0.0016; +0.016, P = 0.55; and +0.17, P < 0.00001). As evidence of the genetic nature of these associations, the slope of the association between the two variables attained greater values the more conducive were the breeding system and linkage pattern for the development of linkage disequilibrium. Simulations with selfing, for instance, resulted in slopes steeper than −1 and + 1 (data not shown). Only under complete outcrossing and random assortment of loci did grouping and cooperation fail to become associated.
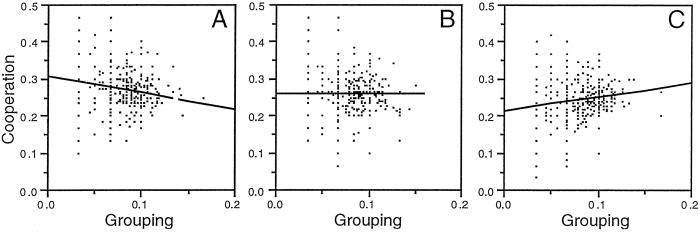
Fig 3.
An example of the periodic change in the sign of the association between cooperative and grouping tendencies for a case with complete outcrossing and full linkage. Each dot represents the average cooperative tendencies of a group as a function of its grouping tendencies. (A) When freeloaders were common, the relationship between the two variables was negative [slope (±standard error) = −0.44 ± 0.08, F(1,498) = 27.8, P < 0.0001]. (B) Because freeloaders were being purged from the population, the relationship disappeared [slope = 0.006 ± 0.08, F(1,498) = 0.006, P = 0.94]. (C) Because freeloaders became rarer, the relationship became positive [slope = 0.38 ± 0.07, F(1,498) = 24.9, P < 0.0001]. Data shown correspond to generations 2,189 (A), 2,221 (B), and 2,268 (C) of the simulation shown in Fig. 4. As discussed in the text, the group-level associations shown reflect linkage disequilibrium at the level of individual genomes.
The changing sign of the association between grouping and cooperation was apparently a reflection of the fact that freeloader frequencies did not remain constant through time but rather oscillated around their equilibrium value (Fig. 4A). Fourier analyses showed that the oscillations in the proportion of freeloaders (Fig. 4A), as well as of the slope of the association between grouping and cooperation, had intrinsic periodicity and were not the result of white noise (Fisher's κ = 62.8, P ≪ 0.00001, for the slope time series, data not shown). Similar oscillations, also with intrinsic periodicity, characterized the cooperation, grouping tendencies, and average group-size series (Fig. 4 B–D). It would be important to investigate the nature of the time lag that may be responsible for these oscillations, the apparent correspondence among the time series (Fig. 4), and how the amplitude and period of the oscillations may be affected by different factors. Such topics, however, are beyond the scope of this paper.
The oscillations of the various variables and the changing sign in the association between cooperation and grouping tendencies are consistent with a scenario in which within- and between-group frequency-dependent selection would be responsible for the maintenance of cooperation at a dynamic equilibrium. When common (Fig. 3A), freeloaders would suffer from the low productivity (Fig. 2B) of the groups they overburden with their presence and thus would be partially purged from the population. When rare (Fig. 3C), however, freeloaders would take advantage (Fig. 2A) of the more numerous cooperators within their groups without having as large an impact on group productivity. Freeloader frequencies then would rise again. The observed oscillations in freeloader frequencies thus would be direct but independent consequences of the lower cooperative and higher grouping tendencies of freeloaders, in interaction with a nonlinear fitness function.
A final point to note is that greater average group sizes had a negative effect on equilibrium levels of cooperation, as evidenced by the lower cooperative tendencies that evolved when greater group-carrying capacities allowed the formation of larger groups (Fig. 1 A and B; see also ref. 13). This effect reflects a change in the amount of genetic variance present within and among groups (Fig. 1D), which in turn would influence the strength of selection at the group and individual level (4, 14, 31–34). In the simulations, for a given relative fitness cost of cooperation, the between-group component of the total variance decreased as the group carrying capacity and, thus, group size increased (Fig. 1D). This result points to a different aspect of the model that may help explain the evolution of costly altruistic behaviors in groups of nonrelatives—the coevolution between grouping tendencies and group size on the one hand and cooperation on the other. In the simulations, as the costs of cooperation increased, not only lower levels of cooperation but also lower grouping tendencies and smaller groups evolved (Fig. 1). With smaller groups, the between-group component of the total variance increased (Fig. 1D). This effect, in turn, should have affected the balance of selection at the two levels, thus allowing the evolution of greater levels of cooperation than might have been possible had group size remained fixed.
The stability of cooperation given the possibility of freeloading and cheating, in particular when the groups consist of nonrelatives, has long been a concern of evolutionary biologists (8, 10, 35, 36). The results here presented suggest that the negative effect that freeloaders have on group productivity (both by failing to contribute to communal activities and by making groups too large) should be sufficient to maintain cooperation under a broad range of realistic conditions even among nonrelatives and even in the presence of relatively steep fitness costs of cooperation. I would argue, therefore, that mechanisms such as policing or punishment, shown to be of relevance in the evolution of cooperation (15, 16), are not necessary for its maintenance. Their presence, however, could serve to temper the relatively wide-amplitude oscillations (Fig. 4 A–C) that apparently would be involved in maintaining cooperation through frequency-dependent selection mechanisms as those discussed here. Policing and punishment thus might arise eventually but only to reinforce processes already in place.
Acknowledgments
P. Abbot and A. Cutter assisted in coding the model. For comments on the manuscript I thank A. Cutter, A. Joshi, W. Maddison, A. Planavski, J. Pepper, and K. S. Powers. This work was supported by National Science Foundation Grant DEB-9815938 (to L.A.) and completed while L.A. was a fellow at the Wissenschaftskolleg zu Berlin (Institute for Advanced Studies, Berlin).
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Alexander R. D. (1974) Annu. Rev. Ecol. Syst. 5, 325-383. [Google Scholar]
- 2.Wilson E. O., (1975) Sociobiology: The New Synthesis (Belknap Press of Harvard Univ. Press, Cambridge, MA).
- 3.Hamilton W. D. (1964) J. Theor. Biol. 7, 1-16. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton W. D. (1975) in Biosocial Anthropology, ed. Fox, R. (Wiley, New York), pp. 133–155.
- 5.Wade M. J. (1980) Science 210, 665-667. [DOI] [PubMed] [Google Scholar]
- 6.Queller D. C. (1992) Evolution (Lawrence, Kans.) 46, 376-380. [DOI] [PubMed] [Google Scholar]
- 7.Kelly J. K. (1994) Theor. Popul. Biol. 46, 32-57. [DOI] [PubMed] [Google Scholar]
- 8.Axelrod R. & Hamilton, W. D. (1981) Science 211, 1390-1396. [DOI] [PubMed] [Google Scholar]
- 9.Boyd R. & Richerson, P. J. (1988) J. Theor. Biol. 132, 337-356. [DOI] [PubMed] [Google Scholar]
- 10.Mesterton-Gibbons M. & Dugatkin, L. A. (1992) Q. Rev. Biol. 67, 267-281. [Google Scholar]
- 11.Giraldeau L.-A. & Caraco, T. (1993) Evol. Ecol. 7, 429-438. [Google Scholar]
- 12.Higashi M. & Yamamura, N. (1993) Am. Nat. 142, 553-563. [Google Scholar]
- 13.Avilés L., Abbot, P. & Cutter, A. D. (2002) Am. Nat. 159, 115-127. [DOI] [PubMed] [Google Scholar]
- 14.Wilson D. S. & Dugatkin, L. A. (1997) Am. Nat. 149, 336-351. [Google Scholar]
- 15.Frank S. A. (1995) Nature 377, 4294-4295. [DOI] [PubMed] [Google Scholar]
- 16.Boyd R. & Richerson, P. J. (1992) Ethol. Sociobiol. 13, 171-195. [Google Scholar]
- 17.Brown C. R. & Brown, M. B., (1996) Coloniality in the Cliff Swallow: The Effect of Group Size on Social Behavior (Univ. of Chicago Press, Chicago).
- 18.Kukuk P. F., Ward, S. A. & Jozwiak, A. (1998) Naturwissenschaften 85, 445-449. [Google Scholar]
- 19.Bernasconi G. & Strassmann, J. E. (1999) Trends Ecol. Evol. 14, 477-482. [DOI] [PubMed] [Google Scholar]
- 20.Faaborg J., Parker, P. G., Delay, L., De Vries, T., Bednarz, J. C., Paz, S. M., Naranjo, J. & Waite, T. A. (1995) Behav. Ecol. Sociobiol. 36, 83-90. [Google Scholar]
- 21.Packer C., Gilbert, D. A., Pusey, A. E. & Obrien, S. J. (1991) Nature 351, 562-565. [Google Scholar]
- 22.Raffa K. F. & Berryman, A. A. (1987) Am. Nat. 129, 234-262. [Google Scholar]
- 23.Bonner J. T. (1982) Am. Nat. 119, 530-552. [Google Scholar]
- 24.Strassmann J. E., Zhu, Y. & Queller, D. C. (2000) Nature 408, 965-967. [DOI] [PubMed] [Google Scholar]
- 25.Velicer G. J., Kroos, L. & Lenski, R. E. (2000) Nature 404, 598-601. [DOI] [PubMed] [Google Scholar]
- 26.Avilés L. (1999) Evol. Ecol. Res. 1, 459-477. [Google Scholar]
- 27.Uyenoyama M. & Feldman, M. W. (1980) Theor. Popul. Biol. 17, 380-414. [DOI] [PubMed] [Google Scholar]
- 28.Wilson D. S. (1990) Oikos 59, 135-140. [Google Scholar]
- 29.Otnes R. K. & Enochson, L., (1978) Applied Time Series Analysis (Wiley, New York).
- 30.Sall J., Lehman, A. & Creighton, L., (2001) JMP, Statistical Discovery Software (SAS Inst., Pacific Grove, CA), Version 4.
- 31.Price G. R. (1972) Ann. Hum. Genet. 35, 485-490. [DOI] [PubMed] [Google Scholar]
- 32.Wade M. J. (1985) Am. Nat. 125, 61-73. [Google Scholar]
- 33.Pepper J. W. & Smuts, B. B. (1999) in Dynamics in Human and Primate Societies, eds. Kohler, T. & Gumerman, G. (Oxford Univ. Press, Oxford), pp. 45–76.
- 34.Wilson D. S. (2001) in Evolutionary Ecology: Concepts and Case Studies, eds. Fox, C. W., Roff, D. A. & Fairbairn, D. J. (Oxford Univ. Press, Oxford), pp. 222–231.
- 35.Trivers R. L. (1971) Q. Rev. Biol. 46, 35-57. [Google Scholar]
- 36.Connor R. C. (1995) Trends Ecol. Evol. 10, 84-86. [DOI] [PubMed] [Google Scholar]