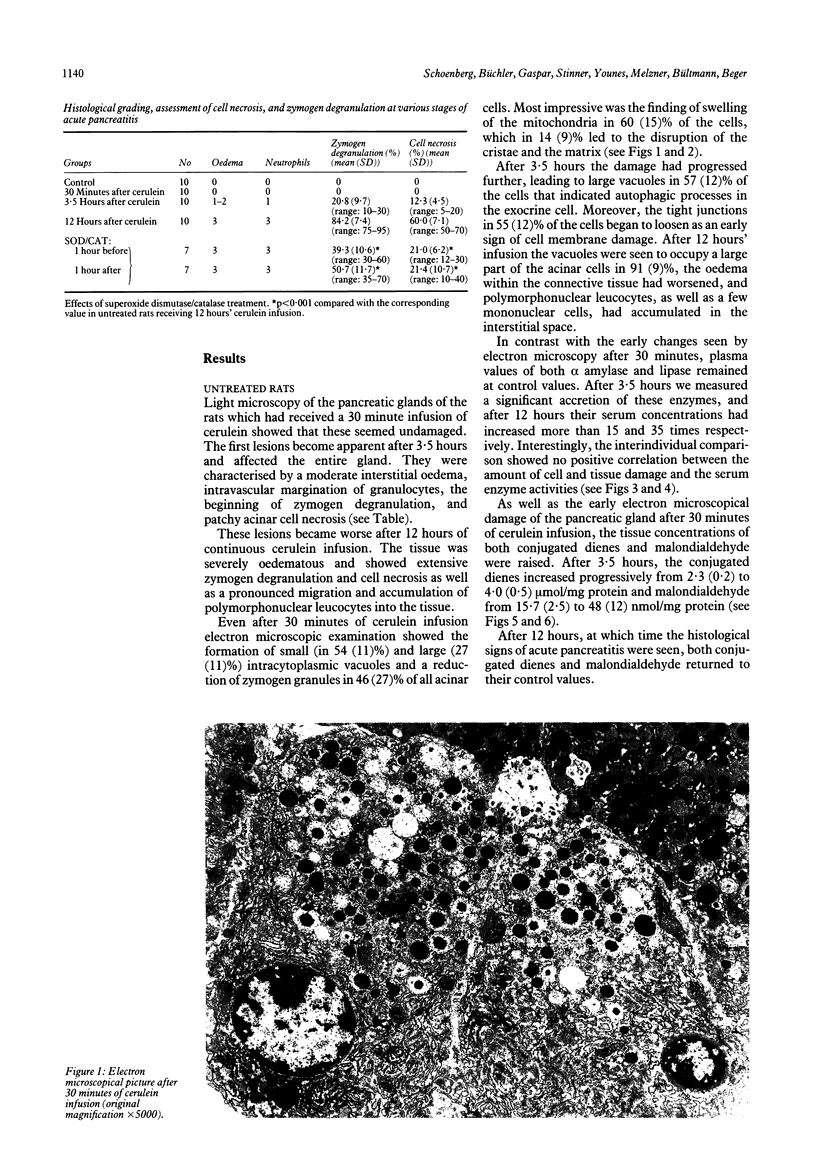
Abstract
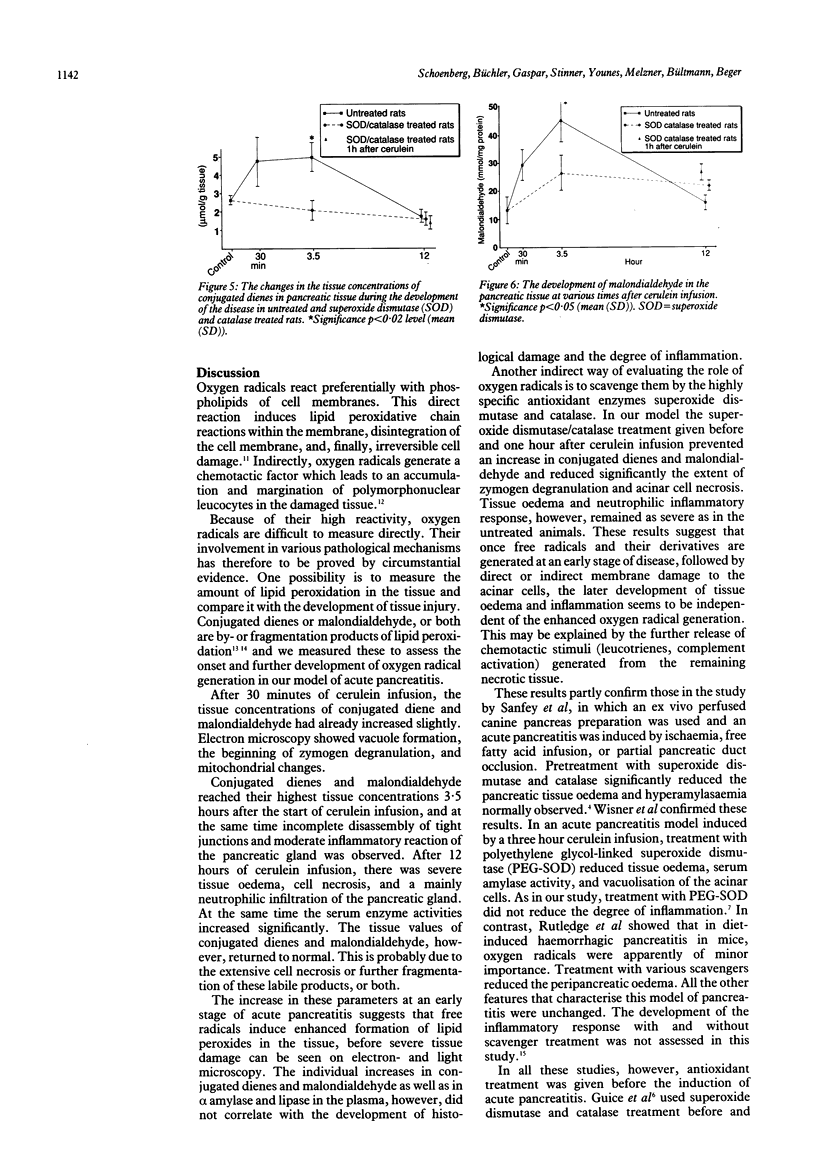
This study aimed to assess the role of oxygen free radicals in acute pancreatitis. Acute pancreatitis was induced in rats by infusion of the CCK-analogue cerulein (5 micrograms/kg per hour) for 30 minutes, 3.5 hours, and 12 hours. After the infusion, serum enzymes and conjugated tissue dienes and malondialdehyde were measured and tissue samples were subjected to electron and light microscopy. Electron microscopy after 30 minutes showed moderate intracellular alterations. After 3.5 hours of cerulein infusion interstitial oedema and intravascular margination of granulocytes in the pancreatic gland were seen. After 12 hours histological evaluation showed pronounced zymogen degranulation, extensive tissue necrosis, and migration of granulocytes into the tissue. Amylase and lipase activities increased 15 and 35-fold respectively during this time. After 30 minutes of cerulein infusion conjugated dienes and malondialdehyde increased, they reached their peak after 3.5 hours and decreased to normal values after 12 hours. Treatment with superoxide dismutase (100,000 U/kg/hour) and catalase (400,000 U/kg/hour) either before or after the start of the cerulein infusion prevented lipid peroxidation and reduced zymogen degranulation and tissue necrosis. Tissue oedema and inflammatory response, however, were not affected in any of the treated rats. Oxygen free radicals are instrumental in the development of acute pancreatitis. Even after its onset, scavenger treatment reduced the tissue damage normally observed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler G., Hupp T., Kern H. F. Course and spontaneous regression of acute pancreatitis in the rat. Virchows Arch A Pathol Anat Histol. 1979 May 14;382(1):31–47. doi: 10.1007/BF01102739. [DOI] [PubMed] [Google Scholar]
- Aho H. J., Nevalainen T. J., Havia V. T., Heinonen R. J., Aho A. J. Human acute pancreatitis: a light and electron microscopic study. Acta Pathol Microbiol Immunol Scand A. 1982 Sep;90(5):367–373. [PubMed] [Google Scholar]
- Braganza J. M., Holmes A. M., Morton A. R., Stalley L., Ku R., Kisher R. Acetylcysteine to treat complications of pancreatitis. Lancet. 1986 Apr 19;1(8486):914–915. doi: 10.1016/s0140-6736(86)91017-2. [DOI] [PubMed] [Google Scholar]
- Braganza J. M., Wickens D. G., Cawood P., Dormandy T. L. Lipid-peroxidation (free-radical-oxidation) products in bile from patients with pancreatic disease. Lancet. 1983 Aug 13;2(8346):375–379. doi: 10.1016/s0140-6736(83)90347-1. [DOI] [PubMed] [Google Scholar]
- Braganza J., Rinderknecht H. Free radicals and acute pancreatitis. Gastroenterology. 1988 Apr;94(4):1111–1112. doi: 10.1016/0016-5085(88)90599-9. [DOI] [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Bulkley G. B. The role of oxygen free radicals in human disease processes. Surgery. 1983 Sep;94(3):407–411. [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Guice K. S., Miller D. E., Oldham K. T., Townsend C. M., Jr, Thompson J. C. Superoxide dismutase and catalase: a possible role in established pancreatitis. Am J Surg. 1986 Jan;151(1):163–169. doi: 10.1016/0002-9610(86)90027-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge P. L., Saluja A. K., Powers R. E., Steer M. L. Role of oxygen-derived free radicals in diet-induced hemorrhagic pancreatitis in mice. Gastroenterology. 1987 Jul;93(1):41–47. doi: 10.1016/0016-5085(87)90311-8. [DOI] [PubMed] [Google Scholar]
- Sanfey H., Bulkley G. B., Cameron J. L. The pathogenesis of acute pancreatitis. The source and role of oxygen-derived free radicals in three different experimental models. Ann Surg. 1985 May;201(5):633–639. doi: 10.1097/00000658-198505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey H., Bulkley G. B., Cameron J. L. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann Surg. 1984 Oct;200(4):405–413. doi: 10.1097/00000658-198410000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F. Free-radical mechanisms in tissue injury. Biochem J. 1984 Aug 15;222(1):1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner J., Green D., Ferrell L., Renner I. Evidence for a role of oxygen derived free radicals in the pathogenesis of caerulein induced acute pancreatitis in rats. Gut. 1988 Nov;29(11):1516–1523. doi: 10.1136/gut.29.11.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes M., Schoenberg M. H., Jung H., Fredholm B. B., Haglund U., Schildberg F. W. Oxidative tissue damage following regional intestinal ischemia and reperfusion in the cat. Res Exp Med (Berl) 1984;184(4):259–264. doi: 10.1007/BF01852385. [DOI] [PubMed] [Google Scholar]