Abstract
The presence of genetic variation for learning ability in animals opens the way for experiments asking how and under what ecological circumstances improved learning ability should evolve. Here we report experimental evolution of learning ability in Drosophila melanogaster. We exposed experimental populations for 51 generations to conditions that we expected to favor associative learning with regard to oviposition substrate choice. Flies that learned to associate a chemical cue (quinine) with a particular substrate, and still avoided this substrate several hours after the cue had been removed, were expected to contribute more alleles to the next generation. From about generation 15 on, the experimental populations showed marked ability to avoid oviposition substrates that several hours earlier had contained the chemical cue. The improved response to conditioning was also expressed when the flies were faced with a choice of novel media. We demonstrate that these behavioral changes are caused by the evolution of both a higher learning rate and a better memory.
Learning ability is known to respond readily to direct artificial selection on a particular conditioned behavior (1–5). In such experiments the conditionability of the focal behavior is the sole criterion that determines whether an individual is allowed to breed. However, in natural populations learning and memory may entail fitness costs, if only because of the energy needed to maintain neuronal information and underlying structures (6). It remains unclear how readily learning evolves under natural selection, when its contribution to reproductive success is indirect and has to be set against its potential costs (7–12).
To address this issue, we kept populations of Drosophila melanogaster under ecological conditions that we expected to favor the evolution of learning ability in the context of oviposition substrate choice. The choice of a suitable oviposition substrate is an ecologically important decision with a direct impact on fitness. It may be modified by experience because in nature Drosophila females lay eggs over extended time, potentially on many different substrates, which are also fed on by the adults. They can thus assess the quality of the oviposition medium, which, together with relatively well developed associative memory (13), opens an opportunity for learning to contribute to Darwinian fitness (11, 12, 14).
In our experiment, the flies had a choice between two oviposition media, one of which had previously contained an aversive chemical (gustatory) cue. An individual would increase its contribution to the next generation by laying a greater number of eggs on the medium that had not contained the cue. This could be achieved in two ways. First, the individual could increase the total number of eggs laid within the oviposition period on both media, independently of the cue. This change would potentially be counteracted by tradeoffs such as reduced offspring quality. Second, a better ability to associate a medium with the aversive cue (i.e., better learning, better memory, and/or a better ability to discriminate between the media) would enable the individual to lay a greater proportion of eggs on the appropriate medium. These changes would not be favored if they entailed a sufficient cost in terms of reduced fecundity, such that the actual number of eggs laid on the appropriate medium would not increase. Thus, in contrast to artificial selection, under which a learning score is the only criterion used to decide whether an animal is allowed to breed, our design allows processes other than learning to increase an individual's contribution to the next generation. How the populations would respond was likely to depend on the available genetic variation and the balance between costs and benefits. Our experimental populations did evolve a marked ability to modify their oviposition substrate preference in response to conditioning. We have been able to show that they achieved this by evolving both faster learning and improved memory but not by any detectable improvement of discrimination ability.
Materials and Methods
Stock.
Our base stock population of D. melanogaster was derived from 2,000 flies caught in Basel (Switzerland) and maintained for 6 months in the laboratory before the beginning of the experiment. All flies used during the experiments were 14 days old (counted from egg).
Experimental Evolution Design.
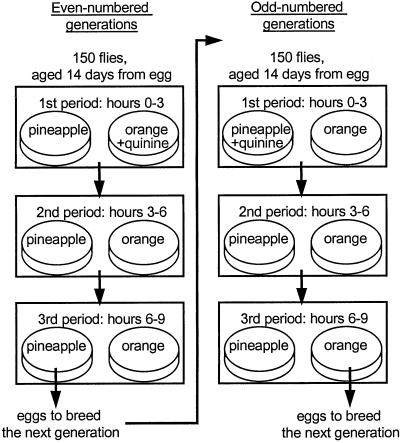
Every generation, 150 adult flies from each of eight experimental and eight control populations were transferred to cages (19 × 12 × 13 cm) and allowed to oviposit during three consecutive periods of 3 h (Fig. 1) in complete darkness, 25°C and 70% relative humidity. During each period, we offered the flies a choice between two oviposition substrates: an orange medium and a pineapple medium. These media were prepared from 100% orange or pineapple juice from concentrate with 6.6 g/liter agar added. At the bottom of the cage, one Petri dish with 10 ml of the orange medium and one with 10 ml of the pineapple medium were attached at the end of plastic tubes (height 5 cm, diameter 6 cm). A fresh set of Petri dishes with the media was provided at the beginning of each period; their position was randomized.
Fig 1.
Design of the experimental evolution: selection regime in the experimental lines at even and odd-numbered generations. Only eggs laid in period 3 on one medium (orange in odd and pineapple in even generations) were used to breed the next generation. The regime experienced by the control lines was identical except that quinine was never added to any medium.
One of the media offered to the experimental populations in period 1 (alternately, pineapple in odd-numbered generations, orange in even-numbered generations) additionally contained quinine hydrochloride (4 g/liter). At this concentration, quinine in the fruit medium had no effect on fly fecundity (F.M., unpublished data). From the first generation, the flies showed a strong avoidance of quinine: only 1.8 ± 0.9% of the eggs were laid on the quinine-containing medium. This avoidance did not change throughout the experiment, indicating that the results we describe below are not due to improved quinine recognition. During this first period (the training period), flies from the experimental populations were thus expected to associate one of the two media with the presence of quinine. Quinine was not added to any medium offered during periods 2 and 3. The choice of oviposition medium in periods 2 and 3 (test periods) is therefore expected to reflect the conditioning that occurred in period 1. Two test periods were used to assess decay of the conditioned response with time (due to forgetting or extinction). Oviposition preference was scored as the proportion of eggs laid on each medium. The next generation was bred from 250 eggs laid in period 3 on the medium that had not contained quinine in period 1 (i.e., orange in odd-numbered, pineapple in even-numbered generations; see Fig. 1). These eggs were rinsed with water and transferred to a 250-ml bottle containing 21 ml of a standard cornmeal medium. Larvae were thus always reared on the same medium, which precluded any preferences induced by larval medium. The control flies were treated in the same way except that they were never given any medium containing quinine.
Experimental flies could thus learn to use the presence of quinine in period 1 as a cue indicating which medium they should avoid for oviposition in period 3, when no cue was present. The cue was not available to the control flies.
The experiment has now been running for 57 generations. Because of technical problems (accidental insecticide poisoning in the laboratory), at generation 27 one experimental (line 4) and one control line (line 3) were lost, and the population size of some other lines was temporarily reduced (in one case to only about 20 adults). To facilitate recovery, selection regime was suspended for generations 27–31; selection was also not applied at generations 11, 35, and 44 for other reasons. At those generations flies laid eggs on a standard cornmeal medium.
Response to Conditioning.
At generations 23 and 46, we simultaneously assayed the effect of conditioning on the oviposition preference of flies from each experimental and control line, as well as from the stock population (kept on a standard cornmeal medium). Each line was divided in three samples. The flies of each group were introduced into cages and, as before, allowed to oviposit for 3 periods of 3 h each. One sample was tested without conditioning (quinine not present in any medium), another sample with conditioning to avoid pineapple (quinine present in the pineapple medium in period 1), and the last sample with conditioning to avoid orange (quinine present in the orange medium in period 1).
At generation 43, we also tested the learning ability of all control and experimental lines when faced with two novel fruit media. These were an apple medium and a tomato medium, both prepared from juice and agar. This test used the same design as described above.
Comparison of the Rate of Learning.
The aim of this assay, performed at generation 47, was to obtain information about the time course of the learning process, i.e., to see how the conditioned response depends on the amount (length) of conditioning. To be better able to control the time spent on a medium with quinine (i.e., the effective conditioning time), we used another conditioning regime. This conditioning regime, in contrast to that described above, did not allow the flies to switch freely between the quinine-containing and quinine-free medium. It involved cycles of a 45-min “resting” period, during which the flies were kept in an empty vial, followed by a 45-min conditioning period, during which the flies were kept in a vial with a quinine-containing orange or pineapple medium (quinine hydrochloride 4 g/liter). The treatments consisted of exposure to one, three, five, or seven such consecutive resting–conditioning cycles (corresponding to 45, 135, 225, and 315 min of total conditioning time). Before the first resting–conditioning cycle, the flies used in the treatments with one, three, and five cycles were maintained in empty vials for additional 540, 360, and 180 min, respectively. That way the total time spent during the conditioning phase in empty vials plus in vials with quinine-containing medium was the same in all treatments (630 min). Therefore, when their conditioned response was assayed, all flies had been prevented from egg laying for the same length of time and their motivation to oviposit should be similar (flies oviposit neither in a quinine-containing medium, nor in empty vials). All cycles of a given treatment involved conditioning to avoid the same medium (either orange or pineapple). In other words, the flies repeatedly encountered either quinine-containing orange medium or quinine-containing pineapple medium during conditioning, but no medium without quinine. There were thus eight treatment combinations (four durations of conditioning × orange or pineapple medium). Each of these eight treatment combinations was applied to a different sample of 50 flies (males + females) from each of five randomly selected experimental and five control lines (control lines 1, 2, 4, 5, and 7; experimental lines 1, 3, 5, 6, and 8). All fly transfers were done without anesthesia. The response to conditioning was assessed immediately after the last resting–conditioning cycle. Each sample of flies was allowed to oviposit for 1 h in a cage with one Petri dish of the orange medium and one Petri dish of the pineapple medium, neither containing quinine. The proportion of eggs laid on each medium was scored.
Decay of the Conditioned Response.
The aim of this assay (done at generation 48) was to study how the conditioned response diminishes with time elapsed since conditioning. Differences in the rate of this decay between the experimental and control populations would suggest evolved differences in their memory. We used the same five experimental and five control lines as in the assay described in the preceding paragraph. We also used the same type of conditioning as described in the preceding paragraph, except that all flies were exposed to five resting–conditioning cycles. We knew from a previous assay that after five resting–conditioning cycles, experimental and control lines show a similar conditioned response if tested immediately after conditioning (see Results). Four samples of 50 flies from each line were conditioned in that manner to avoid orange. The oviposition preference of one of the four samples was tested immediately after the last learning–conditioning cycle; the remaining three samples were transferred for 1, 2, and 3 h, respectively, to empty vials (“forgetting period”) before being tested. Another set of four samples of 50 flies were conditioned in an analogous way to avoid pineapple and tested in the same manner. The oviposition preference of all eight samples was assessed by allowing them to oviposit for 1 h in cages with one Petri dish of orange and one Petri dish of pineapple medium, both without quinine.
Dose–Response Curve.
The aim of this assay (done at generation 56) was to test whether salience of the pineapple and orange medium to elicit oviposition is greater in the experimental than control populations. We again used the same subset of five experimental and control lines. One hundred flies from each line were presented for 3 h with one Petri dish with a pure agar medium (7 g/liter) and one Petri dish with a diluted fruit medium. The fruit medium was composed of agar (7 g/liter) and orange or pineapple fruit juice diluted with water. We tested the following dilution series: 1 (pure juice), 1/4, 1/8, 1/16, 1/32, 1/64, 1/128, 1/256. As flies refrain from laying eggs on a pure agar medium, the number of eggs laid on the fruit medium of a given concentration reflects the ability of the flies to detect this concentration and its salience to stimulate oviposition. To compare the dose–response curves of experimental and control populations we fitted a Poisson regression of the number of eggs laid on the fruit medium on −log2(concentration), with treatment (experimental versus control) as a main effect, and treatment × −log2(concentration) interaction testing for a difference of the slope. We also tested whether differences among the five experimental lines in the response to declining medium concentration were correlated with differences in their learning ability. To this end we calculated Pearson's correlation between the coefficients of Poisson regression, fitted separately to each line, and a learning score. The learning score for each line was estimated as a differences between between the proportion of eggs laid on orange when conditioned to avoid pineapple and when conditioned to avoid orange, assayed at the same generation as the dose–response curve (but using different individuals). The conditioning involved six resting–conditioning cycles of the type described in Comparison of the Rate of Learning.
Analysis.
SPSS statistical package was used for all analyses except for the Poisson regression, which was done with proc genmod of SAS statistical software. The analysis treated the proportions of eggs laid on the orange medium by each line in each period as raw data. Because all proportion values fell in the range 0.30 − 0.84, they were not transformed before the analysis (15). Where the data from periods 2 and 3 were analyzed together, repeated-measures ANOVA was used, with line treated as the subject and period as the within-subject effect. Where appropriate, the residuals were checked for normality with the Shapiro–Wilk test. No deviations from normality were detected, except in the case mentioned in Fig. 2.
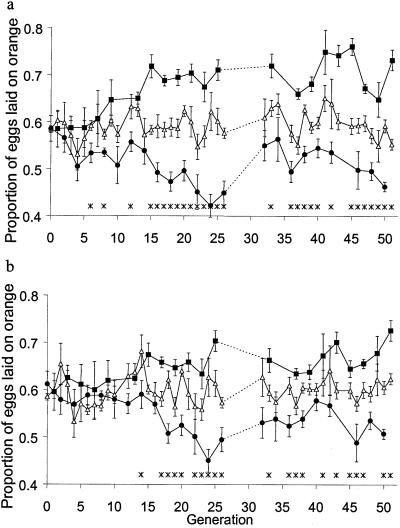
Fig 2.
The proportion of eggs laid on the orange medium in the course of the experimental evolution (means ± standard errors). (a) Period 2. (b) Period 3. Circles indicate experimental populations at even-numbered generations (conditioned to avoid orange); squares indicate experimental populations at odd-numbered generations (conditioned to avoid pineapple); triangles indicate control populations (not conditioned). Data for generations 11, 27–31, 35, and 44 are missing. An asterisk indicates a significant difference (t test, P < 0.05, not corrected for multiple comparisons) between the experimental and control lines. The data only deviated from normality at generations 32 and 45 (0.05 > P > 0.01).
Results
Changes in the Course of Experimental Evolution.
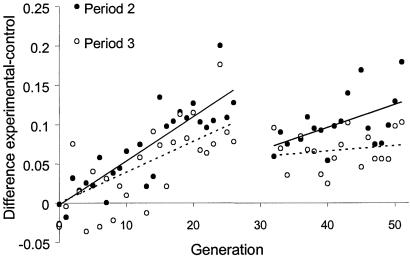
Fig. 2 shows the proportion of eggs laid on the orange medium by the experimental and control lines in the course of the experiment. At the beginning (generation 0), the proportion of eggs laid on each medium during periods 2 and 3 was not affected by the presence of quinine during period 1 (repeated measures analysis of variance, F1,16 = 0.06, P > 0.8). After several generations of experimental evolution, the experimental populations began to show an effect of conditioning on oviposition substrate preference. In periods 2 and 3, they laid an increasing proportion of their eggs on the “correct” medium, i.e., the medium that had not contained quinine in period 1 (Fig. 2). From generation 15 until selection was suspended at generation 27, the experimental flies consistently laid in period 2 a significantly greater proportion of their eggs on the “correct” medium than the control flies; for the third period this held from generation 17. After the selection regime had been resumed at generation 32, the difference was less pronounced at some generations, but still consistent. The increasing difference between the experimental and control lines with respect to the proportion of eggs laid on the “correct” medium (Fig. 3) illustrates the evolution of improved learning ability. The difference was typically smaller in period 3 than in period 2 (paired t test, P = 0.008), suggesting that with time the flies either tended to forget the association between quinine and the medium or learned that quinine was now absent from the medium (extinction). We did not observe any change of fecundity of the experimental or control flies over generations (regression analysis; control: F = 0.04, P > 0.8; experimental: F = 0.67, P > 0.4).
Fig 3.
The difference between the experimental and control lines in the mean proportion of eggs laid on the “correct” medium (orange in odd-numbered, pineapple in even-numbered generations). Separate regression lines were fitted for the two testing periods (period 2 and period 3). Because selection was suspended at generations 27–31, regression lines for generations 1–26 and 32–51 were fitted separately; for generations 1–26, the lines were forced through the origin. For generations 1–26, both regression slopes are significantly positive (P < 0.001); after generation 32, only the regression for period 2 is significantly positive (P < 0.01).
Response to Conditioning.
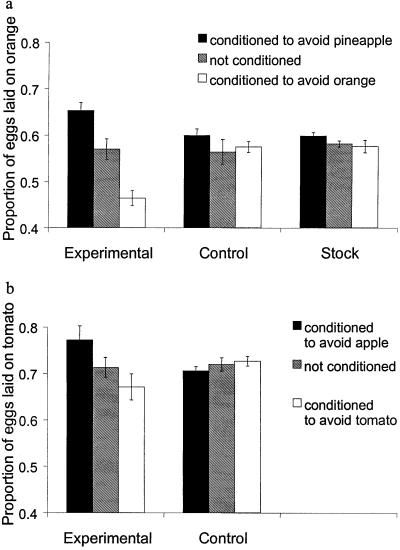
The assays done at generation 23 and 46 showed that, in the absence of conditioning, the preference of the experimental flies did not differ from the control and stock (Fig. 4a; repeated-measures ANOVA, F2,24 = 0.19, P > 0.8 and F2,21 = 2.45, P = 0.11 for generation 23 and 46, respectively). This indicates that no evolutionary changes of genetically based (innate) preference occurred during the experiment. However, the oviposition substrate preference of the experimental populations was strongly affected by conditioning (F2,24 = 24.9, P < 0.001 and F2,21 = 41.1 P < 0.001 for generation 23 and 46, respectively), whereas the control and stock flies showed no detectable response to conditioning (Fig. 4a; generation 23: control: F2,24 = 0.87, P > 0.4; stock: F2,24 = 1.42, P > 0.2; generation 46: control: F2,21 = 0.31, P > 0.7; stock: F2,21 = 0.72, P > 0.4). There was no difference between the control and stock flies, indicating that the control flies did not evolve learning ability in the course of the experiment. For the experimental treatment, we did not observe any difference of conditionability between generations 23 and 46.
Fig 4.
Response of oviposition substrate preference to conditioning, measured on two sets of media: orange versus pineapple (generation 46) (a); apple versus tomato (generation 43) (b). “Conditioned to avoid pineapple” means that quinine was present in the pineapple medium offered in period 1. The proportion of eggs laid on the orange medium was averaged over periods 2 and 3; bars represent standard errors.
When faced with two novel fruit media (apple and tomato), flies of the experimental lines still responded to conditioning (repeated-measures ANOVA, F2,21 = 5.09, P = 0.018), whereas flies of the control lines showed no response to conditioning (Fig. 4b; repeated-measures ANOVA, F2,21 = 0.82, P > 0.4). The improved learning ability of the experimental populations was thus also manifested when they were faced with oviposition media other than those used in the course of selection.
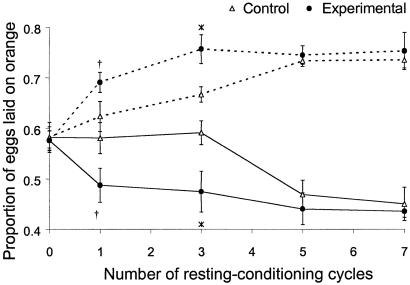
Comparison of the Rate of Learning (Generation 47).
As in the previous assay, in the absence of conditioning the oviposition substrate preference (the proportion of eggs laid on the orange medium) did not differ between the experimental and control flies (P > 0.2). The response of the oviposition substrate preference was a decelerating function of the total conditioning time (the number of resting–conditioning cycles), irrespective of whether the flies were conditioned to avoid orange or pineapple (Fig. 5). Flies from the experimental populations responded to conditioning faster. They showed a significant change of their oviposition preference already after a single 45-min conditioning event (t = 3.01, P = 0.014 and t = 5.11, P < 0.001 for flies conditioned to avoid orange and pineapple, respectively). For the control flies the response was significant at P < 0.05 only after three (conditioned to avoid pineapple) or five (conditioned to avoid orange) resting–conditioning cycles, corresponding to a total conditioning time of 135 and 225 min, respectively. As a consequence, the experimental flies exposed to 45 and 135 min of total conditioning time showed a stronger response to conditioning than control flies, although for 45 min this difference was only marginally significant (Fig. 5). However, when total conditioning time was longer, the response of the control flies became as large as that of experimental flies. After 315 min of conditioning the control flies showed almost exactly the same oviposition substrate preference as the experimental flies, both when conditioned to avoid orange and when conditioned to avoid pineapple (Fig. 5). To summarize, although the control flies learned more slowly than the control flies (they showed a weaker response after a short conditioning time), their maximum response to conditioning seemed to be the same as that of the experimental flies.
Fig 5.
Comparison of the rate of learning (generation 47). The response of experimental and control populations to conditioning as a function of the total conditioning time (the number of resting–conditioning cycles). Solid lines, flies conditioned to avoid orange; dashed lines, flies conditioned to avoid pineapple. Bars represent ± one standard error; the asterisk indicates a significant difference (t test, P < 0.05) between the experimental and control lines for a given conditioning treatment; the dagger indicates 0.05 < P < 0.08.
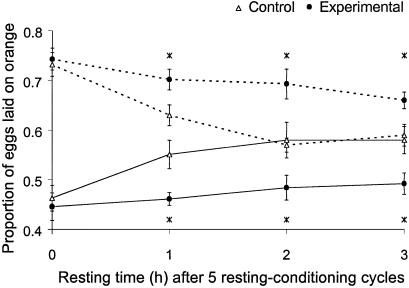
Decay of the Conditioned Response (Generation 48).
The response of experimental and control flies to five cycles of resting and conditioning (total conditioning time 225 min) was statistically undistinguishable when tested immediately after conditioning (Fig. 6). This result is consistent with the results from generation 47 described in the previous paragraph. In both experimental and control populations, the effect of conditioning on oviposition site preference diminished with time elapsed since the end of conditioning, but this decay was faster in the controls (Fig. 6). As a consequence, whenever there was a delay between conditioning and testing, the experimental flies laid a smaller proportion of eggs than the control flies on the medium they were conditioned to avoid. This held irrespective of the length of the delay (1, 2, or 3 h) and of the medium they were conditioned to avoid (orange or pineapple). The experimental flies still showed a significant effect of conditioning if tested 3 h after termination of conditioning (t = 2.85, P = 0.011 and t = 6.68, P < 0.001 for flies conditioned to avoid orange and pineapple, respectively). In contrast, no effect of conditioning could be detected in control flies already after 2 h, nor after 3 h. The faster decay of the conditioned response with time was also manifested in a significant interaction between time since conditioning and experimental versus control selection regime (two-way analysis of covariance, F = 5.27, P = 0.024 and F = 5.08, P = 0.026, for flies conditioned to avoid orange and pineapple, respectively). This finding indicates that the experimental flies had a better memory than the controls.
Fig 6.
Decay of the conditioned response with time elapsed since the last conditioning cycle (generation 48). All flies were exposed to five resting–conditioning cycles, with total conditioning time of 225 min. Symbols are as in Fig. 5.
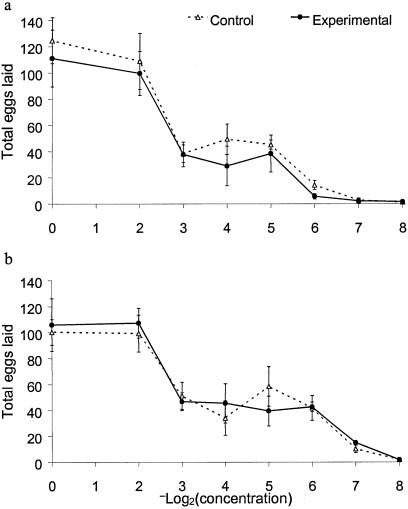
Dose–Response Curve.
The experimental and control lines responded similarly to decreasing concentration of juice in the medium, with a characteristic plateau at intermediate concentrations (Fig. 7). We detected no differences between the treatments in the intercept or slope of the Poisson regression of the number of eggs laid on the −log2(concentration) on either medium (all P > 0.5). The differences among the replicate experimental lines in their response to a declining medium concentration were not correlated with differences in their learning score (Pearson's r = −0.06, P > 0.9 and r = 0.11, P > 0.8 for orange and pineapple medium, respectively).
Fig 7.
Dose–response curve measuring the potential of diluted fruit medium (orange in a and pineapple in b) to stimulate oviposition (generation 56). Bars are ± one standard error.
Discussion
When exposed to ecological conditions thought to favor learning, our experimental fly populations evolved an improved ability to associate the taste or smell of an oviposition medium with an aversive chemical cue (quinine) and to avoid this medium several hours later, when the cue was no longer present (Fig. 4a). We did not observe any significant increase in the number of eggs laid in both treatments over generations. Most of the evolutionary change occurred within the first 20 generations of selection (Figs. 2 and 3). The change was partially reversed when the selection regime was suspended, and some populations went through a mild bottleneck at generations 27–31. The subsequent slowdown of evolutionary change is likely to reflect loss of genetic variation.
Although the control lines did not show a detectable response to conditioning under the design used during the experimental evolution (Fig. 4), they did respond to a more intensive and prolonged conditioning regime (Fig. 5). The experimental flies developed an association between the chemical cue and the medium faster than the control flies, i.e., had a higher learning rate (Fig. 5). They also remembered the association longer (Fig. 6). Hence, both faster learning and better memory contributed to the improved ability of the experimental lines to respond to conditioning. An improved response to conditioning was also observed when the flies were faced with novel fruit media (Fig. 4b), i.e., was not limited to the media used during selection.
We can exclude the most plausible alternative explanations. First, even though the experimental lines learned faster than the controls, the conditioned response of both reached the same plateau (Fig. 5), which implies that the effectiveness of quinine as reinforcer (16) does not differ between the two types of lines. (Note that the fact that both lines detect quinine equally well would be insufficient to validate this conclusion; ref. 16.) Second, in the absence of conditioning, both types of lines laid the same proportion of eggs on the orange medium, and this proportion was significantly different from 50% (Fig. 4a). Similarly, when faced with two novel media, apple and tomato, both experimental and control flies laid ≈72% of eggs on the tomato medium in the absence of conditioning (Fig. 4b), suggesting that the experimental populations did not evolve a substantially better ability to discriminate between the media. However, a firm conclusion on discrimination ability would require a “cross-adaptation” approach (17). Third, the experimental and control lines showed the same oviposition response to decreasing concentration of the media (Fig. 7), and among the experimental lines there was no relationship between this response and the learning ability. Thus, the faster learning response of the experimental flies is not due to greater salience (i.e., perceived intensity) of the odors or tastes of the media as stimuli eliciting oviposition (16).
We cannot yet say how specific the improved learning ability is, i.e., whether it would also be manifested in other learning tasks. The possibility that the improved learning ability of different replicate lines may have different genetic or physiological basis also remains to be addressed. Nonetheless, our study demonstrates that fruit flies can readily evolve improved learning ability and better memory under ecologically relevant circumstances. It supports the theoretical prediction that learning should be favored when the environment is temporally or spatially variable (8) and the animal can get reliable cues (10). It also demonstrates that fruit flies can use their learning ability to modify oviposition substrate choice, with direct consequences for fitness. Other things being equal, under our selection regime the contribution of a fly to the next generation was proportional to the percentage of eggs laid on the favored medium. Based on this assumption, by the 23rd generation the improved learning ability would already give the flies from the experimental populations a 15% advantage over the control flies in terms of geometric mean fitness, which is the appropriate measure under a temporally varying selection regime (18).
Although using single-locus large-effect mutants is likely to be the most effective approach to uncovering the molecular bases of learning (19), our approach offers an opportunity to study the genetic bases of quantitative variation for learning ability segregating in natural populations (20). It also opens new avenues of research on the ecological consequences and fitness costs of learning.
Acknowledgments
We thank D. Ebert, B. Gerber, N.Grillenzoni, M. Kirkpatrick, D. Meyer, R. Lenski, A. Ramaekers, I. Sanders, A. Schneider, R. Stocker, and two anonymous reviewers for comments on the manuscript, and N. Cueni, T. Flatt, and N. Vouilloz for assistance with the experiments. This work was supported by the Swiss National Science Foundation.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tryon R. C. (1940) Yk. Natl. Soc. Stud. Educ. 39, 111-119. [Google Scholar]
- 2.McGuire T. R. & Hirsch, J. (1977) Proc. Natl. Acad. Sci. USA 74, 5193-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandes C., Frisch, B. & Menzel, R. (1988) Anim. Behav. 36, 981-985. [Google Scholar]
- 4.Lofdahl K. L., Holliday, M. & Hirsch, J. (1992) J. Comp. Psychol. 106, 172-183. [DOI] [PubMed] [Google Scholar]
- 5.Reif M., Linsenmair, K. E. & Heisenberg, M. (2002) Anim. Behav. 63, 143-155. [Google Scholar]
- 6.Dukas R. (1999) J. Theor. Biol. 197, 41-50. [DOI] [PubMed] [Google Scholar]
- 7.Papaj D. R. & Prokopy, R. J. (1989) Annu. Rev. Entomol. 34, 315-350. [Google Scholar]
- 8.Dukas R. (1998) in Cognitive Ecology, ed. Dukas, R. (University of Chicago Press, Chicago), pp. 129–164.
- 9.Stephens D. W. (1991) Behav. Ecol. 2, 77-89. [Google Scholar]
- 10.Parmesan C., Singer, M. C. & Harris, I. (1995) Anim. Behav. 50, 161-175. [Google Scholar]
- 11.Dukas R. & Bernays, E. A. (2000) Proc. Natl. Acad. Sci. USA 97, 2637-2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egas M. & Sabelis, M. W. (2001) Ecol. Lett. 4, 190-195. [Google Scholar]
- 13.Dubnau J. & Tully, T. (1998) Annu. Rev. Neurosci. 21, 407-444. [DOI] [PubMed] [Google Scholar]
- 14.Roitberg R. D., Reid, M. L. & Li, C. (1993) in Insect Learning: Ecological and Evolutionary Perspectives, eds. Papaj, D. R. & Lewis, A. C. (Chapman and Hall, New York), pp. 174–194.
- 15.Sokal R. R. & Rohlf, F. J., (1995) Biometry: The Principles and Practice of Statistics in Biological Research (Freeman, New York).
- 16.Rescorla R. A. (1988) Annu. Rev. Neurosci. 11, 329-352. [DOI] [PubMed] [Google Scholar]
- 17.Cobb M. & Domain, I. (2000) Proc. R. Soc. London Ser. B 267, 2119-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillespie J. H. (1973) Genet. Res. 21, 115-120. [Google Scholar]
- 19.Tully T. (1996) Proc. Natl. Acad. Sci. USA 93, 13460-13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toma D. P., White, K. P., Hirsch, J. & Greenspan, R. J. (2002) Nat. Genet. 31, 349-353. [DOI] [PubMed] [Google Scholar]