Abstract
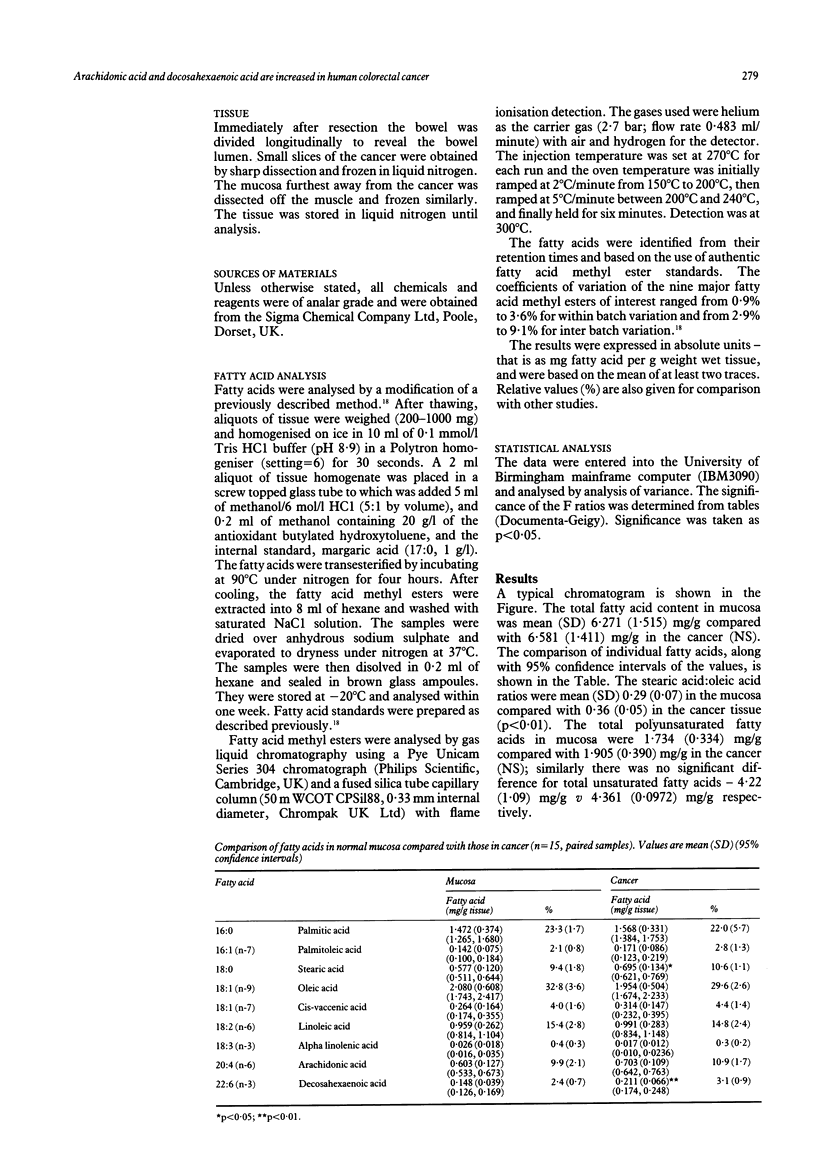
Increased arachidonic acid concentrations in experimental rodent colonic cancer have been described recently. In humans, a reduced erythrocyte stearic acid to oleic acid ratio has been reported in patients with colorectal cancer and it has been proposed that similar changes exist in the cancer tissue. The long chain fatty acids in the cancers of 15 patients with colorectal cancer were measured and compared with values in the unaffected mucosa. The values were expressed as mean (SD) mg fatty acid/g tissue and compared by analysis of variance. In the cancer tissue arachidonic acid was increased (0.703 (0.109) mg/g v 0.603 (0.127) mg/g, p less than 0.05) as was docosahexaenoic acid (0.211 (0.066) mg/g v 0.148 (0.039) mg/g, p less than 0.001). In contrast, the stearic acid to oleic acid ratio in the cancer tissue was increased rather than decreased, as previously suggested (0.36 (0.05) v 0.29 (0.7), p less than 0.01). Increased arachidonic acid and docosahexaenoic acid concentrations may be related to reduced lipid peroxidation, which is a feature of rapidly growing cells. Alternatively, the increased arachidonic acid values could be due to enhanced desaturase activity upon linoleic and linolenic acid, leading perhaps to increased formation of prostaglandins and other lipoxygenase products.
Full text
PDF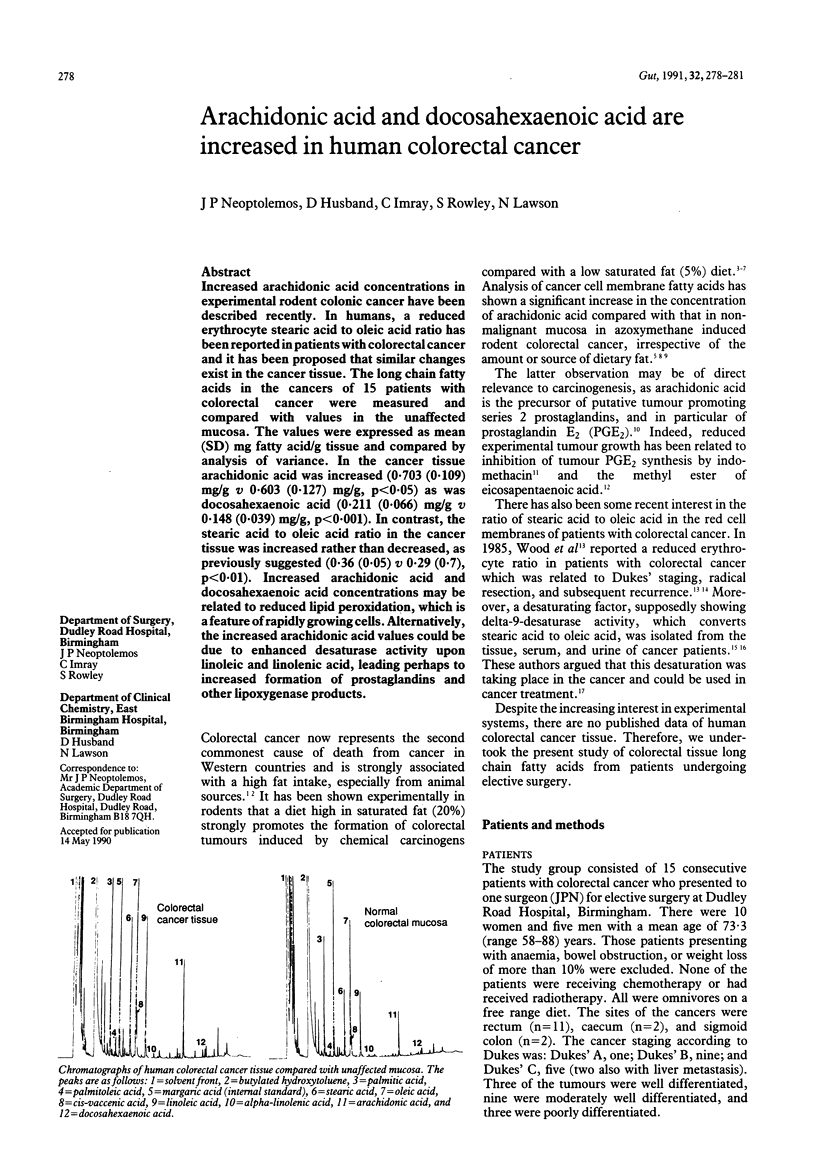


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong B., Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975 Apr 15;15(4):617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- Bennett A., Tacca M. D., Stamford I. F., Zebro T. Prostaglandins from tumours of human large bowel. Br J Cancer. 1977 Jun;35(6):881–884. doi: 10.1038/bjc.1977.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasitus T. A., Davidson N. O., Schachter D. Variations in dietary triacylglycerol saturation alter the lipid composition and fluidity of rat intestinal plasma membranes. Biochim Biophys Acta. 1985 Jan 25;812(2):460–472. doi: 10.1016/0005-2736(85)90321-9. [DOI] [PubMed] [Google Scholar]
- Bull A. W., Soullier B. K., Wilson P. S., Hayden M. T., Nigro N. D. Promotion of azoxymethane-induced intestinal cancer by high-fat diet in rats. Cancer Res. 1979 Dec;39(12):4956–4959. [PubMed] [Google Scholar]
- Bégin M. E., Ells G., Das U. N., Horrobin D. F. Differential killing of human carcinoma cells supplemented with n-3 and n-6 polyunsaturated fatty acids. J Natl Cancer Inst. 1986 Nov;77(5):1053–1062. [PubMed] [Google Scholar]
- Bégin M. E., Ells G., Das U. N. Selected fatty acids as possible intermediates for selective cytotoxic activity of anticancer agents involving oxygen radicals. Anticancer Res. 1986 Mar-Apr;6(2):291–295. [PubMed] [Google Scholar]
- Bégin M. E., Ells G., Horrobin D. F. Polyunsaturated fatty acid-induced cytotoxicity against tumor cells and its relationship to lipid peroxidation. J Natl Cancer Inst. 1988 Apr 6;80(3):188–194. doi: 10.1093/jnci/80.3.188. [DOI] [PubMed] [Google Scholar]
- Cheeseman K. H., Collins M., Proudfoot K., Slater T. F., Burton G. W., Webb A. C., Ingold K. U. Studies on lipid peroxidation in normal and tumour tissues. The Novikoff rat liver tumour. Biochem J. 1986 Apr 15;235(2):507–514. doi: 10.1042/bj2350507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. J., Jarrett F., Boyle P., Indran M., Carr K., Owen R. W., George W. D. Morphological and cell kinetic effects of dietary manipulation during colorectal carcinogenesis. Gut. 1987 Jun;28(6):754–763. doi: 10.1136/gut.28.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Habib N. A., Hershman M. J., Salem R., Barker W., Apostolov K., Wood C. B. Increased erythrocyte stearic acid desaturation in rats with chemically induced colorectal carcinomas. Int J Colorectal Dis. 1987 Feb;2(1):12–14. doi: 10.1007/BF01648990. [DOI] [PubMed] [Google Scholar]
- Habib N. A., Wood C. B., Apostolov K., Barker W., Hershman M. J., Aslam M., Heinemann D., Fermor B., Williamson R. C., Jenkins W. E. Stearic acid and carcinogenesis. Br J Cancer. 1987 Oct;56(4):455–458. doi: 10.1038/bjc.1987.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib N. A., Wood C. B., Apostolov K., Thompson A., Barker W., Mentha G., Coutilno J., Hershman M. Desaturation-producing factor present in the tissue, blood, and urine of cancer patients. Cancer Detect Prev. 1987;10(1-2):57–61. [PubMed] [Google Scholar]
- Jeffcoat R., Roberts P. A., James A. T. The control of lipogenesis by dietary linoleic acid and its influence on the deposition of fat. Eur J Biochem. 1979 Nov;101(2):447–453. doi: 10.1111/j.1432-1033.1979.tb19738.x. [DOI] [PubMed] [Google Scholar]
- Lawson N., Husband D., McGuigan J., Watson D. C., Collins F. J., Pandov H. I. Increased levels of vaccenic acid in bronchogenic carcinoma tissue. Ann Clin Biochem. 1989 Mar;26(Pt 2):125–131. doi: 10.1177/000456328902600206. [DOI] [PubMed] [Google Scholar]
- Minoura T., Takata T., Sakaguchi M., Takada H., Yamamura M., Hioki K., Yamamoto M. Effect of dietary eicosapentaenoic acid on azoxymethane-induced colon carcinogenesis in rats. Cancer Res. 1988 Sep 1;48(17):4790–4794. [PubMed] [Google Scholar]
- Narisawa T., Sato M., Sano M., Niwa M., Takahashi M., Ito T., Tanida N., Shimoyama T. Prevention of colon polyposis and carcinomas by right hemicolectomy and indomethacin in animal model. Cancer. 1985 Oct 1;56(7):1719–1724. doi: 10.1002/1097-0142(19851001)56:7<1719::aid-cncr2820560742>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Neoptolemos J. P., Clayton H., Heagerty A. M., Nicholson M. J., Johnson B., Mason J., Manson K., James R. F., Bell P. R. Dietary fat in relation to fatty acid composition of red cells and adipose tissue in colorectal cancer. Br J Cancer. 1988 Nov;58(5):575–579. doi: 10.1038/bjc.1988.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoptolemos J. P., Thomas B. S. Erythrocyte membrane stearic acid: oleic acid ratios in colorectal cancer using tube capillary column gas liquid chromatography. Ann Clin Biochem. 1990 Jan;27(Pt 1):38–43. doi: 10.1177/000456329002700108. [DOI] [PubMed] [Google Scholar]
- Nigro N. D., Singh D. V., Campbell R. L., Sook M. Effect of dietary beef fat on intestinal tumor formation by azoxymethane in rats. J Natl Cancer Inst. 1975 Feb;54(2):439–442. [PubMed] [Google Scholar]
- Nishida T., Miwa H., Shigematsu A., Yamamoto M., Iida M., Fujishima M. Increased arachidonic acid composition of phospholipids in colonic mucosa from patients with active ulcerative colitis. Gut. 1987 Aug;28(8):1002–1007. doi: 10.1136/gut.28.8.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHNISHI T. Lipid peroxide formation and phospholipid in normal and tumor tissues. Gan. 1958 Dec;49(4):233–248. [PubMed] [Google Scholar]
- Reddy B. S., Sugie S. Effect of different levels of omega-3 and omega-6 fatty acids on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Res. 1988 Dec 1;48(23):6642–6647. [PubMed] [Google Scholar]
- Reddy B. S., Watanabe K., Weisburger J. H. Effect of high-fat diet on colon carcinogenesis in F344 rats treated with 1,2-dimethylhydrazine, methylazoxymethanol acetate, or methylnitrosourea. Cancer Res. 1977 Nov;37(11):4156–4159. [PubMed] [Google Scholar]
- Sakaguchi M., Hiramatsu Y., Takada H., Yamamura M., Hioki K., Saito K., Yamamoto M. Effect of dietary unsaturated and saturated fats on azoxymethane-induced colon carcinogenesis in rats. Cancer Res. 1984 Apr;44(4):1472–1477. [PubMed] [Google Scholar]
- Tisdale M. J., Mahmoud M. B. Activities of free radical metabolizing enzymes in tumours. Br J Cancer. 1983 Jun;47(6):809–812. doi: 10.1038/bjc.1983.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFSON N., WILBUR K. M., BERNHEIM F. Lipid peroxide formation in regenerating rat liver. Exp Cell Res. 1956 Apr;10(2):556–558. doi: 10.1016/0014-4827(56)90031-3. [DOI] [PubMed] [Google Scholar]
- Wood C. B., Habib N. A., Thompson A., Bradpiece H., Smadja C., Hershman M., Barker W., Apostolov K. Increase of oleic acid in erythrocytes associated with malignancies. Br Med J (Clin Res Ed) 1985 Jul 20;291(6489):163–165. doi: 10.1136/bmj.291.6489.163. [DOI] [PMC free article] [PubMed] [Google Scholar]


