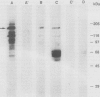
Abstract
The adhesive properties of tumour cells to laminin, the major glycoprotein of basement membranes, play a crucial part in the complex process of tumour invasion and metastasis. We therefore investigated the expression of laminin binding proteins in isolated basolateral cell membranes of human colorectal carcinomas and the adjacent normal colonic mucosa. Cell membrane binding assays and immunoblotting experiments showed appreciable quantitative and qualitative differences in the expression of these proteins in neoplastic and normal tissue. Epithelial basolateral cell membranes of colorectal carcinomas bound five to eight times more radioactive labelled laminin than basolateral cell membranes of the adjacent normal colonic epithelium. The expression of laminin binding proteins with Mr 66,000-69,000 daltons corresponding to the so called 'Mr 67,000 dalton laminin receptor' was three to four times higher in colorectal carcinomas than in normal colonic epithelium. In addition, laminin binding proteins with higher molecular weights, which may be related to the family of integrins, were also increased in colorectal carcinomas. In particular, laminin binding proteins with Mr 180,000 daltons were exclusively expressed on neoplastic epithelial cells of human colorectal carcinomas. Our data suggest that certain classes of laminin binding proteins may be selectively expressed on colonic tumour cells, leading to an increased capacity for migration, invasion, and metastasis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada S. S., Yamada K. M. Characterization of a 140-kD avian cell surface antigen as a fibronectin-binding molecule. J Cell Biol. 1986 Feb;102(2):442–448. doi: 10.1083/jcb.102.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- August D. A., Ottow R. T., Sugarbaker P. H. Clinical perspective of human colorectal cancer metastasis. Cancer Metastasis Rev. 1984;3(4):303–324. doi: 10.1007/BF00051457. [DOI] [PubMed] [Google Scholar]
- Dillner L., Dickerson K., Manthorpe M., Ruoslahti E., Engvall E. The neurite-promoting domain of human laminin promotes attachment and induces characteristic morphology in non-neuronal cells. Exp Cell Res. 1988 Jul;177(1):186–198. doi: 10.1016/0014-4827(88)90036-5. [DOI] [PubMed] [Google Scholar]
- Engel J., Odermatt E., Engel A., Madri J. A., Furthmayr H., Rohde H., Timpl R. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J Mol Biol. 1981 Jul 25;150(1):97–120. doi: 10.1016/0022-2836(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Gehlsen K. R., Dillner L., Engvall E., Ruoslahti E. The human laminin receptor is a member of the integrin family of cell adhesion receptors. Science. 1988 Sep 2;241(4870):1228–1229. doi: 10.1126/science.2970671. [DOI] [PubMed] [Google Scholar]
- Goodman S. L., Deutzmann R., von der Mark K. Two distinct cell-binding domains in laminin can independently promote nonneuronal cell adhesion and spreading. J Cell Biol. 1987 Jul;105(1):589–598. doi: 10.1083/jcb.105.1.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Hahn U., Stallmach A., Hahn E. G., Riecken E. O. Basement membrane components are potent promoters of rat intestinal epithelial cell differentiation in vitro. Gastroenterology. 1990 Feb;98(2):322–335. doi: 10.1016/0016-5085(90)90821-h. [DOI] [PubMed] [Google Scholar]
- Hand P. H., Thor A., Schlom J., Rao C. N., Liotta L. Expression of laminin receptor in normal and carcinomatous human tissues as defined by a monoclonal antibody. Cancer Res. 1985 Jun;45(6):2713–2719. [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Greggs R., Decker C., Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985 Dec;101(6):2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Greggs R., Decker C., Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985 Dec;101(6):2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Ogle R. C., Cannon F. B., Little C. D., Sweeney T. M., Luckenbill-Edds L. Laminin receptors for neurite formation. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1282–1286. doi: 10.1073/pnas.85.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Malinoff H. L., Wicha M. S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475–1479. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. B., Skubitz A. P., Palm S. L., Furcht L. T. Metastasis inhibition of different tumor types by purified laminin fragments and a heparin-binding fragment of fibronectin. J Natl Cancer Inst. 1988 Mar 16;80(2):108–116. doi: 10.1093/jnci/80.2.108. [DOI] [PubMed] [Google Scholar]
- Orsenigo M. N., Tosco M., Esposito G., Faelli A. The basolateral membrane of rat enterocyte: its purification from brush border contamination. Anal Biochem. 1985 Feb 1;144(2):577–583. doi: 10.1016/0003-2697(85)90156-3. [DOI] [PubMed] [Google Scholar]
- Palm S. L., McCarthy J. B., Furcht L. T. Alternative model for the internal structure of laminin. Biochemistry. 1985 Dec 17;24(26):7753–7760. doi: 10.1021/bi00347a038. [DOI] [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Runyan R. B., Versalovic J., Shur B. D. Functionally distinct laminin receptors mediate cell adhesion and spreading: the requirement for surface galactosyltransferase in cell spreading. J Cell Biol. 1988 Nov;107(5):1863–1871. doi: 10.1083/jcb.107.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Kleinman H. K., Huber H., Deutzmann R., Yamada Y. Laminin, a multidomain protein. The A chain has a unique globular domain and homology with the basement membrane proteoglycan and the laminin B chains. J Biol Chem. 1988 Nov 15;263(32):16536–16544. [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Stallmach A., Riecken E. O. Laminin-cell membrane binding proteins in small intestinal epithelial cells. Digestion. 1990;46 (Suppl 2):31–39. doi: 10.1159/000200364. [DOI] [PubMed] [Google Scholar]
- Stallmach A., Schuppan D., Dax J., Hanski C., Riecken E. O. Identification of laminin binding proteins in cell membranes of a human colon adenocarcinoma cell line. Gut. 1990 Jan;31(1):70–76. doi: 10.1136/gut.31.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Liotta L. A., Russo R. G., Martin G. R. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982 Jun;42(6):2265–2269. [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. R., Weiser M. M. Rat small intestinal laminin-binding proteins. Digestion. 1990;46 (Suppl 2):22–30. doi: 10.1159/000200363. [DOI] [PubMed] [Google Scholar]
- Yow H. K., Wong J. M., Chen H. S., Lee C. G., Davis S., Steele G. D., Jr, Chen L. B. Increased mRNA expression of a laminin-binding protein in human colon carcinoma: complete sequence of a full-length cDNA encoding the protein. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6394–6398. doi: 10.1073/pnas.85.17.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler R. G., Devesa S. S., Fraumeni J. F., Jr Epidemiologic patterns of colorectal cancer. Important Adv Oncol. 1986:209–232. [PubMed] [Google Scholar]
- von der Mark K., Risse G. Isolation and characterization of laminin receptors. Methods Enzymol. 1987;144:490–507. doi: 10.1016/0076-6879(87)44197-9. [DOI] [PubMed] [Google Scholar]