Abstract
γδ intraepithelial T lymphocytes (IEL) represent a major T cell population within the intestine of unclear functional relevance. The role of intestinal γδ IEL was evaluated in the dextran sodium sulfate (DSS) induced mouse colitis model system. Large numbers of γδ T cells, but not αβ T cells, were localized at sites of DSS-induced epithelial cell damage. γδ IEL in DSS treated mice expressed keratinocyte growth factor (KGF), a potent intestinal epithelial cell mitogen. γδ cell-deficient mice (TCRδ−/−) and KGF-deficient mice (KGF−/−), but not αβ cell-deficient mice (TCRα−/−), were more prone than wild-type mice to DSS-induced mucosal injury and demonstrated delayed tissue repair after termination of DSS treatment. Termination of DSS treatment resulted in vigorous epithelial cell proliferation in wild-type mice but not in TCRδ−/− mice or KGF−/− mice. These results suggest that γδ IEL help preserve the integrity of damaged epithelial surfaces by providing the localized delivery of an epithelial cell growth factor.
The intestine contains, in contrast to the lymphoid system, a prominent T cell population expressing the γδ-form of the T cell antigen receptor (TCR). The role of intestinal γδ intraepithelial T lymphocytes (IEL) under normal and disease conditions remains controversial (1–7). Activated but not resting intestinal γδ IEL express the epithelial cell growth factor KGF (keratinocyte growth factor; ref. 8). γδ IEL isolated from other tissues such as skin also produced KGF after activation (8). In contrast, resting and activated intestinal αβ IEL do not express KGF. Thus, the ability to produce KGF appears to be a conserved feature of γδ IEL populations, suggesting that these cells share a similar specialized role in their tissue of residence.
KGF, a member of the fibroblast growth factor (FGF) family, recognizes a splice variant of one of the FGF receptor (FGFR) expressed by epithelial cells (9–12). Administration of recombinant KGF (rKGF) to animals promote the growth and differentiation of epithelial cells located in various organs (13–16). In particular, epithelial cells lining the gastrointestinal tract are highly responsive to rKGF (17). Furthermore, rKGF promote skin, lung, and intestinal tissue regeneration in model systems of injury (15, 16, 18, 19). Supporting a role for KGF in tissue repair, both KGF and KGFR are overexpressed in intestinal tissues obtained from inflammatory bowel disease (IBD) patients (20, 21). The cellular origin of KGF in human IBD remains ambiguous but fibroblasts and T lymphocytes were identified as judged from an analysis of the topographic distribution of these cells and KGF mRNA (20). These data suggest that KGF is an important growth factor to help maintain and/or restore the integrity of epithelial tissues after injury. Because activated γδ IEL represent a cellular source of KGF normally found in close physical contact with intestinal epithelial cells, we investigated whether this T cell population has a role in maintaining the integrity of the intestinal epithelium under stress conditions.
Intestinal epithelial cell damage was induced in mice by using dextran sodium sulfate (DSS) administered in drinking water (22–24). In this established model system, lesions to the large intestine occur in the absence of T cells and are detectable within the first few days after initiation of DSS treatment. Termination of DSS treatment leads to the progressive repair of mucosal lesions. These features of DSS-induced colitis were deemed appropriated to evaluate the role of γδ IEL during destruction and repair of the intestinal mucosa. Here, we present evidence that γδ IEL help maintain intestinal integrity by promoting the repair of epithelial lesions. Furthermore, we show, by using several approaches, that γδ IEL-derived KGF is a component of this protective mechanism. Our study documents, by using a whole animal model system, the importance of γδ IEL for preserving the intestinal epithelium under stress conditions.
Materials and Methods
Animal Studies.
Groups of 3–6 C57BL/6 mice were fed 2.5% DSS (molecular weight 40,000; ICN Biomedicals) in drinking water ad libitum. The presence of blood in feces could be detected after 2 days of treatment in 50% of mice. By day 4, 100% of DSS-treated mice were positive for the presence of occult blood in stools (Hemoccult; SmithKline Diagnostics, San Jose, CA). In regeneration studies, mice were treated with DSS for 5 days and returned to regular drinking water for an additional 2–60 days. For histology, the large intestine was dissected from its mesentery and segments (1–2 cm) were fixed in 10% neutral buffered formalin and paraffin-embedded. Sections (5 μm) were stained with periodic acid/Schiff (PAS) reaction and hematoxylin. Alternately, tissue segments were embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA), frozen in liquid nitrogen, and cut into 5- to 8-μM sections.
Grading of Colitis.
Tissue sections were graded for the extent of crypt damage and regeneration. In brief, the sections were graded with a range from 0 to 4 as to the amount of crypt damage or regeneration as follows. For crypt damage: 0 = none; 1 = basal 1/3; 2 = basal 2/3; 3 = only surface epithelium intact; 4 = entire crypt and epithelium lost. For regeneration: 4 = no tissue repair; 3 = surface epithelium not intact; 2 = regeneration with crypt depletion; 1 = almost complete regeneration; 0 = complete regeneration or normal tissue. Each section was then assigned a final score for each feature separately by establishing the product of the grade for that feature and the percentage involvement where 1 = 1–25%; 2 = 26–50%; 3 = 51–75%; 4 = 76–100%.
Immunohistochemistry.
Anti-TCR γδ (biotin-GL3, PharMingen) and anti-TCR αβ (biotin-H57-597, PharMingen) antibodies were deposited onto acetone-fixed, avidin-biotin blocked (Vector) frozen sections at a final concentration of 5 μg/ml in PBS containing 2% FBS for 2 h. Slides were washed, and then incubated with peroxidase-conjugated streptavidin (Jackson ImmunoResearch) for 1 h. Slides were then incubated in metal-enhanced diaminobenzidine (Pierce) to detect bound antibody complexes, and were counterstained with hematoxylin. Staining specificity for appropriate antigens was documented by a demonstrable lack of reactivity with species matched control antibodies and in the absence of primary antibody.
In Situ Hybridization.
For details regarding in situ hybridization, see supporting information, which is published on the PNAS web site, www.pnas.org.
Southern (cDNA) Blots.
Mononuclear cells were isolated by Percoll discontinuous-gradient centrifugation from the large intestine of mice essentially as described (4). γδ IEL and αβ IEL were sorted by flow cytometry, and the resulting cell populations were more than 98% homogeneous (8). Total RNA was extracted from purified intestinal T cell populations employing TRIzol reagent (GIBCO/BRL). RT-PCR using KGF and β-actin specific primers were performed as described (8). PCR conditions for 30 cycles were 94°C for 1 min, 55°C for 30 s, and 72°C for 30 s. PCR-generated cDNA was resolved by agarose gel electrophoresis, transferred to nylon membrane (Hybond-N, Amersham Pharmacia) and processed for hybridization and detection as directed by the manufacturer. A mouse KGF cDNA probe was labeled to a specific activity of 109 cpm/μg with α-32P-labeled 2′-deoxycytosine 5′-triphosphate by the random priming method (8).
Cellular Proliferation.
Cells undergoing DNA replication in vivo were labeled with 5-bromo-2′-deoxyuridine (BrdUrd; 50 mg/kg of body weight) from a freshly made stock solution (5 mg/ml) dissolved in PBS. In brief, BrdUrd was administered by i.p. injection to groups of 3–6 mice three times at 8-h intervals. At 24-h, mice were killed, and the large intestine was dissected from the mesentery. Sections (5–8 μm) from frozen tissue were air-dried, fixed with 70% ethanol, treated sequentially with 2N HCl for 20 min and 0.1 M Borax for 2 min, followed by washes in PBS, 1% Tween-20 in PBS, and 2% FBS in PBS. Slides were then incubated for 1 h with monoclonal rat anti-BrdUrd antibody (MAS 250b, Harlan Sera-Lab, Loughborough, U.K.) followed by further incubations with biotinylated monoclonal mouse anti-rat IgG antibody (Jackson ImmunoResearch), peroxidase-conjugated streptavidin (Jackson ImmunoResearch), and metal-enhanced diaminobenzidine (Pierce).
Results
Characterization of DSS-Induced Colitis in C57BL/6 Mice.
Previous studies characterized the clinical symptoms and intestinal pathology induced by DSS fed to Swiss–Webster, Balb/C and CBA/J mice (22, 24). These studies and others have revealed significant variations in disease severity between mouse strains. Because several gene-knockout mouse strains of potential interest for this study were available to us on the C57BL/6 genetic background, we initially characterized DSS-induced murine colitis in wild-type C57BL/6 mice. C57BL/6 mice fed DSS at a concentration of 2.5% (wt/vol) developed colitis after 4 days of treatment as judged from the presence of occult blood in feces and weight loss. Treatment with DSS for more than 7 days caused death in some mice. Thus, DSS experiments reported here were arbitrarily limited to a maximum treatment period of 5 days.
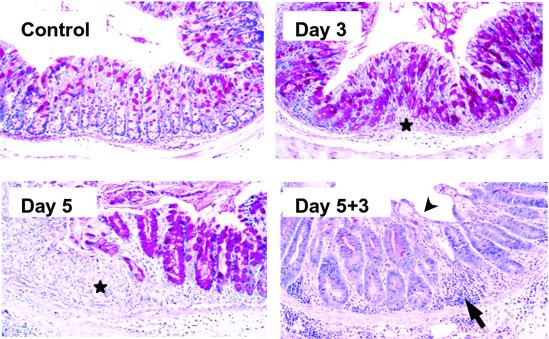
Microscopic examinations of the gastrointestinal tract established that C57BL/6 mice fed DSS for defined time periods had reproducible ulcerations of the large intestine (Fig. 1). The colon displayed signs of mucosal erosion including epithelial cell loss and crypt shortening as early as 3 days after initiation DSS treatment. Crypt dropout and edema were evident by day 5 of DSS treatment (Fig. 1) Lesions to the intestinal mucosa occurred as discrete areas surrounded by relatively normal epithelium. Epithelial cell damage was confined to the colon and did not involve other regions of the gastrointestinal tract, such as the small intestine (data not shown). Inflammation was also clearly evident by day 5 of DSS treatment. This finding is in agreement with previous observations that inflammation in the DSS-induced colitis model systems is a rather late event that follows histological evidence of tissue damage (24, 25).
Fig 1.
Histopathology of the colon in C57BL/6 mice subjected to DSS treatment. Representative areas of normal mucosa, ulcerated mucosa after 3 days and 5 days of DSS treatment, and mucosal regeneration 3 days after termination of DSS treatment. Ulcerated areas are shown by stars, mononuclear cell infiltration is shown by an arrow, and areas of surface re-epithelialization are shown by an arrowhead. Similar results were obtained from at least four independent experiments. (Original magnification = ×200.)
Repair of DSS-induced lesions was evaluated by returning DSS-treated mice to regular drinking water and analyzing intestinal tissue at defined intervals for a maximum period of 4 weeks. Clear signs of mucosal healing, including surface re-epithelialization and hyperplastic crypts, were evident as early as 3 days after termination of DSS treatment (Fig. 1).
Mice Lacking γδ T Cells Display a Severe DSS Colitis.
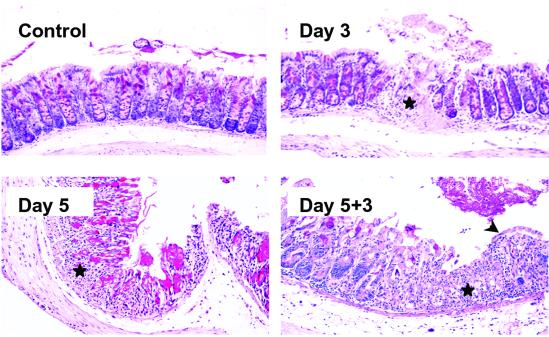
The ability of γδ T cells to protect the intestinal mucosa from DSS damage was evaluated by using TCRδ−/− mice (26). Several major differences in disease severity were observed between DSS-treated TCRδ−/− mice (Fig. 2) and wild-type mice (Fig. 1). TCRδ−/− mice had a more severe erosion of the mucosa at day 3 after initiation of DSS treatment (Fig. 2). These lesions consisted of focal transmural ulcerations typically distinct from the limited epithelial cell loss observed in wild-type mice. This feature was consistently seen in DSS-treated TCRδ−/− mice but was rarely observed in C57BL/6 mice (<10%). More interesting, repair of damaged epithelium was dramatically impaired in TCRδ−/− mice as shown by minimal surface re-epithelialization 3 days after termination of DSS treatment (Fig. 2). At this time point, crypts were only beginning to reappear within eroded areas. In contrast, regions of damaged mucosa in identically treated wild-type mice already contained numerous hyperplastic crypts. These data suggest that γδ IEL have a beneficial role in maintaining and/or restoring the colonic epithelium after DSS treatment.
Fig 2.
Heightened susceptibility of TCRδ−/− mice to DSS-induced colitis. Representative areas of normal mucosa, ulcerated mucosa after 3 days and 5 days of DSS treatment, and mucosal regeneration 3 days after termination of DSS treatment. Ulcerated areas are indicated by stars, and areas of surface re-epithelialization are indicated by an arrowhead. TCRδ−/− mice had consistently more severe colonic damage compared with wild type. Evidence of regeneration, and in particular surface re-epithelialization, was scant 3 days after termination of DSS treatment. Similar results were obtained in three independent experiments. (Original magnification = ×200.)
Examination of DSS-treated mice mutant lacking αβ T cells (TCRα−/−) did not reveal significant differences in tissue damage and repair when compared with C57BL/6 mice (data not shown). This finding suggests a minor role for αβ T cells in the initiation of DSS colitis and in the mechanisms influencing tissue repair after DSS removal. It is important to note here that TCRα−/− mice 12 weeks old and older have been reported to develop a spontaneous colitis involving an unusual T cell population expressing a TCR β-chain homodimer (27–30). All TCRα−/− mice used in our study were ≈8 weeks old and had no obvious intestinal disease before DSS treatment.
KGF−/− Mice and TCRδ−/− Mice Share a Heightened Susceptibility to DSS Colitis.
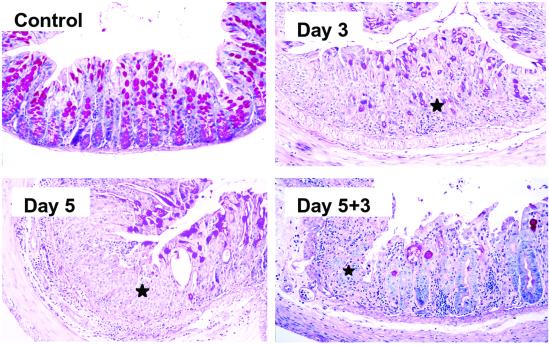
We and others previously suggested that KGF may be protective for epithelial tissues (8, 15, 16, 31, 32). Supporting this possibility, we determined that KGF−/− mice (33) are more susceptible to DSS colitis (Fig. 3) than wild-type animals (Fig. 1). The phenotype displayed by KGF−/− mice after DSS treatment was similar to that observed for TCRδ−/− mice. However, compared with TCRδ−/− mice, KGF−/− mice had more widespread mucosal lesions, which included the distal portion of the small intestine (data not shown). This phenotype probably reflects the complete lack of KGF expression in the mucosa of KGF−/− mice compared with the potential for KGF expression from mucosal fibroblasts in the TCRδ−/− mice.
Fig 3.
KGF−/− mice suffer from a severe form of DSS colitis. Representative areas of normal mucosa, ulcerated mucosa after 3 days of DSS treatment, ulcerated mucosa after 5 days of DSS treatment, and mucosal regeneration 3 days after termination of DSS treatment. Ulcerated areas are indicated by stars. Almost complete ulceration of the colonic mucosa occurred after 3 days of DSS treatment. Repair of colonic lesions was significantly impaired compared with that in wild-type mice, and was similar to that observed in TCRδ−/− mice. Similar results were obtained in three independent experiments. (Original magnification = ×200.)
Delayed Repair of Intestinal Lesions in TCRδ−/− Mice and KGF−/− Mice.
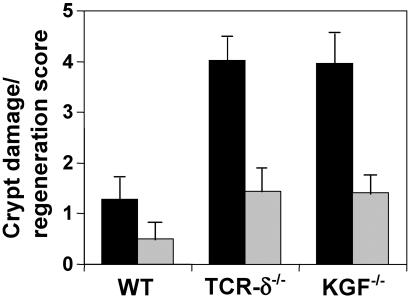
In wild-type mice, essentially complete repair of all intestinal lesions was observed 14 days after termination of DSS treatment (Fig. 4). A clear differences in tissue repair between wild-type mice and either TCRδ−/− mice or KGF−/− mice was still evident at day 14 after termination of DSS treatment (Fig. 4). At this time, the colon of similarly treated KGF−/− mice resembled that of TCRδ−/− mice. In these mice surface re-epithelialization was well-established but, in contrast to wild-type mice, crypts were still mostly absent from DSS-damaged areas. In fact, TCRδ−/− and KGF−/− mice required more than 4 weeks to attain the level of intestinal regeneration displayed in C57BL/6 mice after 2 weeks (data not shown).
Fig 4.
Histological grading of tissue damage and regeneration. Bars represent the ratio of the colonic damage score divided by the tissue regeneration score 3 days (black bars) and 14 days (gray bars) after termination of DSS treatment. TCRδ−/− mice and KGF−/− have significantly more colonic damage and less tissue regeneration compared with wild-type mice. Data are expressed as the mean and SD of a minimum of 12 sections representing the distal and medial colon of at least 3 mice per group. Data are representative of three experiments.
Intestinal γδ T Cells in DSS-Treated Mice Localize Near Damaged Areas and Express KGF.
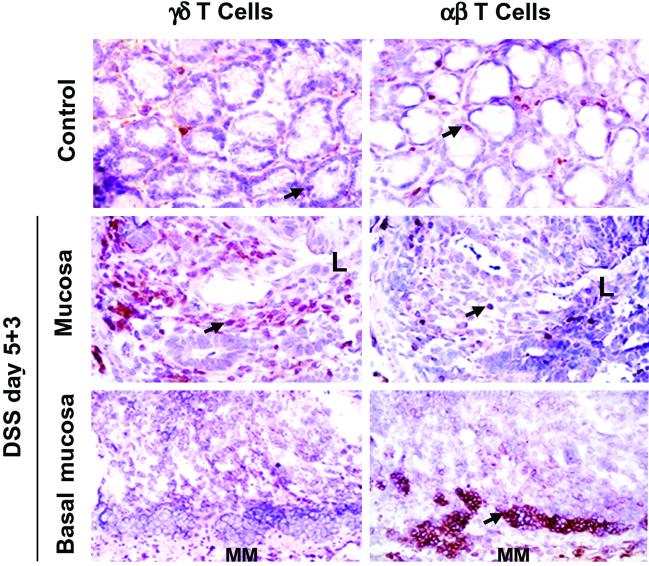
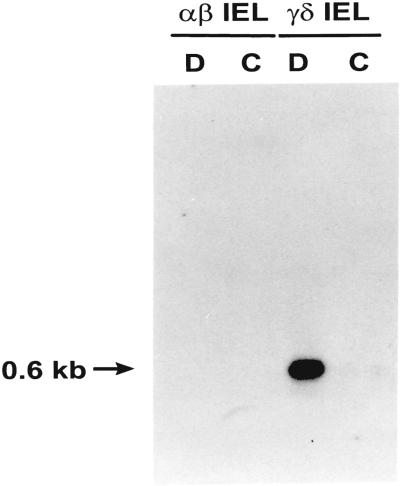
Experiments were designed to localized γδ IEL within the large intestine of DSS-treated C57BL/6 mice. Immunohistochemical studies in DSS-treated mice showed an elevated number of γδ IEL, but not αβ IEL in close proximity to areas of damaged intestinal epithelia (Fig. 5). A progressive return to normal γδ IEL densities correlated with the appearance of regenerative epithelium and histological evidence of tissue repair (data not shown). In contrast, αβ T cells localized to lymphocyte aggregates present near the basement membrane that contained relatively few γδ T cells (Fig. 5). These aggregates are a classical feature of DSS-induced colitis of unknown functional importance. Thus, our results show that γδ IEL are found in large numbers predominantly within intestinal areas undergoing active tissue repair after termination of DSS treatment. Next, we evaluated the potential for KGF mRNA expression by freshly isolated γδ IEL recovered from DSS-treated wild-type mice. KGF mRNA expression was detected in purified γδ IEL, but not in purified αβ IEL from DSS-treated mice (Fig. 6). In contrast, purified γδ and αβ IEL from untreated mice did not express KGF (Fig. 6). We next determined the localization of KGF mRNA positive cells in the colon of control and DSS-treated mice by in situ hybridization. KGF mRNA was expressed in a constitutive fashion in control mice by cells corresponding to fibroblasts by morphological criteria (Fig. 8, which is published as supporting information on the PNAS web site). These cells were detected in wild-type, TCRδ−/− and TCRα−/− mice. We found no evidence that IEL expressed KGF mRNA in control wild-type mice. After termination of DSS treatment, KGF mRNA expression was strongly up-regulated in wild-type mice and TCRα−/− mice, but not in TCRδ−/− mice (Fig. 8). In wild-type and TCRα−/− mice, zones of intense KGF expression colocalized with areas identified as containing high-densities of γδ IEL by immunohistochemistry (Figs. 5 and 8). Taken together, these data strongly suggest that intestinal γδ IEL are activated in vivo to express KGF after DSS treatment.
Fig 5.
Localization of γδ T cells and αβ T cells in the colon of DSS-treated wild-type mice. Arrowheads point to representative positive cells. In wild-type mice recovering from DSS-colitis, γδ T cells were found in large numbers within areas of mucosal damage, whereas αβ T cells were detected predominantly in aggregates near the basal mucosa. Within damaged crypts, the number of γδ T cells averaged 24.06 ± 14.40, and the number of αβ T cells was 1.72 ± 1.67 (t test, P < 0.001, n = 18). Increased numbers of γδ T cells were detected as early as 3 days after initiation of DSS treatment (data not shown). L, the orientation of the section shown relative to the lumen; MM, muscularis mucosae. (Original magnification = ×400.)
Fig 6.
KGF expression in the intestine of DSS-treated mice. KGF mRNA expression by purified intestinal γδ IEL isolated from DSS-treated C57BL/6 mice. D, IEL recovered from the intestine 3 days after termination of DSS treatment; C, IEL isolated from control untreated mice. Similar results were obtained in two independent experiments.
Decreased Intestinal Epithelial Cell Proliferation in TCRδ−/− Mice and KGF−/− Mice After Termination of DSS Treatment.
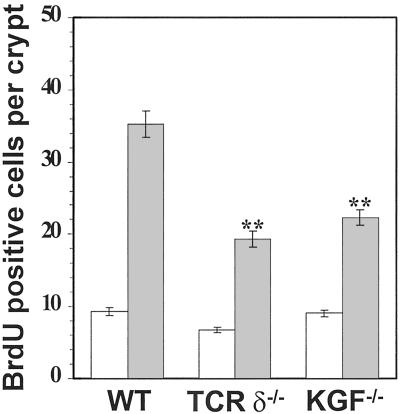
The rate of epithelial cell proliferation in DSS-treated mice was determined by administration of the DNA precursor analog BrdUrd. Preliminary experiments established that DSS treatment reduced the average number of proliferating cells per colonic crypt. In wild-type mice, the average number of BrdUrd-positive cells dropped from 10 cells per crypt in untreated control mice to less than 1 cell per crypt after 5 days of DSS treatment (data not shown). This reduced rate of epithelial cell proliferation was observed as early as 2 days after initiation of DSS treatment. Thus, even though the exact mode of action for DSS in vivo remains unknown, we show here that this chemical compound effectively down-regulates intestinal epithelial cell proliferation. This result is consistent with a previous report of reduced proliferation and viability of epithelial cells cultured in the presence of DSS (34). Termination of DSS treatment in wild-type mice resulted in a dramatic increased in the number of BrdUrd-labeled intestinal epithelial cells above the constitutive proliferation rate (Fig. 7). In contrast, a significantly smaller proportion of epithelial cells were found to undergo cell division in the large intestine of untreated TCRδ−/− mice (Fig. 7). A similar observation was previously reported in a study of epithelial cell proliferation in the small intestine of TCRδ−/− mice (4). Importantly, the absolute number of BrdUrd+ cells intestinal epithelial cells in TCRδ−/− mice and KGF−/− mice was significantly reduced after termination of DSS-treatment. These data suggest that γδ IEL-derived KGF delivered at sites of intestinal injury may enhance the rate of tissue repair by stimulating epithelial cell proliferation.
Fig 7.
Decreased intestinal epithelial cell proliferation in TCRδ−/− mice and KGF−/− mice recovering from DSS-induced colitis. A minimum of 4 representative longitudinal sections of the large intestine for each animal group were enumerated for BrdUrd-positive cells. The data are presented for untreated mice (white bars) and for mice 3 days after termination of DSS treatment (gray bars). Data are expressed as the mean BrdUrd-positive cells per crypt and SD; **, P < 0.01.
Discussion
Despite intense efforts, the role played by γδ IEL in health and disease remains unclear. Several putative functions have been proposed for γδ IEL including cytolytic removal of damaged cells and supply of epithelial cell growth factors (35–38). The data presented here define a specialized function for intestinal γδ T cells in preserving the integrity of stressed or injured intestinal mucosa. We propose that the apparent specificity of γδ IEL for stress-induced self antigen(s) may explain the activation of γδ IEL observed here in DSS-treated wild-type mice (39–42). This specificity would allow activated γδ IEL to release KGF near damaged epithelial cells. Our data suggest that this release of KGF promotes localized epithelial cell proliferation. However, KGF is also know to take part in epithelial cell differentiation and such a role may also be relevant in tissue repair (43). This possibility is being addressed by monitoring the expression of epithelial cell differentiation markers during intestinal regeneration in the various mouse strains used in this study.
Our data also show that regeneration of the intestinal mucosa is significantly delayed but not abrogated in TCRδ−/− and KGF−/− mice. The existence of parallel or compensatory mechanisms operating to maintain intestinal homeostasis may account for the ability of those mice to repair intestinal lesions. These alternative pathways may involve other cytokines including members of the FGF family other than KGF. Of interest, FGF-10 was recently shown to be functionally similar to KGF despite limited amino acid sequence identity (44, 45). Presumably, this can be accounted for by the finding that both FGF-10 and KGF interact with the same FGFR splice variant (46).
Finally, γδ IEL residing in skin, another epithelial tissue, were recently shown to perform a role similar to that described here in the intestine (47). Thus, the events described here may be part of a general immune mechanism involving γδ IEL that serves to monitor and maintain the integrity of epithelial tissues.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (to R.B. and W.L.H.), the Crohn's and Colitis Foundation of America (to R.B.), the Strohm IBD Center at Scripps Clinic (to R.B.), and the Leukemia and Lymphoma Society (to W.L.H.). Y.C. was a fellow of the Crohn's and Colitis Foundation of America. This is manuscript 11762-IMM from The Scripps Research Institute.
Abbreviations
IEL, intraepithelial T lymphocytes
DSS, dextran sodium sulfate
TCR, T cell antigen receptor
KGF, keratinocyte growth factor
rKGF, recombinant KGF
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Trejdosiewicz L. K., Calabrese, A., Smart, C. J., Oakes, D. J., Howdle, P. D., Crabtree, J. E., Losowsky, M. S., Lancaster, F. & Boylston, A. W. (1991) Immunology 84, 440-444. [PMC free article] [PubMed] [Google Scholar]
- 2.Fukashima K., Masuda, T., Ohtani, H., Sasaki, I., Funayama, Y., Matsuno, S. & Nagura, H. (1991) Gastroenterology 101, 670-678. [DOI] [PubMed] [Google Scholar]
- 3.Giacommelli R., Parzanese, I., Frieri, G., Passacantando, A., Pizzuto, F., Pimpo, T., Cipriani, P., Viscido, A., Caprilli, R. & Tonietti, G. (1994) Clin. Exp. Immunol. 98, 83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komano H., Fujiura, Y., Kawaguchi, M., Matsumoto, S., Hashimoto, Y., Obana, S., Mombaerts, P., Tonegawa, S., Yamamoto, H., Itohara, S., et al. (1995) Proc. Natl. Acad. Sci. USA 92, 6147-6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soderstrom K., Bucht, A., Halapi, E., Gronberg, A., Magnusson, I. & Kiessling, R. (1996) J. Immunol. 156, 2331-2339. [PubMed] [Google Scholar]
- 6.McVay L. D., Li, B., Biancaniello, R., Creighton, M. A., Bachwich, D., Lichtenstein, G., Rombeau, J. L. & Carding, S. R. (1997) Mol. Med. 3, 183-203. [PMC free article] [PubMed] [Google Scholar]
- 7.Kagnoff M. F. (1998) Am. J. Physiol. 274, G455-G458. [DOI] [PubMed] [Google Scholar]
- 8.Boismenu R. & Havran, W. L. (1994) Science 266, 1253-1255. [DOI] [PubMed] [Google Scholar]
- 9.Finch P. W., Rubin, J. S., Miki, T., Ron, D. & Aaronson, S. A. (1989) Science 245, 752-755. [DOI] [PubMed] [Google Scholar]
- 10.Miki T., Fleming, T. P., Bottaro, D. P., Rubin, J. S., Ron, D. & Aaronson, S. A. (1991) Science 251, 72-75. [DOI] [PubMed] [Google Scholar]
- 11.Miki T., Bottaro, D. P., Fleming, T. P., Smith, C. L., Burgess, W. H., Chan, A. M.-L. & Aaronson, S. A. (1992) Proc. Natl. Acad. Sci. USA 89, 246-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yayon A., Zimmer, Y., Guo-Hong, S., Avivi, A., Yarden, Y. & Givol, D. (1992) EMBO J. 11, 1885-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulich T. R., Yi, E. S., Longmuir, K., Yin, S., Biltz, R., Morris, C. F., Housley, R. M. & Pierce, G. F. (1994) J. Clin. Invest. 93, 1298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulich T. R., Yi, E. S., Cardiff, R., Yin, S., Bikhazi, N., Biltz, R., Morris, C. F. & Pierce, G. F. (1994) Am. J. Pathol. 144, 862-868. [PMC free article] [PubMed] [Google Scholar]
- 15.Staiano-Coico L., Krueger, J. G., Rubin, J. S., D'limi, S., Vallat, V. P., Valentino, L., Fahey, T., Hawes, A., Kingston, G., Madden, M. R., et al. (1993) J. Exp. Med. 178, 865-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce G. F., Yanagihara, D., Klopchin, K., Danilenko, D. M., Hsu, E., Kenney, W. C. & Morris, C. F. (1994) J. Exp. Med. 179, 831-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Housley R. M., Morris, C. F., Boyle, W., Ring, B., Biltz, R., Tarpley, J. E., Aukerman, S. L., Devine, P. L., Whitehead, R. H. & Pierce, G. F. (1994) J. Clin. Invest. 94, 1764-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi E. S., Williams, S. T., Lee, H., Malicki, D. M., Chin, E. M., Yin, S., Tarpley, J. & Ulich, T. R. (1996) Am. J. Pathol. 149, 1963-1970. [PMC free article] [PubMed] [Google Scholar]
- 19.Zeeh J. M., Procaccino, F., Hoffmann, P., Aukerman, S. L., McRoberts, J. A., Soltani, S., Pierce, G. F., Lakshmanan, J., Lacey, D. & Eysselein, V. E. (1996) Gastroenterology 110, 1077-1083. [DOI] [PubMed] [Google Scholar]
- 20.Finch P. W., Pricolo, V., Wu, A. & Finkelstein, S. D. (1996) Gastroenterology 110, 441-451. [DOI] [PubMed] [Google Scholar]
- 21.Brauchle M., Madlener, M., Wagner, A. D., Angermeyer, K., Lauer, U., Hofschneider, P. H., Gregor, M. & Werner, S. (1996) Am. J. Pathol. 149, 521-529. [PMC free article] [PubMed] [Google Scholar]
- 22.Okayasu I., Hatakeyama, S., Yamada, M., Ohkusa, T., Inagaki, Y. & Nakaya, R. (1990) Gastroenterology 98, 694-702. [DOI] [PubMed] [Google Scholar]
- 23.Elson C. O., Sartor, R. B., Tennyson, G. S. & Riddell, R. H. (1995) Gastroenterology 109, 1344-1367. [DOI] [PubMed] [Google Scholar]
- 24.Cooper H. S., Murthy, S. N. S., Shah, R. S. & Sedergran, D. J. (1993) Lab. Invest. 69, 238-249. [PubMed] [Google Scholar]
- 25.Axelsson L. G., Landstrom, E., Goldschmidt, T. J., Gronberg, A. & Bylund-Fellinius, A.-C. (1996) Inflamm. Res. 45, 181-191. [DOI] [PubMed] [Google Scholar]
- 26.Itohara S., Mombaerts, P., Lafaille, J. J., Iacomini, J., Nelson, A., Clarke, A. R., Hooper, M. L., Farr, A. & Tonegawa, S. (1993) Cell 72, 337-348. [DOI] [PubMed] [Google Scholar]
- 27.Mizoguchi A., Mizoguchi, E., Chiba, C., Spiekermann, G. M., Tonegawa, S., Nagler-Anderson, C. & Bhan, A. K. (1996) J. Exp. Med. 183, 847-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi T., Kiyono, H. & Hamada, S. (1997) Gastroenterology 112, 1876-1886. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi I., Iijima, H., Katashima, R., Itakura, M. & Kiyono, H. (1999) J. Immunol. 162, 1843-1850. [PubMed] [Google Scholar]
- 30.Mizoguchi A., Mizoguchi, E. & Bhan, A. K. (1999) Gastroenterology 116, 320-326. [DOI] [PubMed] [Google Scholar]
- 31.Werner S., Smola, H., Liao, X., Longaker, M. T., Krieg, T., Hofschneider, P. H. & Williams, L. T. (1994) Science 266, 819-822. [DOI] [PubMed] [Google Scholar]
- 32.Werner S., Peters, K. G., Longaker, M. T., Fuller-Pace, F., Banda, M. J. & Williams, L. T. (1992) Proc. Natl. Acad. Sci. USA 89, 6896-6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo L., Degenstein, L. & Fuchs, E. (1996) Genes Dev. 10, 165-175. [DOI] [PubMed] [Google Scholar]
- 34.Dieleman L. A., Ridwan, B. U., Tennyson, G. S., Beagley, K. W., Bucy, R. P. & Elson, C. O. (1994) Gastroenterology 107, 1643-1652. [DOI] [PubMed] [Google Scholar]
- 35.Shires J., Theodoridis, E. & Hayday, A. C. (2001) Immunity 15, 419-434. [DOI] [PubMed] [Google Scholar]
- 36.Fahrer A. M., Konigshofer, Y., Kerr, E. M., Ghandour, G., Mack, D. H., Davis, M. M. & Chien, Y. H. (2001) Proc. Natl. Acad. Sci. USA 98, 10261-10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carding S. R. & Egan, P. J. (2002) Nat. Rev. Immunol. 2, 336-345. [DOI] [PubMed] [Google Scholar]
- 38.Hayday A., Theodoridis, E., Ramsburg, E. & Shires, J. (2001) Nat. Immunol. 2, 997-1003. [DOI] [PubMed] [Google Scholar]
- 39.Havran W. L., Chien, Y.-H. & Allison, J. P. (1991) Science 252, 1430-1432. [DOI] [PubMed] [Google Scholar]
- 40.Catalfamo M., Roura-Mir, C., Sospedra, M., Aparicio, P., Costagliola, S., Ludgate, M., Pujol-Borrell, R. & Jaraquemada, D. (1996) J. Immunol. 156, 804-811. [PubMed] [Google Scholar]
- 41.Maeurer M. J., Martin, D., Walter, W., Liu, K., Zitvogel, L., Halusczcak, K., Rabinowich, H., Duquesnoy, R., Storkus, W. & Lotze, M. T. (1996) J. Exp. Med. 183, 1681-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groh V., Steinle, A., Bauer, S. & Spies, T. (1998) Science 279, 1737-1740. [DOI] [PubMed] [Google Scholar]
- 43.Byrne F. R., Farrell, C. L., Aranda, R., Rex, K. L., Scully, S., Brown, H. L., Flores, S. A., Gu, L. H., Danilenko, D. M., Lacey, D. L., et al. (2002) Am. J. Physiol. Gastrointest. Liver Physiol. 282, G690-G701. [DOI] [PubMed] [Google Scholar]
- 44.Beer H. D., Florence, C., Dammeier, J., McGuire, L., Werner, S. & Duan, D. R. (1997) Oncogene 15, 2211-2218. [DOI] [PubMed] [Google Scholar]
- 45.Tagashira S., Harada, H., Katsumata, T., Itoh, N. & Nakatsuka, M. (1997) Gene 197, 399-404. [DOI] [PubMed] [Google Scholar]
- 46.Igarashi M., Finch, P. W. & Aaronson, S. A. (1998) J. Biol. Chem. 273, 13230-13235. [DOI] [PubMed] [Google Scholar]
- 47.Jameson J., Ugarte, K., Chen, N., Yachi, P., Fuchs, E., Boismenu, R. & Havran, W. L. (2002) Science 296, 747-749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.