Abstract
Lymphocyte-like cells in the intestine of the sea lamprey, Petromyzon marinus, were isolated by flow cytometry under light-scatter conditions used for the purification of mouse intestinal lymphocytes. The purified lamprey cells were morphologically indistinguishable from mammalian lymphocytes. A cDNA library was prepared from the lamprey lymphocyte-like cells, and more than 8,000 randomly selected clones were sequenced. Homology searches comparing these ESTs with sequences deposited in the databases led to the identification of numerous genes homologous to those predominantly or characteristically expressed in mammalian lymphocytes, which included genes controlling lymphopoiesis, intracellular signaling, proliferation, migration, and involvement of lymphocytes in innate immune responses. Genes closely related to those that in gnathostomes control antigen processing and transport of antigenic peptides could be ascertained, although no sequences with significant similarity to MHC, T cell receptor, or Ig genes were found. The data suggest that the evolution of lymphocytes in the lamprey has reached a stage poised for the emergence of adaptive immunity.
Vertebrates with jaws (gnathostomes) possess an adaptive (anticipatory) immune system that is absent in nonvertebrates (1). The hallmark of the system is its capacity to anticipate an encounter with any protein or carbohydrate foreign to the body and to retain a memory of this encounter. This memory enables the organism to respond in an accelerated manner after reexposure to the same antigenic stimulus. The adaptive immune system is comprised of a complex array of different cell types and molecules intricately interacting with one another. In this array, three cell types and three types of molecules are central to the immune-system function. These include T and B lymphocytes as well as the dendritic cells, all derived from a common hemopoietic stem cell (2), and the T cell receptor (TCR), B cell receptor (BCR, Ig), and MHC molecules. Lymphocytes of jawed vertebrates are small cells in which the nucleus, characterized by a densely compacted, coarse chromatin, occupies ≈90% of the cell volume, and the cytoplasm is limited to a thin rim (3). They possess the ability to respond to stimuli by enlarging their volume (lymphoblast transformation) and dividing repeatedly. Although some of the activated lymphocytes terminally differentiate into effector cells, others return to the state of small, resting cells (1). The proliferative phase multiplies those lymphocytes that express TCR or Ig receptors specific for the stimulating antigen and thus expands them into clones of cells that express the same receptor. The return to the morphology of small lymphocytes marks the generation of memory cells. The differentiated lymphocytes can kill infected target cells (cytotoxic T lymphocytes), assist other cells in their development (helper T lymphocytes), or become Ig (antibody)-secreting plasma cells (B lymphocytes).
TCR, Ig, and MHC receptors have been found thus far only in jawed vertebrates. Attempts to demonstrate their presence in living jawless vertebrates, represented by lampreys and hagfishes, have failed (4). On the other hand, small cells with darkly staining nuclei and scanty cytoplasm have been observed in histological sections prepared from either lamprey or hagfish tissues (5). Because of the failure to obtain clear evidence for the existence of the adaptive immune system in jawless fishes, the identity of these cells has remained in doubt. The aim of the present study was to exploit contemporary methods of cell separation to isolate the agnathan lymphocyte-like cells to characterize them morphologically and by the genes they express.
Materials and Methods
Source and Preparation of Cell Suspension.
Ammocoete larvae (8–13 cm long) of the sea lamprey, Petromyzon marinus (from Lake Huron, MI) were dissected along the ventral side to extract the intestine and the associated typhlosole (spiral valve). Cells harvested by maceration of these tissues between two glass slides were suspended in one part water and two parts PBS. Lamprey intestinal cells with forward and sideward light-scattering characteristics of a control population of intraepithelial lymphocytes from the mouse intestine were identified and sorted by using the MoFlo cytometer (Cytomation, Fort Collins, CO). Sorted cells were prepared for electron microscopy by fixation with 2.5% glutaraldehyde in 0.1 M cacodylate buffer for 1 h before centrifugation and resuspension in the same buffer. For light-microscopic evaluation, sorted cells were coated onto glass slides by using a cytocentrifuge, stained with Wright stain, washed, and photographed.
Construction of cDNA Library.
Total RNA was extracted from 1 × 106 sorted cells with the help of the RNeasy mini kit (Qiagen, Hilden, Germany). To construct a directional cDNA library with high representation of 5′ ends, the SMART cDNA library-construction kit (BD Biosciences/CLONTECH, Heidelberg, Germany) was used according to manufacturer protocol. Briefly, ≈200–300 ng of total RNA were reverse-transcribed by the PowerScript reverse transcriptase (BD Biosciences/CLONTECH), and the transcript was subjected to cDNA amplification in 25 cycles of long-distance PCR. The cDNA was size-fractionated by chromatography on CHROMA SPIN-400 columns, and fractions containing cDNA larger than 500 bp were pooled and directionally inserted into the λTriplEx2 vector. Ligated DNA was packaged into phage particles by using the Gigapack Gold in vitro packaging kit (Stratagene, Amsterdam) and transfected into the Escherichia coli strain XL1-Blue (BD Biosciences/CLONTECH), which then were plated. A total of 2 × 106 independent plaque-forming units was obtained. Not all inserts represented full-length cDNA sequences, presumably because of incomplete DNA synthesis and preferential amplification of small molecules in the PCR step used to increase the yield of cDNA. Insert sizes ranged from 500 bp to 4 kb, the majority being between 500 bp and 1.5 kb.
Analysis of Individual Clones.
The unamplified library was plated at a dilution yielding single, well isolated plaques. A total of 9,312 clones was picked manually and resuspended in 500 μl of lambda dilution buffer (100 mM NaCl/10 mM MgCl2/35 mM Tris⋅HCl, pH 7.5/0.01% gelatin). Insert DNA from 1 μl of the phage suspension was PCR-amplified in a 25-μl reaction volume with the primer pair TriplEx2LD5 (5′-CTCGGGAAGCGCGCCATTGTGTTGGT-3′) and TriplEx2LD3 (5′-ATACGACTCACTATAGGGCGAATTGGCC-3′) by either HotStar Taq polymerase (Qiagen) or the Expand long-template PCR system (Roche Diagnostics) using the manufacturer's buffer systems. Cycling conditions in a PTC-200 Peltier thermal cycler (MJResearch and Biozym, Hessisch Oldendorf, Germany) were: initial denaturation at 94°C for 2 min (Expand long-template PCR system) or 15 min (HotStar Taq polymerase), followed by 10 cycles of denaturation at 94°C for 10 sec, primer annealing at 65°C for 30 sec, and elongation at 68°C (Expand long-template PCR system) or 72°C (HotStar Taq polymerase) for 6 min. Twenty more PCR cycles then were added under the same conditions except for an incremental increase of 20 sec in the elongation step of each cycle. The PCR was completed by a final elongation for 7 min at 72°C. The PCR products were purified and subjected to single-pass sequencing by the custom-sequencing service of MediGenomix (Martinsried, Germany) using the pTripl5Seq2 (5′-GAAGCGCGCCATTGTGTT-3′) primer annealing next to the 5′ end of the insert cDNA. The sequences varied in length and quality, with useful sequences generally not exceeding 800 bp. Of the 9,312 sequences, 8,043 judged to be of high quality and free of di- or trinucleotide repeats were chosen for further analysis.
Sequence Analysis.
Insert sequences were subjected to BLASTX searches (6) against the nonredundant protein database at the National Center for Biotechnology Information (NCBI). Immunologically relevant sequence matches to database entries were examined in more detail. By using the NCBI BLASTCLUST program, the sequences were grouped into clusters of identical or overlapping sequences. In this step, 1,735 sequences were combined into clusters containing between 2 and 210 sequences.
Results and Discussion
Physical Characteristics of Lamprey Lymphocyte-Like Cells.
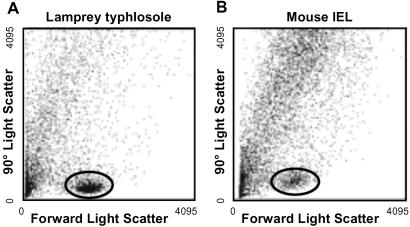
The typhlosole, an invaginated spiral valve spanning the length of the lamprey intestine, was the primary tissue source for the cells in this analysis. Cells derived from this tissue were analyzed in parallel with mouse intestinal epithelial cells by automated flow cytometry. The lamprey typhlosole contains a discrete subpopulation of cells with light-scatter characteristics that closely resemble those of the lymphocytes in the mouse intestinal epithelium (Fig. 1). This population of lamprey cells was isolated with an automated cell sorter for further analysis. Both light (Fig. 2) and electron (Fig. 3) microscopy reveal the purified lamprey cells to represent a fairly homogeneous population in terms of size, staining properties, and structure. The diameter of the cells ranges from 6 to 9 μm, mainly because of variation in the amount of cytoplasm present. Most of the cell volume is taken up by the darkly staining nucleus, the scanty, pale cytoplasm often being limited to a narrow rim in the light-microscopic preparations. In electron-microscopic preparations the nucleus is quite dense, filled with compacted chromatin. The cytoplasm is rich in ribosomes, both free and attached to the rough endoplasmic reticulum. The surface of most of the cells appears to be largely smooth. All these characteristics are histological hallmarks of “small lymphocytes” (7). Indeed, even experienced morphologists would be unable to distinguish the lamprey cells from mouse lymphocytes if presented with unmarked preparations of the two. Nevertheless, in the absence of sufficient information about the functional properties of the lamprey cells, we refer to them as “lymphocyte-like.”
Fig 1.
Fractionation of lamprey intestine cells by light-scattering characteristics using flow cytometry: forward (180°) and sideward (90°) light-scatter diagrams of lamprey typhlosole cells (A) and mouse intestinal intraepithelial lymphocytes (IEL, B). The populations containing small lymphocyte-like cells are encircled.
Fig 2.
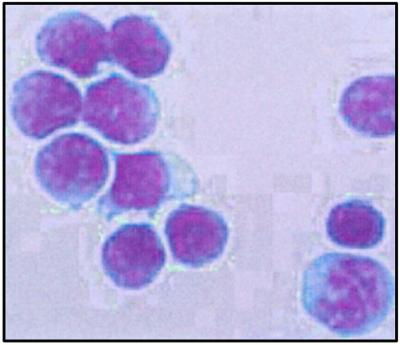
Light-microscopic view of sorted lamprey lymphocyte-like cells stained with Wright stain. The large, darkly stained nuclei and the thin rim of agranular cytoplasm is highly reminiscent of mammalian lymphocytes.
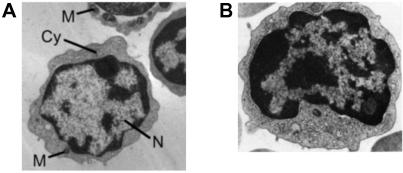
Fig 3.
Transmission electron-microscopic views of lamprey lymphocyte-like cells (A) and mouse intestinal epithelial lymphocytes (B). The cells contain large nuclei (N) with heterochromatin forming a peripheral rim adjacent to the nuclear envelope. The nuclei are surrounded by a thin layer of cytoplasm (Cy) containing several small spherical mitochondria (M).
Gene-Expression Profile.
The purified population of the lamprey lymphocyte-like cells was used as a source for RNA isolation, production of a cDNA library, and sequencing of randomly selected cDNA clones. In the collection of 8,043 sequences remaining after the exclusion of poor-quality sequences from the initial number of 9,312, there were 1,735 that were represented more than once in the set. Of these, sequences of 73 genes were found twice, 24 three times, and 18 four times. Some 15% of the 8,043 sequences were found in >10 copies. Most abundant in this set were mitochondrial RNA-derived sequences and rRNA, followed by a group of 178 ESTs of an unidentifiable gene, 157 sequences encoding ribosomal protein L41, and 135 ESTs of the ferritin heavy chain. The remaining clusters contained mostly heat-shock protein genes, genes encoding transcription- and translation-associated factors, as well as other housekeeping genes. The large number of unique sequences in the data set (6,308/8,043 or 78.4%) indicates that the library covered a wide range of genes expressed at low abundance. However, although the stringency of the BLASTX search was set rather low (a discrimination threshold of E < 0.009) so as to detect as many similarities between lamprey and mammalian genes as possible, the identity of only 2,590 of the 6,308 unique sequences (≈40%) could be established. This is an indication that a fraction of the ESTs may be derived from 3′ UTRs. The remaining 60% of unidentified sequences may represent either novel ESTs or sequences with very low similarity to known genes. As would be expected for metabolically active cells, ESTs of genes involved in the control of gene expression and protein synthesis were highly represented in the set. They included 36 genes encoding transcription factors, 15 genes participating in RNA synthesis and processing, 944 ribosomal protein-encoding genes, 81 genes specifying translation factors, and 6 genes functioning in signal peptide recognition (Table 1).
Table 1.
Classification of ESTs according to BLASTX search results
| Similarity group | Frequency |
|---|---|
| Mitochondrial-derived RNA | 0.032 |
| Ribosomal proteins | 0.260 |
| Signal transduction-associated proteins | 0.019 |
| Calcium-binding proteins | 0.007 |
| Retroelement-associated proteins and transposons | 0.031 |
| Ion channels and transporters molecules | 0.003 |
| Chaperones | 0.034 |
| Nuclear DNA-associated proteins | 0.013 |
| Transcription factors | 0.009 |
| Translation factors | 0.020 |
| Cell proliferation and differentiation-associated proteins | 0.010 |
| Structural proteins | 0.020 |
| Metabolic proteins | 0.057 |
| Other proteins | 0.090 |
| Ferritin | 0.043 |
| Proteins without significant similarity | 0.349 |
Below we highlight those ESTs that are homologous to mammalian genes prominently expressed in lymphocytes and that may be relevant to immune responses. Because of space limitations, we do not describe the genes from which the ESTs are derived here, nor do we provide evidence for their orthology with mammalian genes. For five of these genes, this information is given in the accompanying paper (8); for others it will be given in publications that are in preparation. The purpose of the present survey is to provide an overview necessary for drawing conclusions about the possible involvement of the lamprey lymphocyte-like cells in immunity. For convenience, the survey is divided into several functional categories.
Genes Controlling Lymphocyte Ontogeny.
The differentiation of mammalian lymphocytes is driven by a set of transcription factors that includes proteins of the Spi (9) and Ikaros (10, 11) families. Members of both families have been demonstrated in the lamprey (12–14), and evidence for their expression in tissues rich in lymphocyte-like cells has been provided. To demonstrate the expression of Spi and Ikaros genes in these cells directly, we performed RT-PCR with lamprey Spi-B and Ikaros-specific primers by using extracted total RNA. A strong band representing the expected PCR product was present in each case but was absent when the reverse-transcription step was omitted (data not shown). The lamprey EST set also contains sequences specifying other factors less specifically involved in the control of hemopoiesis (Table 2). They are exemplified by the mast/stem cell growth factor receptor, a c-Kit tyrosine kinase-type protein of the colony-stimulating factor 1/platelet-derived growth factor receptor family (15).
Table 2.
Results of BLASTX searches: Catalogue of proteins expressed in hematopoietic and lymphocyte-like cells
| Clone | Similarity to | Species | Accession no. | Function |
|---|---|---|---|---|
| Genes controlling lymphocyte ontogeny | ||||
| Spi transcription factor | Petromyzon marinus | AAF78904 | Transcription factor | |
| Ikaros transcription factors | Petromyzon marinu | AAL67302/AAL62094 | Transcription factors | |
| 42A10 | Mast/stem cell growth factor receptor (tyrosine-protein kinase c-Kit) | Gallus gallus | Q08156 | Tyrosine protein kinase |
| 38A03 | Uncharacterized hematopoietic stem/progenitor cells protein MDS003 | Homo sapiens | NP_060938 | Unknown |
| Genes involved in lymphocyte signaling | ||||
| 26A03 | Human leukocyte common antigen CD45 | Homo sapien | NP_002829 | Protein-tyrosine phosphatase |
| 25F12 | B cell phosphoinositide 3-kinase adaptor (BCAP) | Mus musculus | NP_113553 | Links BCR-associated kinases with phosphatidylinositol 3-kinase |
| 51E10 | CD3 ɛ associated protein; antisense to ERCC-1 (CAST) | Homo sapiens | NP_036231 | T-cell activation |
| 11C05 | Activated protein kinase C receptor (RACK1) | Rattus norvegicus | A36986 | PRKC-mediated signaling |
| 12D11 | Thymosin β-10 | Rattus norvegicus | AAB37101 | Actin monomer sequestering protein |
| 79D04 | IgG Fc-binding protein | Homo sapiens | AAD39266 | Role in intestinal immune protection and inflammation |
| O8F12 | c-Kit-related kinase | Xenopus laevis | I51703 | Tyrosine protein kinase |
| 43B05 | Kit-like A | Lethenteron reissneri | BAA84745 | Tyrosine protein kinase |
| 14C11 | Tumor necrosis factor receptor superfamily member | Mus musculus | NP_035739 | Binding of tumor necrosis factor |
| 18A11 | Progesterone receptor-related protein p23 | Gallus gallus | B56211 | Chaperone, disrupting receptor-mediated transcriptional activation |
| 18B09 | Ran GTP-binding protein | Xenopus laevis | BAA89696 | GTP-binding protein |
| 30G05 | Protein tyrosine phosphatase | Mus musculus | CAA76755 | Protein tyrosine phosphatase |
| 34A04 | fms-related tyrosine kinase 3 | Homo sapiens | XP_012297 | Tyrosine kinase |
| 34E11 | Myc-binding protein | Xenopus laevis | I51586 | Myc binding, regulation of gene expression during differentiation |
| 36G05 | ATP-stimulated glucocorticoid-receptor translocation promoter | Rattus norvegicus | NP_077357 | Increases binding of activated glucocorticoid-receptor to nuclei |
| 48H11 | Lanthionine-synthetase component C-like protein-coupled receptor | Homo sapiens | NP_061167 | G protein-coupled receptor |
| 58B05 | Granalcin | Mus musculus | AAM66720 | Calcium binding |
| 11D11 | Calmodulin 2 | Homo sapiens | NP_001734 | Calcium binding |
| 06F07 | Calcium-dependent protein kinase SK5 | Glycine max | P28583 | Calcium-dependent signal transduction |
| Genes required for lymphocyte proliferation and migration | ||||
| 09D03 | CD98 | Homo sapiens | XP_043189 | Amino acid transporter |
| 87B05/78D10 | CD9/CD81 (target of antiproliferative antibody 1) | Bos taurus | P30932 | Tetraspanin receptors, participation in cell migration and adhesion |
| Rattus norvegicus | NP_037219 | |||
| Genes encoding proteasomal subunits and an ABC transporter | ||||
| 04A09 | ABCB9 | Homo sapiens | NP_062571 | ABC transporter |
| 55B12 | PSMB4 | Xenopus laevis | P28024 | Protein degradation |
| 93B06 | PSMB7 | Homo sapiens | AAH00509 | Protein degradation |
| 97D07 | 26S proteasome subunit pUb-R3 | Mus musculus | BAA97575 | Protein degradation |
| 66F06 | Proteasome subunit α type 2 (proteasome component C3) | Xenopus laevis | P24495 | Protein degradation |
| 23B08 | Proteasome subunit α type 6 | Mus musculus | NP_036098 | Protein degradation |
| 97G10 | Proteasome inhibitor PI31 subunit | Homo sapiens | NP_006805 | Protein degradation |
| Other immunologically relevant genes | ||||
| 06D11 | Chaperone protein dnaJ | Haemophilus ducreyi | P48208 | Chaperone |
| 34G10 | Heat-shock 90-kDa protein 1, β | Homo sapiens | XP_018115 | Chaperone |
| 29C10 | Heat-shock 90-kDa protein 1, α | Homo sapiens | NP_005339 | Chaperone |
| 34D12 | Heat-shock cognate 70 protein | Gallus gallus | CAA06233 | Chaperone |
| 73H07 | dnaK-type molecular chaperone hsp70 | Pleurodeles waltl | JC5642 | Chaperone |
| 84F09 | Heat-shock protein hsp70-related protein | Homo sapiens | XP_028530 | Chaperone |
| 97B07 | Heat-shock protein 86 | Homo sapiens | AAA36024 | Chaperone |
| 87A09 | Macrophage migration inhibitory factor | Gallus gallus | Q02960 | Regulation of macrophage function in inflammation |
| 25B03 | CXC chemokine receptor 4 | Acipenser ruthenus | CAB60252 | Directs migration of leukocytes in lymphoid organs |
| 15G09 | Cathepsin L | Myxine glutinosa | AAF19630 | Cysteine protease |
| 73A07 | Cystatin B thiol proteinase inhibitor | Homo sapiens | NP_000091 | Cysteine protease inhibitor |
| 92D12 | Egg-white cystatin; cystatin F; leukostatin | Gallus gallus | P81061 | Cysteine protease inhibitor |
| Homo sapiens | NP_003642 | |||
| 97A10 | Lysosomal trafficking regulator | Mus musculus | NP_034878 | Lysosomal traffic |
| 15E08 | Lymphocyte antigen 6 complex-related | Mus musculus | NP_034872 | GPI-linked cell-surface glycoprotein |
| 26C02 | Complement component 1, q subcomponent-binding protein | Mus musculus | NP_031599 | Multifunctional protein; regulation of complement system |
| 44BG01 | Hepsin | Homo sapiens | NP_002142 | Blood coagulation, fibrinolysis, complement activation |
| 70H12 | Glutathione-dependent hematopoietic prostaglandin D synthase | Gallus gallus | O73888 | Conversion of prostaglandin PGH2 to PGD2 |
| 49B02 | Zygin 2; fasciculation and elongation protein ζ 2; Pre-T/NK cell-associated protein (3C1) | Homo sapiens | NP_005093 | Axonal fasciculation in neural tissue |
| 13G06 | Mitogen-inducible gene 2 (mig-2) or talin | Homo sapiens | XP_051693 | Connects cytoskeletal structures to plasma membrane |
| 40A01 | Ribosome-associated membrane protein 4 (RAMP4) | Rattus norvegicus | CAB40910 | Controls glycosylation of MHC class II-associated invariant chain |
| 29A06 | Tumor necrosis factor-inducible protein TSG-6, (hyaluronate-binding protein PS4) | Oryctolagus cuniculus | P98065 | Hyaluronan-binding protein |
| 11B12 | Interferon-inducible protein 16 | Rattus norvegicus | NP_110460 | Unknown |
| 11E10 | Slit | Danio rerio | AAG36772 | Inhibits leukocyte chemotaxis |
| 92F05 | Dentritic cell protein DC6 | Homo sapiens | NP_064574 | Unknown |
| 46F05 | RW1 protein | Mus musculus | AAC15232 | Possible role in antiviral immune response |
| 58A07 | ADP-ribosylation-like factor-6 interacting protein (ARL6) | Homo sapiens | Q15041 | Role in hematopoietic maturation |
| 62D09 | Putative human HLA class II-associated protein | Homo sapiens | NP_006296 | Binding of cytoplasmic domain of the HLA DR-α chain |
| 17B05 | Thioredoxin | Oryctolagus cuniculus | P08628 | Redox reactions, augments expression of IL-2 receptor |
| 72D07 | Thioredoxin peroxidase 1 | Rattus norvegicus | NP_058865 | Immunoregulation of natural killer cell activity |
| 25D08 | Peroxidasin | Drosophila melanogaster | AAF47668 | Extracellular matrix-associated peroxidase in hemocytes |
| 75F03 | Peptidyl-prolyl cis-trans-isomerase (cyclophilin D) | Mus musculus | NP_032046 | Binds cyclosporin A |
| 83G05 | B cell translocation gene-2 (BTG2), antiproliferative | Mus musculus | NP_031596 | Cell-cycle progression |
| 84A11 | Tumor protein, translationally controlled 1 | Homo sapiens | NP_003286 | IgE-dependent histamine-releasing factor |
| 77H04 | SEC61, γ subunit of protein translocation complex | Mus musculus | XP_122171 | Protein translocation |
| 09G06 | Ferritin heavy chain | Salmo salar | P49946 | Iron storage and cellular regulation |
| 47B04 | Ferritin H-3 | Oncorhynchus mykiss | BAA13148 | Iron storage and cellular regulation |
| 91F11 | Ferritin light chain 2 | Mus musculus | NP_032075 | Iron storage and cellular regulation |
Genes Involved in Lymphocyte Signaling.
Antigenic stimulation of mammalian lymphocytes activates complex signaling pathways involving a characteristic array of enzymes and adaptor proteins (16, 17). The lamprey EST set contains sequences derived from genes encoding three proteins, CD45, BCAP (B cell adaptor for phosphoinositide 3-kinase), and CAST (CD3ɛ-associated signal transducer), the orthologs of which are essential for the activation of mammalian lymphocytes. Mammalian CD45 (leukocyte common antigen) is a receptor-type protein tyrosine phosphatase that dephosphorylates Srk-family protein tyrosine kinases associated with the TCR–CD3 complex after the engagement of the TCR by an MHC–peptide assembly. Mammalian BCAP is a protein activated by BCR-associated protein tyrosine kinases after the engagement of the BCR by an antigen (18). The activated BCAP mediates the interaction between the phosphatidylinositol 3-kinase and its substrate, phosphatidylinositol-(4,5)-biphosphate. Mammalian CAST is similarly an adaptor protein that, as the name implies, is constitutively associated with the ɛ chain of the TCR–CD3 complex. Upon its activation after the engagement of the TCR by the MHC–peptide assembly, it transduces the activation signal to other proteins in the pathway, leading to the activation of interleukin-2 and other genes (19). The lamprey counterparts of these three proteins are described in the accompanying paper (8). The mammalian proteins are expressed either exclusively or predominantly in lymphocytes or other cells of the hemopoietic progression.
Involvement in lymphocyte signaling also is prognosticated for the receptor for activated protein kinase C (RACK1), the lamprey homolog of which is identified by several ESTs in the set. The mammalian RACK1 is a WD motif-containing protein that binds specifically to the type I IFN receptor chain 2 (20). In addition to these molecules, the lamprey EST set also contains sequences encoding several other proteins, the mammalian homologs of which are involved in the signaling pathways shared by many different cell types including lymphocytes. These include three calcium-binding proteins, calmodulin and calmodulin-related kinase (16 ESTs), as well as grancalcin (1 EST). Mammalian grancalcin is expressed predominantly in myeloid cells, both normal and cancerous (21).
Genes Required for Lymphocyte Proliferation and Migration.
The ability to respond to stimuli by growth, cell division, and migration is one of the functional characteristics of mammalian lymphocytes (1). Many genes control these activities, and the homologs of several of them are indicated by the lamprey EST set. An example of a gene expressed abundantly by mammalian lymphocytes during the proliferation phase of their cell cycle is CD98 (solute carrier family 3 member 2 or SLC3A2, also referred to as T cell-activation antigen; see ref. 22). It seems to have at least two functions. As a heavy chain of a dimeric transporter, it regulates the import of amino acids into the proliferating cells, and by its interaction with integrin adhesion receptors, it influences the stimulation of T lymphocytes and other cells. An example of a molecule that may influence lymphocyte migration is CD9, which is known to associate with integrins and a great number of other molecules. Lamprey orthologs of CD98 and CD9 have been identified in the EST set and characterized (8). Their considerable sequence similarity with their mammalian counterparts suggests that they may be functionally equivalent to the latter.
Genes Encoding Proteasomal Subunits and an ABC Transporter.
Proteasomes are organelles of eukaryotic cells in which proteins are degraded into peptides (23). Stimulated lymphocytes and other immunologically active cells of jawed vertebrates possess a class of proteasomes specialized in producing peptides for loading onto newly synthesized MHC class I molecules. A distinctive feature of these “immunoproteasomes” is the replacement of subunits PSMB5, PSMB6, and PSMB7 of the “housekeeping” proteasomes by subunits PSMB8, PSMB9, and PSMB10, respectively. These last three subunits are encoded in genes that are regulated by IFN-γ (24), and they possess activities necessary for the generation of peptides fitting into the peptide-binding region of the class I molecules. The lamprey EST set contains six sequences encoding proteasomal proteins: PSMB4, PSMB7, 26S proteasome subunit pUb-R3, PSMA2, PSMA6, and PSMF1. Of these, the most interesting is the sequence related to the gene encoding the PSMB7. Analysis of the gene from which the EST is derived has revealed it to be related closely not only to PSMB7 of the housekeeping proteasomes but also to PSMB10 of the immunoproteasomes. The pair is believed to have arisen by gene duplication from a common ancestor, and therefore the lamprey gene could represent the state before the duplication. Phylogenetic analysis, however, favors the possibility that the lamprey gene might actually be PSMB10, which has not come under the control of IFN-γ as yet and therefore has been evolving more slowly than the IFN-γ-regulated genes (25).
In gnathostome lymphocytes the immunoproteasome-generated peptides are delivered to the site of class I molecule synthesis in the endoplasmic reticulum by the ATP-binding cassette transporters ABCB2 (TAP1) and ABCB3 (TAP2; ref. 26). The lamprey EST set contains an ABCB9 sequence that is related closely to these genes (T.U.-o., W.E.M., M.D.C., and J.K., unpublished results). The exact nature of this relationship is unclear. As in the case of PSMB7, the lamprey gene might represent the ancestral condition from which ABCB2 and ABCB3 could have arisen by gene duplication. But the situation again is complicated by the fact the ABCB2 and ABCB3 (but apparently not ABCB9) have also come under the influence of IFN-γ, and this event may have accelerated their evolution.
Other Immunologically Relevant Genes.
In addition to these “high-profile” homologs of genes expressed in mammalian lymphocytes, the lamprey cDNA library yielded an assortment of somewhat less prominent but by no means less important genes participating in immunological responses mostly of the innate type (Table 2). Several highly conserved chaperone-encoding sequences have been found in the library at a frequency of 3.4%; they include Hsp90 and Hsp70/Hsc70-related genes. Cytokine-encoding genes are represented in the EST set by the macrophage-inhibitory factor (A. Sato, T.U.-o., N. Kuroda, W.E.M., N. Takezaki, R. Dongak, F. Figueroa, M.D.C., and J.K., unpublished results) and the chemokine receptor of the CXCR4 type (N. Kuroda, T.U.-o., A. Sato, I. E. Samonte, F. Figueroa, W.E.M., and J.K., unpublished results), which in mammals are expressed predominantly in lymphocytes and macrophages. Lysosomal cysteine proteases are represented in the collection by cathepsin L, which in mammalian lymphocytes plays a critical part in antigen degradation for presentation by MHC class II molecules (27). Cysteine protease inhibitors are exemplified by cystatin 7 (leukostatin), an inhibitor of the papain family proteases, and cystatin B, presumably serving as a protector against cathepsin L and other proteases leaking from lysosomes. The Ly6 family of glycosylphosphatidylinositol-anchored proteins is represented by 14 ESTs. The lamprey genes seem to encode a 109-aa residue protein related to Ly6D and neurotoxin 1. The complement cascade is represented by the component C1q and hepsin, a cell membrane-associated serine protease participating in blood coagulation and fibrinolysis, and the prostaglandin system is epitomized by hemopoietic prostaglandin D2 synthase, the key enzyme in the production of the D and J series of prostanoids (28). Other genes included homologs of (i) zygin 2 expressed in human pre-T and natural killer cells (29), (ii) mitogen-inducible gene 2 (mig-2) or talin expressed in trout leukocytes and presumably involved in cell–cell contacts between lymphocytes (30), and (iii) stress-induced ribosome-associated protein 4 (RAMP4), which in mammalian lymphocytes interacts with nascent MHC class II-associated invariant chains (31).
Interpretation.
The cell population isolated by flow cytometry from the intestine of the sea lamprey fits the description of lymphocytes by a number of criteria. First, morphologically the lamprey cells are indistinguishable from mammalian lymphocytes both at the light- and electron-microscopy levels. They also possess physical characteristics of lymphocytes, thus enabling us to isolate them from other hematopoietic lineage cells on the basis of their distinctive high-scatter characteristics. Second, the lamprey cells are abundant in tissues (intestine, typhlosole) that are believed to have been phylogenetically the locus operandi of lymphocytes (32). Third, judging from their expression of certain transcription factors, the lamprey lymphocyte-like cells are apparently of hemopoietic origin. Fourth, the cells express gene homologous to those prominently or characteristically expressed in mammalian lymphocytes. And fifth, when cultured in the presence of mitogens such as lipopolysaccharide and phytohemagglutinin, the cells respond by blast transformation and limited proliferation (L.A.G., unpublished data).
There is one major criterion that the cells do not seem to fulfill to qualify as bona fide lymphocytes: Thus far, they have shown no indication of expressing MHC, TCR, and BCR, the three molecules considered by some (33) to be the hallmark of lymphocytes. Although the initial homology searches of the EST set described in the present study identified several sequences with weak similarities to one or an other of the three cardinal markers of mammalian lymphocytes, further analysis of these clones proved the correspondence to be spurious. No clone with significant sequence similarity to any of the three genes could be authenticated. Although a negative result of this sort does not prove that the lamprey lacks MHC, TCR, and BCR genes, it increases the likelihood of this being the case. Furthermore, two other observations point in the same direction. First, far smaller sampling of cDNA libraries prepared by the same protocol from tissues of bony fishes yield sequences derived from these three genes (A. Sato and I. E. Samonte, unpublished data). This result may be regarded as a positive control for the experiment reported here. And second, all other efforts to find MHC, TCR, and BCR as well as other genes necessary for the trio's function (e.g., RAG, PSMB8, PSMB9, PSMB10, ABCB2, and ABCB3) also have been unsuccessful (4). Although close relatives of some of these auxiliary genes (e.g., PSBM10, ABCB2, and ABCB3) exist in the lamprey (ref. 25; T.U.-o., W.E.M., M.D.C., and J.K., unpublished results), they do not seem to have the same function as in mammalian lymphocytes.
It therefore may be fair to conclude at this stage that despite their morphological, physical, ontogenetic, and biochemical similarity to mammalian lymphocytes, the lamprey lymphocyte-like cells in the typhlosole are functionally not equivalent to true lymphocytes. They seem to have reached a stage in evolution in which many but not all of the molecules and mechanisms necessary for assuming the function of a key cellular element in the adaptive immune response have been established. What seems to be missing are a few critical genes (the MHC, TCR, BCR genes) and their auxiliaries. If this interpretation is correct, the introduction of these genes into the lamprey lymphocyte-like cells should convert them into genuine lymphocytes.
Acknowledgments
We thank Dr. G. Larry Gartland for help with flow-cytometric analysis and cell sorting, Ms. Jane Kraushaar for editorial assistance, and Dr. James G. Seeley (Hammond Bay Biological Station, U.S. Department of Interior) for ammocoete larvae of the sea lamprey.
Abbreviations
TCR, T cell receptor
BCR, B cell receptor
References
- 1.Klein J. & Horejsi, V., (1997) Immunology (Blackwell Scientific, Oxford).
- 2.Weissman I. L. (1994) Immunity 1 529-531. [DOI] [PubMed] [Google Scholar]
- 3.Weiss L., (1984) The Blood Cells and Hematopoietic Tissues (Elsevier, New York).
- 4.Klein J., Sato, A. & Mayer, W. E. (2000) in Major Histocompatibility Complex: Evolution, Structure, and Function, ed. Kasahara, M. (Springer, Tokyo), pp. 3–26.
- 5.Good R. A., Finstad, J. & Litman, J. (1972) in The Biology of Lampreys, eds. Hardisty, M. W. & Potter, I. C. (Academic, London), Vol. 2, pp. 405–432. [Google Scholar]
- 6.Altschul S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Miller, W. & Lipman, D. J. (1997) Nucleic Acids Res. 25 3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bessis M., (1977) Blood Smears Reinterpreted (Springer, Berlin).
- 8.Uinuk-ool T., Mayer, W. E., Sato, A., Dongak, R., Cooper, M. D. & Klein, J. (2002) Proc. Natl. Acad. Sci. USA 99 14356-14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clevers H. C. & Grosschedl, R. (1996) Immunol. Today 17 336-343. [DOI] [PubMed] [Google Scholar]
- 10.Georgopoulos K. (2002) Nat. Rev. Immunol. 2 162-174. [DOI] [PubMed] [Google Scholar]
- 11.Cortes M., Wong, E., Koipally, J. & Georgopoulos, K. (1999) Curr. Opin. Immunol. 11 167-171. [DOI] [PubMed] [Google Scholar]
- 12.Shintani S., Terzic, J., Sato, A., Saraga-Babic, M., O'hUigin, C., Tichy, H. & Klein, J. (2000) Proc. Natl. Acad. Sci. USA 97 7417-7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haire R. N., Miracle, A. L., Rast, J. P. & Litman, G. W. (2000) J. Immunol. 165 306-312. [DOI] [PubMed] [Google Scholar]
- 14.Mayer W. E., O'hUigin, C., Tichy, H., Terzic, J. & Saraga-Babic, M. (2002) Scand. J. Immunol. 55 162-170. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki E., Okamura, H., Chikamune, T., Kanai, Y., Watanabe, M., Naito, M. & Sakurai, M. (1993) Gene 128 257-261. [DOI] [PubMed] [Google Scholar]
- 16.Kane L. P., Lin, J. & Weiss, A. (2000) Curr. Opin. Immunol. 12 242-249. [DOI] [PubMed] [Google Scholar]
- 17.Kurosaki T. (2002) Curr. Opin. Immunol. 14 341-347. [DOI] [PubMed] [Google Scholar]
- 18.Okada T., Maeda, A., Iwamatsu, A., Gotoh, K. & Kurosaki, T. (2000) Immunity 13 817-827. [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki T., Hamano, Y., Tashiro, H., Itoh, K., Nakano, H., Miyatake, S. & Saito, T. (1999) J. Biol. Chem. 274 18173-18180. [DOI] [PubMed] [Google Scholar]
- 20.Croze E., Usacheva, A., Asarnow, D., Minshall, R. D., Perez, H. D. & Colamonici, O. (2000) J. Immunol. 165 5127-5132. [DOI] [PubMed] [Google Scholar]
- 21.Boyhan A., Casimir, C. M., French, J. K., Teahan, C. G. & Segal, A. W. (1992) J. Biol. Chem. 267 2928-2933. [PubMed] [Google Scholar]
- 22.Devés R. & Boyd, C. A. R. (2000) J. Membr. Biol. 173 165-177. [DOI] [PubMed] [Google Scholar]
- 23.Baumeister W., Walz, J., Zühl, F. & Seemüller, E. (1998) Cell 92 367-380. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi M., Ishibashi, T., Tanaka, K. & Kasahara, M. (1997) J. Immunol. 159 2760-2770. [PubMed] [Google Scholar]
- 25.Takezaki N., Zaleska-Rutczynska, Z. & Figueroa, F. (2002) Gene 282 179-187. [DOI] [PubMed] [Google Scholar]
- 26.Abele R. & Tampé, R. (1999) Biochim. Biophys. Acta 1461 405-419. [DOI] [PubMed] [Google Scholar]
- 27.Lennon-Duménil A.-M., Bakker, A. H., Wolf-Bryant, P., Ploegh, H. L. & Lagaudrière-Gesbert, C. (2002) Curr. Opin. Immunol. 14 15-21. [DOI] [PubMed] [Google Scholar]
- 28.Kanaoka Y., Ago, H., Inagaki, E., Nanayama, T., Miyano, M., Kikuno, R., Fujii, Y., Eguchi, N., Toh, H., Urade, Y., et al. (1997) Cell 90 1085-1095. [DOI] [PubMed] [Google Scholar]
- 29.Ranes-Goldberg M. G., Hori, T., Mohan-Peterson, S. & Spits, H. (1993) J. Immunol. 151 5810-5821. [PubMed] [Google Scholar]
- 30.Rees D. J., Ades, S. E., Singer, S. J. & Hynes, R. O. (1990) Nature 347 685-689. [DOI] [PubMed] [Google Scholar]
- 31.Schröder K., Martoglio, B., Hofmann, M., Hölscher, C., Hartmann, E., Prehn, S., Rapoport, T. A. & Dobberstein, B. (1999) EMBO J. 18 4804-4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fichtelius K. E. (1970) Lymphology 1 50-59. [PubMed] [Google Scholar]
- 33.Klein J. (1988) in The Pharmacology of Lymphocytes, eds. Bray, M. A. & Morley, J. (Springer, Berlin), pp. 11–36.