Abstract
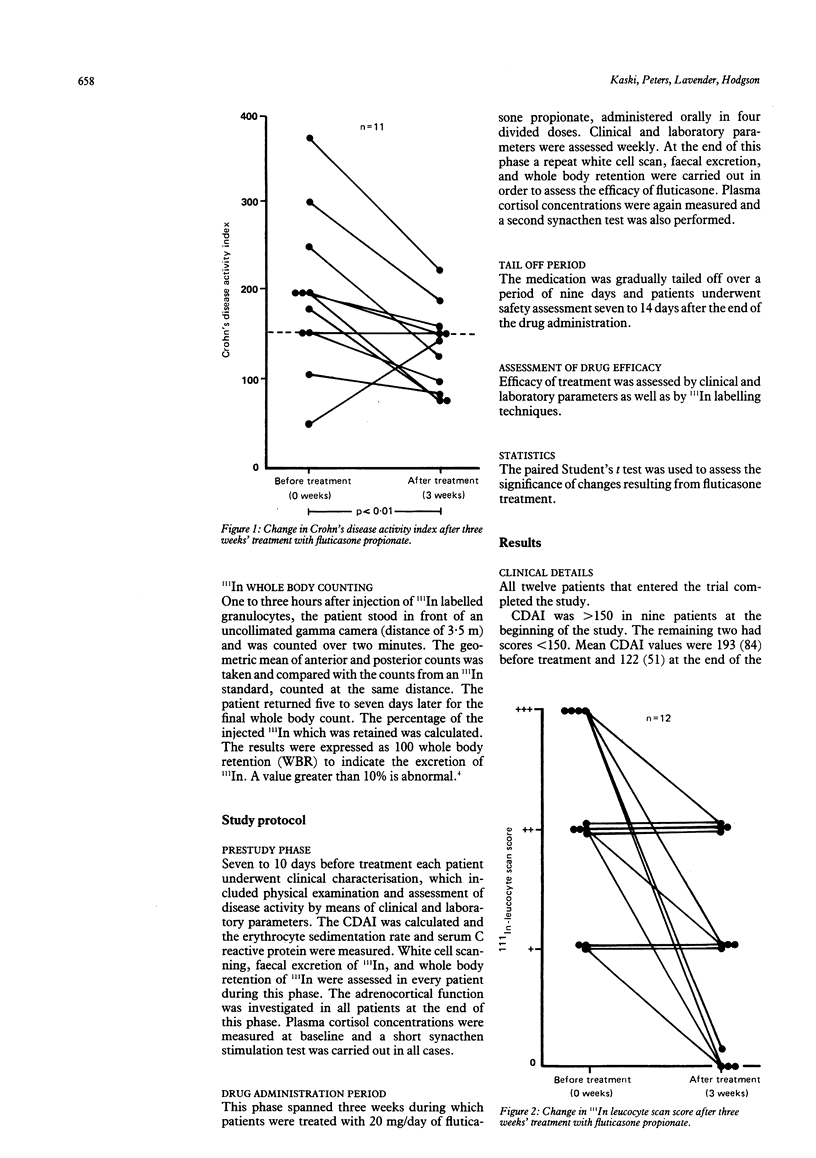
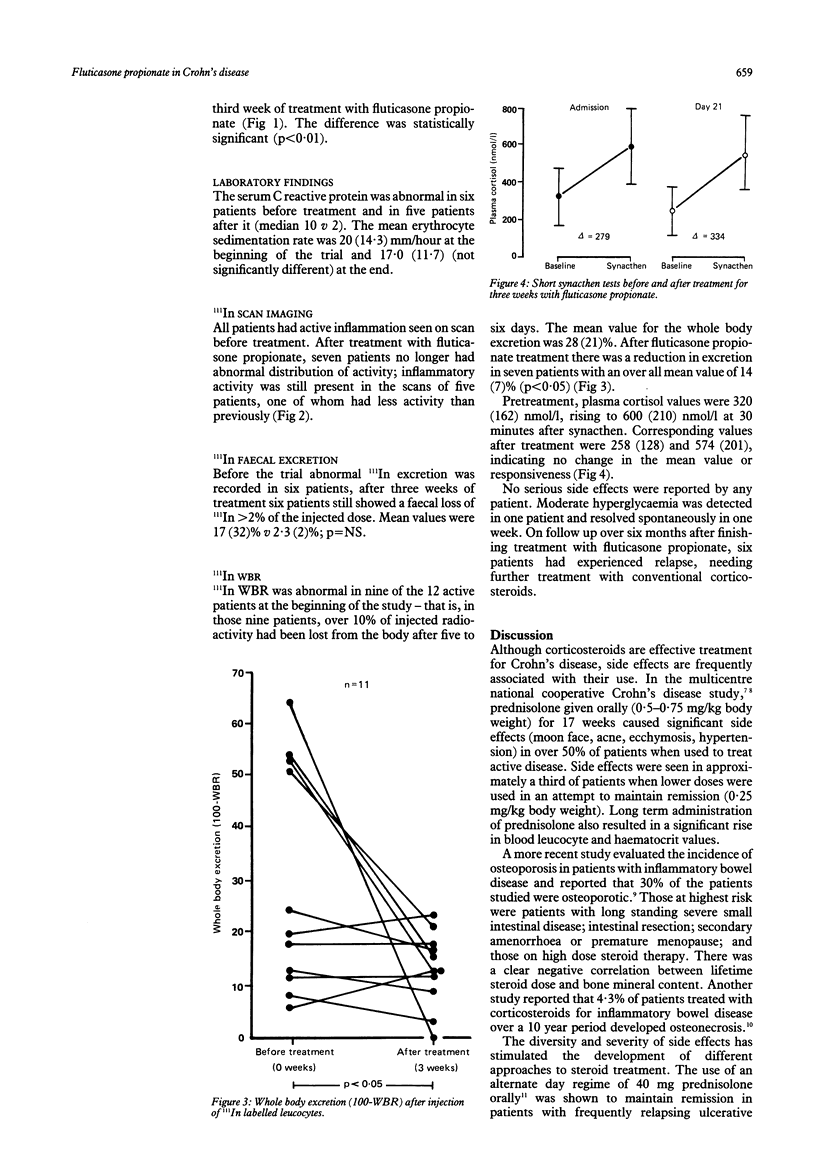
Fluticasone propionate, a topically active corticosteroid of low systemic bioavailability after oral administration, has been used in a pilot study for the treatment of mild and moderately active Crohn's disease. Twelve patients received oral fluticasone propionate for three weeks, and the effects were monitored using the Crohn's disease activity index and by 111In granulocyte scanning, assessing inflammation from scan appearances, four day faecal excretion of radioactivity, and whole body excretion of radioactivity. All patients completed the trial. No serious side effects were reported. There was a significant fall in Crohn's disease activity index values over the three week treatment period (193 (84) v 122 (51), p less than 0.01). 111In leucocyte scan images were improved (seven patients) or unchanged (five patients). There was a significant fall in excretion of injected radioactivity calculated from whole body data (28 (21)% v 14 (0.7)%, p less than 0.05). There were no changes in plasma cortisol values, either basal or synacthen stimulated. Fluticasone propionate is a promising therapeutic agent for Crohn's disease that offers the possibility of controlling inflammation without inducing systemic corticosteroid side effects and which merits evaluation in a double blind trial versus conventional corticosteroids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best W. R., Becktel J. M., Singleton J. W. Rederived values of the eight coefficients of the Crohn's Disease Activity Index (CDAI). Gastroenterology. 1979 Oct;77(4 Pt 2):843–846. [PubMed] [Google Scholar]
- Compston J. E., Judd D., Crawley E. O., Evans W. D., Evans C., Church H. A., Reid E. M., Rhodes J. Osteoporosis in patients with inflammatory bowel disease. Gut. 1987 Apr;28(4):410–415. doi: 10.1136/gut.28.4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson A., Hellers G., Lyrenäs E., Löfberg R., Nilsson A., Olsson O., Olsson S. A., Persson T., Salde L., Naesdal J. A controlled randomized trial of budesonide versus prednisolone retention enemas in active distal ulcerative colitis. Scand J Gastroenterol. 1987 Oct;22(8):987–992. doi: 10.3109/00365528708991947. [DOI] [PubMed] [Google Scholar]
- Kumana C. R., Seaton T., Meghji M., Castelli M., Benson R., Sivakumaran T. Beclomethasone dipropionate enemas for treating inflammatory bowel disease without producing Cushing's syndrome or hypothalamic pituitary adrenal suppression. Lancet. 1982 Mar 13;1(8272):579–583. doi: 10.1016/s0140-6736(82)91747-0. [DOI] [PubMed] [Google Scholar]
- Larochelle P., Du Souich P., Bolte E., Lelorier J., Goyer R. Tixocortol pivalate, a corticosteroid with no systemic glucocorticoid effect after oral, intrarectal, and intranasal application. Clin Pharmacol Ther. 1983 Mar;33(3):343–350. doi: 10.1038/clpt.1983.43. [DOI] [PubMed] [Google Scholar]
- Malchow H., Ewe K., Brandes J. W., Goebell H., Ehms H., Sommer H., Jesdinsky H. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology. 1984 Feb;86(2):249–266. [PubMed] [Google Scholar]
- Peters A. M., Saverymuttu S. H., Reavy H. J., Danpure H. J., Osman S., Lavender J. P. Imaging of inflammation with indium-111 tropolonate labeled leukocytes. J Nucl Med. 1983 Jan;24(1):39–44. [PubMed] [Google Scholar]
- Powell-Tuck J., Bown R. L., Chambers T. J., Lennard-Jones J. E. A controlled trial of alternate day prednisolone as a maintenance treatment for ulcerative colitis in remission. Digestion. 1981;22(5):263–270. doi: 10.1159/000198667. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S. H., Camilleri M., Rees H., Lavender J. P., Hodgson H. J., Chadwick V. S. Indium 111-granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111-granulocyte excretion. Gastroenterology. 1986 May;90(5 Pt 1):1121–1128. doi: 10.1016/0016-5085(86)90376-8. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S. H., Peters A. M., Lavender J. P., Hodgson H. J., Chadwick V. S. 111Indium autologous leucocytes in inflammatory bowel disease. Gut. 1983 Apr;24(4):293–299. doi: 10.1136/gut.24.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton J. W., Law D. H., Kelley M. L., Jr, Mekhjian H. S., Sturdevant R. A. National Cooperative Crohn's Disease Study: adverse reactions to study drugs. Gastroenterology. 1979 Oct;77(4 Pt 2):870–882. [PubMed] [Google Scholar]
- Summers R. W., Switz D. M., Sessions J. T., Jr, Becktel J. M., Best W. R., Kern F., Jr, Singleton J. W. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology. 1979 Oct;77(4 Pt 2):847–869. [PubMed] [Google Scholar]
- Vakil N., Sparberg M. Steroid-related osteonecrosis in inflammatory bowel disease. Gastroenterology. 1989 Jan;96(1):62–67. doi: 10.1016/0016-5085(89)90764-6. [DOI] [PubMed] [Google Scholar]
- van der Heide H., van den Brandt-Gradel V., Tytgat G. N., Endert E., Wiltink E. H., Schipper M. E., Dekker W. Comparison of beclomethasone dipropionate and prednisolone 21-phosphate enemas in the treatment of ulcerative proctitis. J Clin Gastroenterol. 1988 Apr;10(2):169–172. doi: 10.1097/00004836-198804000-00013. [DOI] [PubMed] [Google Scholar]


