Abstract
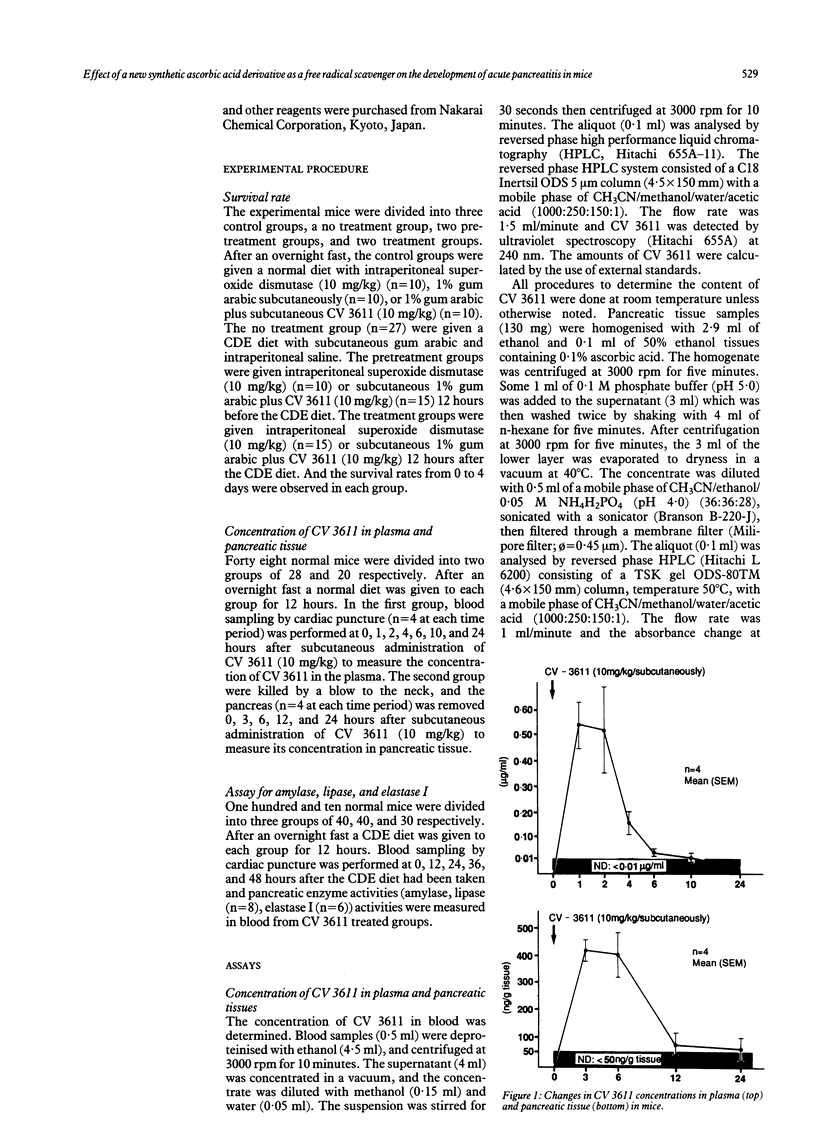
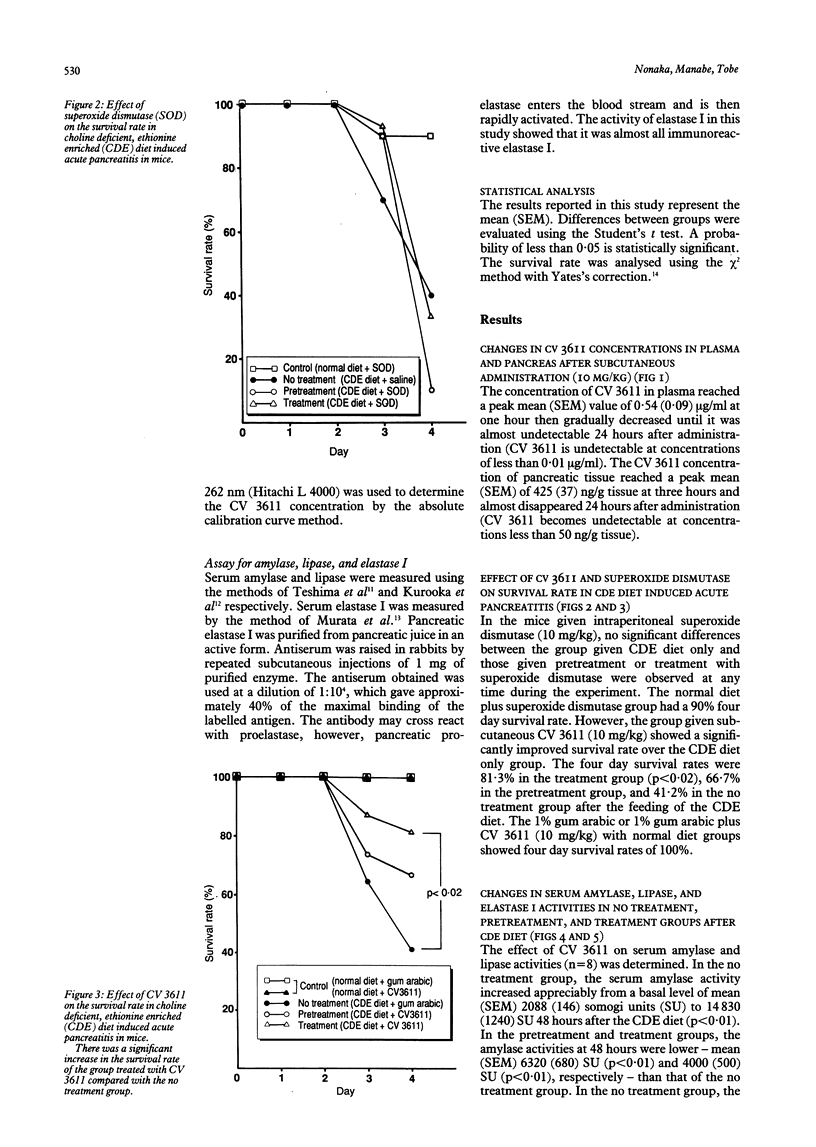
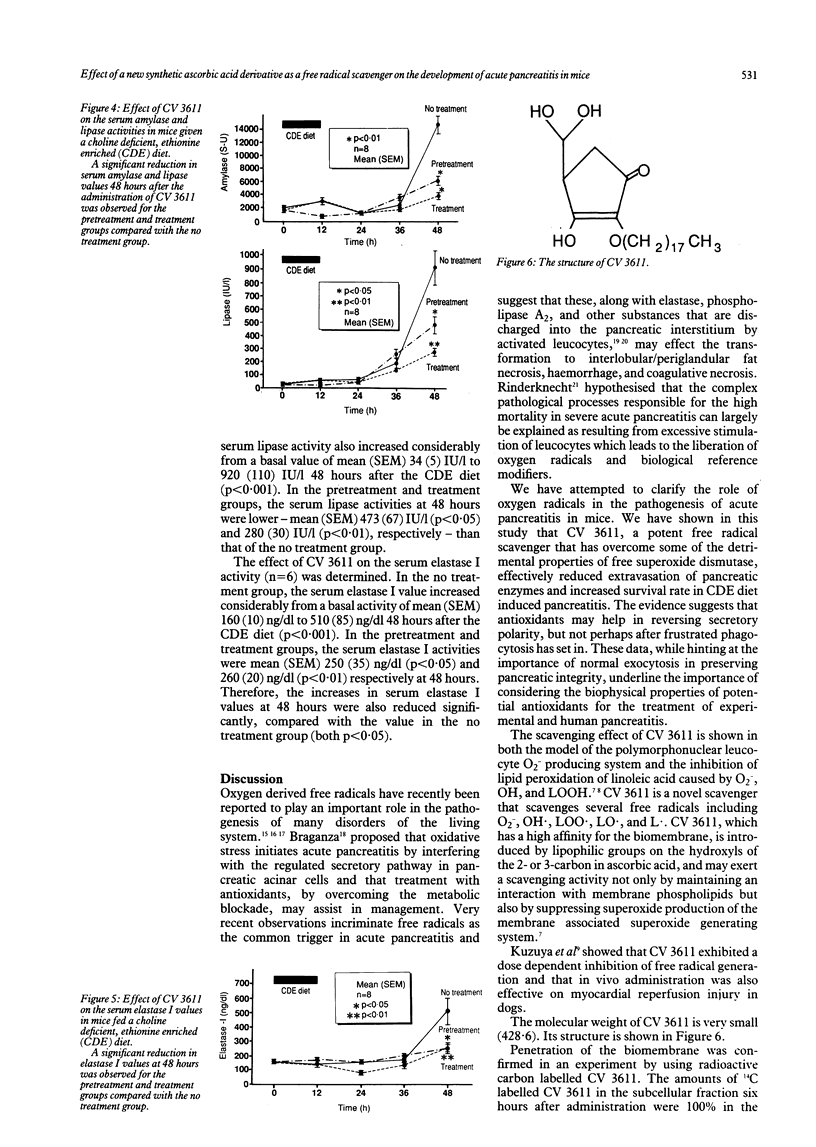
The therapeutic effects of CV 3611, a new synthetic free radical scavenger prepared from an ascorbic acid derivative, on choline deficient, ethionine enriched (CDE) diet induced acute pancreatitis in mice were evaluated and compared with those of superoxide dismutase. Time/course studies after subcutaneous injection of CV 3611 in normal mice showed a peak plasma concentration of mean (SEM) 0.54 (0.09) micrograms/ml at one hour, with a gradual decrease over the next 10 hours, while a peak concentration in pancreatic tissue of mean (SEM) 425 (33) ng/g tissue was achieved at three hours and the drug was undetectable at 12 hours. Survival rates and activities of pancreatic enzymes (amylase, lipase, elastase I) were compared in control mice and animals that received CV 3611 before or at the time of feeding the CDE diet. The survival rate was observed in a no treatment group and mice given pretreatment or treatment with CV 3611 or superoxide dismutase. The survival rate was significantly better in the treatment group given CV 3611 (p less than 0.02), but superoxide dismutase had no significant effect on survival. The increases in the three serum enzyme activities were significantly less at 48 hours in the groups given pretreatment or treatment with CV 3611 than in the no treatment group. These results indicate that CV 3611, which has been proved to pass through the cell membrane and to have a long half life in plasma and tissue, had an important therapeutic effect on the development of acute pancreatitis. They also suggest that oxygen derived free radicals may play an important role in the development of acute pancreatitis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Guice K. S., Miller D. E., Oldham K. T., Townsend C. M., Jr, Thompson J. C. Superoxide dismutase and catalase: a possible role in established pancreatitis. Am J Surg. 1986 Jan;151(1):163–169. doi: 10.1016/0002-9610(86)90027-9. [DOI] [PubMed] [Google Scholar]
- Kato K., Terao S., Shimamoto N., Hirata M. Studies on scavengers of active oxygen species. 1. Synthesis and biological activity of 2-O-alkylascorbic acids. J Med Chem. 1988 Apr;31(4):793–798. doi: 10.1021/jm00399a019. [DOI] [PubMed] [Google Scholar]
- Kurooka S., Okamoto S., Hashimoto M. A novel and simple colorimetric assay for human serum lipase. J Biochem. 1977 Feb;81(2):361–369. doi: 10.1093/oxfordjournals.jbchem.a131467. [DOI] [PubMed] [Google Scholar]
- Kuzuya T., Hoshida S., Nishida M., Kim Y., Fuji H., Kitabatake A., Kamada T., Tada M. Role of free radicals and neutrophils in canine myocardial reperfusion injury: myocardial salvage by a novel free radical scavenger, 2-octadecylascorbic acid. Cardiovasc Res. 1989 Apr;23(4):323–330. doi: 10.1093/cvr/23.4.323. [DOI] [PubMed] [Google Scholar]
- Lombardi B., Estes L. W., Longnecker D. S. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by DL-ethionine fed with a choline-deficient diet. Am J Pathol. 1975 Jun;79(3):465–480. [PMC free article] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Murata A., Ogawa M., Fujimoto K., Kitahara T., Matsuda Y., Kosaki G. Radioimmunoassay of human pancreatic elastase 1. In vitro interaction of human pancreatic elastase 1 with serum protease inhibitors. Enzyme. 1983;30(1):29–37. doi: 10.1159/000469542. [DOI] [PubMed] [Google Scholar]
- Nonaka A., Manabe T., Tamura K., Asano N., Imanishi K., Tobe T. Changes of xanthine oxidase, lipid peroxide and superoxide dismutase in mouse acute pancreatitis. Digestion. 1989;43(1-2):41–46. doi: 10.1159/000199859. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N. Role of oxygen-derived free radicals in digestive tract diseases. Surgery. 1983 Sep;94(3):415–422. [PubMed] [Google Scholar]
- Rinderknecht H. Fatal pancreatitis, a consequence of excessive leukocyte stimulation? Int J Pancreatol. 1988 Mar;3(2-3):105–112. doi: 10.1007/BF02798921. [DOI] [PubMed] [Google Scholar]
- Rutledge P. L., Saluja A. K., Powers R. E., Steer M. L. Role of oxygen-derived free radicals in diet-induced hemorrhagic pancreatitis in mice. Gastroenterology. 1987 Jul;93(1):41–47. doi: 10.1016/0016-5085(87)90311-8. [DOI] [PubMed] [Google Scholar]
- Sanfey H., Bulkley G. B., Cameron J. L. The pathogenesis of acute pancreatitis. The source and role of oxygen-derived free radicals in three different experimental models. Ann Surg. 1985 May;201(5):633–639. doi: 10.1097/00000658-198505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner J., Green D., Ferrell L., Renner I. Evidence for a role of oxygen derived free radicals in the pathogenesis of caerulein induced acute pancreatitis in rats. Gut. 1988 Nov;29(11):1516–1523. doi: 10.1136/gut.29.11.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Flynn J., Demling R. H. Role of oxygen radicals in endotoxin-induced lung injury. Arch Surg. 1984 Jan;119(1):77–82. doi: 10.1001/archsurg.1984.01390130059011. [DOI] [PubMed] [Google Scholar]


