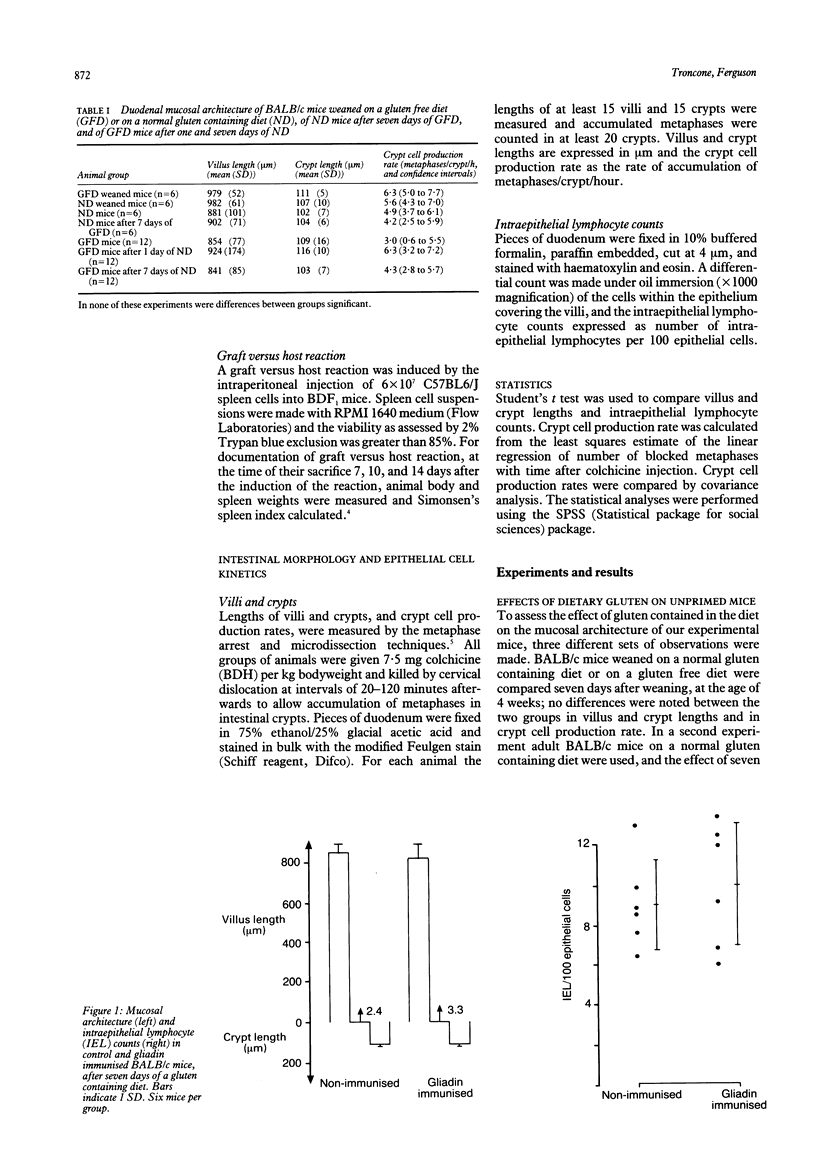
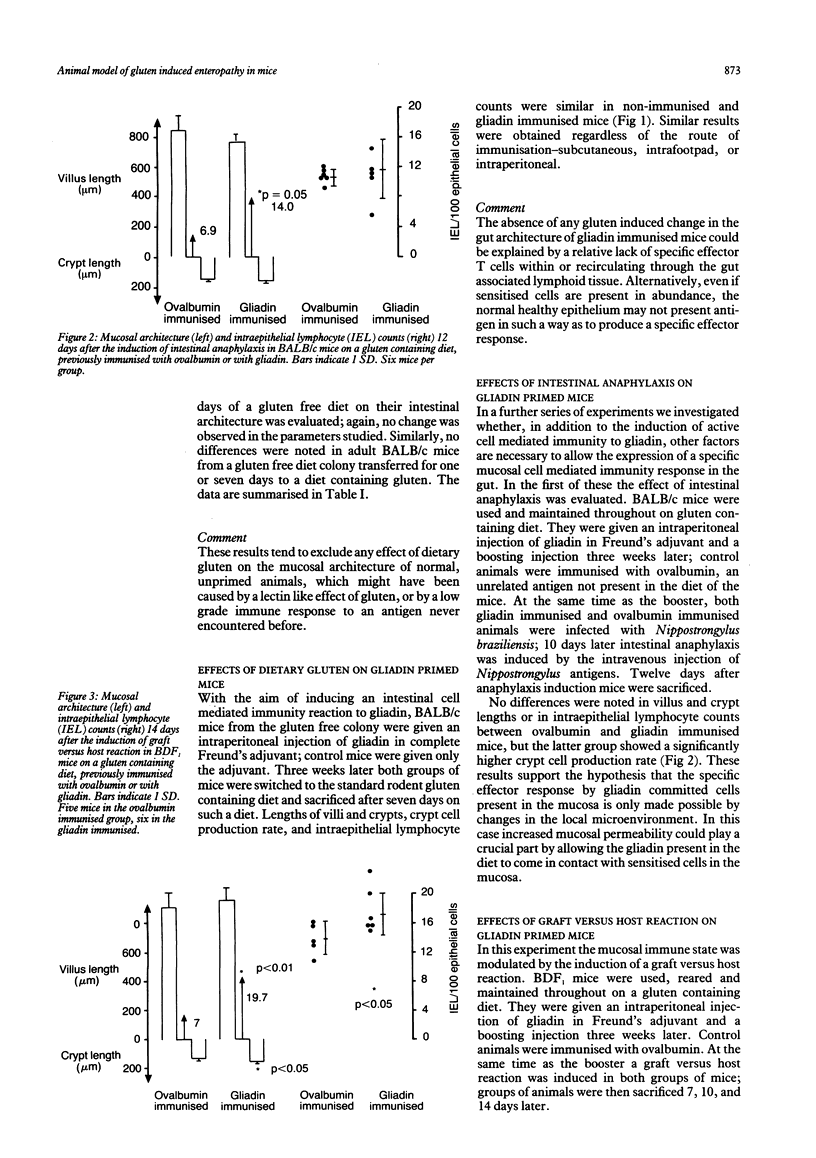
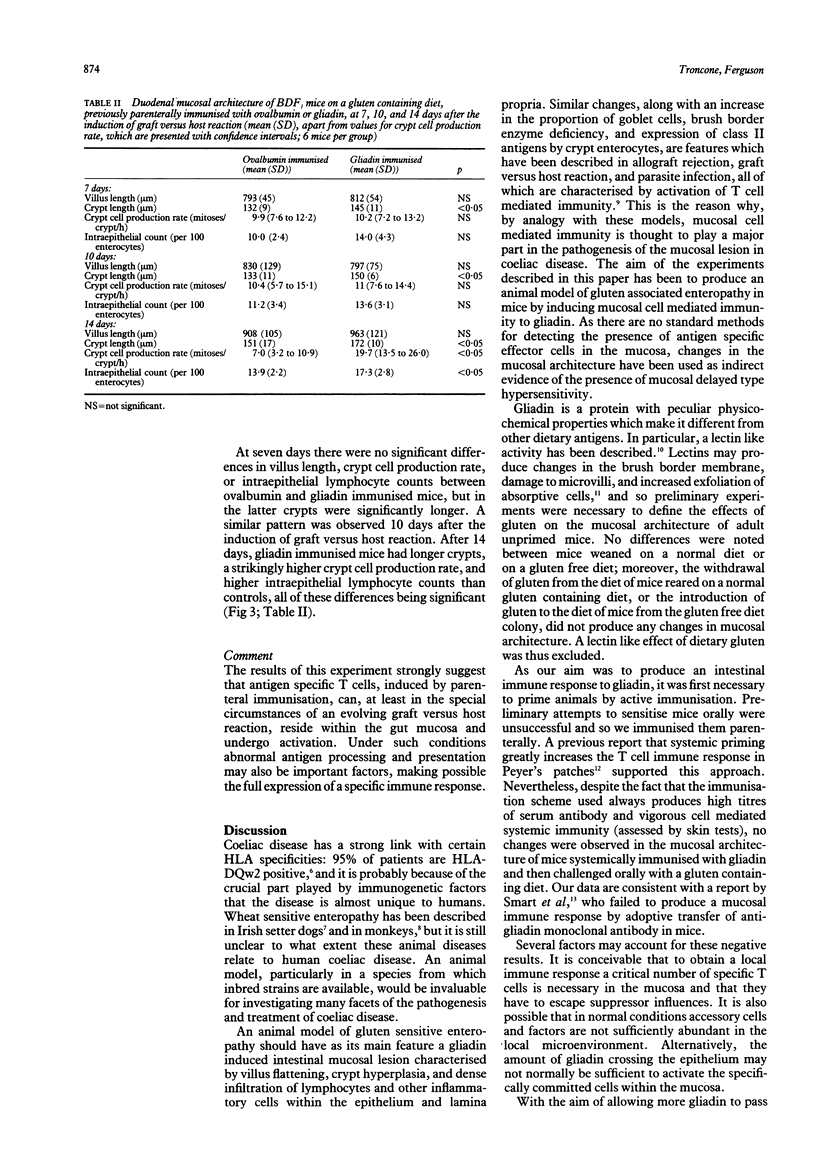
Abstract
The aim of our experiments was to produce a local T cell mediated immune response to gliadin in the mouse small intestine as a possible animal model of gluten sensitive enteropathy, coeliac disease. BALB/c and BDF1 mice were immunised systemically with gliadin in complete Freund's adjuvant. The jejunal mucosa was challenged by feeding a gluten containing diet, and villus and crypt lengths, crypt cell production rate, and intraepithelial lymphocyte counts were determined to assess mucosal cell mediated immunity. In some animals permeability and local immunity were modulated by concurrent intestinal anaphylaxis or a graft versus host reaction. There were no changes in the jejunal mucosa of BALB/c mice fed a gluten containing diet after having been parenterally immunized. When, however, mice were parenterally immunised with gliadin, fed a gluten containing diet, rendered hypersensitive to helminth antigen by infection with the nematode parasite Nippostrongylus brasiliensis, and challenged intravenously to produce intestinal anaphylaxis crypt cell production rate was significantly higher than in ovalbumin immunized controls at 12 days after parasite challenge. Finally, graft versus host reaction was induced in BDF1 mice that had been parenterally immunised with gliadin and were on a gluten containing diet. Two weeks later these mice had significantly longer crypts and a higher crypt cell production rate and intraepithelial lymphocyte count than control, unimmunized mice with graft versus host reaction. We conclude that active immunization with gliadin does not in itself produce intestinal cell mediated immunity to gliadin contained in the diet, or enteropathy. Additional factors, such as those occurring during intestinal anaphylaxis (increase intestinal permeability), or during graft versus host reaction (enhanced antigen presentation), seem to be necessary for the full expression of a jejunal mucosal reaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry R. E., Baker P., Read A. E. Coeliac disease. The clinical presentation. Clin Gastroenterol. 1974 Jan;3(1):55–69. [PubMed] [Google Scholar]
- Batt R. M., McLean L., Carter M. W. Sequential morphologic and biochemical studies of naturally occurring wheat-sensitive enteropathy in Irish setter dogs. Dig Dis Sci. 1987 Feb;32(2):184–194. doi: 10.1007/BF01297107. [DOI] [PubMed] [Google Scholar]
- Clarke R. M. Mucosal architecture and epithelial cell production rate in the small intestine of the albino rat. J Anat. 1970 Nov;107(Pt 3):519–529. [PMC free article] [PubMed] [Google Scholar]
- Doherty M., Barry R. E. Gluten-induced mucosal changes in subjects without overt small-bowel disease. Lancet. 1981 Mar 7;1(8219):517–520. doi: 10.1016/s0140-6736(81)92860-9. [DOI] [PubMed] [Google Scholar]
- Douglas A. P. The binding of a glycopeptide component of wheat gluten to intestinal mucosa of normal and coeliac human subjects. Clin Chim Acta. 1976 Dec 1;73(2):357–361. doi: 10.1016/0009-8981(76)90183-2. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Blackwell J. N., Barnetson R. S. Effects of additional dietary gluten on the small-intestinal mucosa of volunteers and of patients with dermatitis herpetiformis. Scand J Gastroenterol. 1987 Jun;22(5):543–549. doi: 10.3109/00365528708991895. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Vassalli P. Gut injury in mouse graft-versus-host reaction. Study of its occurrence and mechanisms. J Clin Invest. 1986 May;77(5):1584–1595. doi: 10.1172/JCI112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzsonn V., Olsen W. A. In vivo responses of rat intestinal epithelium to intraluminal dietary lectins. Gastroenterology. 1982 May;82(5 Pt 1):838–848. [PubMed] [Google Scholar]
- Marsh M. N. Studies of intestinal lymphoid tissue. XI--The immunopathology of cell-mediated reactions in gluten sensitivity and other enteropathies. Scanning Microsc. 1988 Sep;2(3):1663–1684. [PubMed] [Google Scholar]
- O'Mahony S., Vestey J. P., Ferguson A. Similarities in intestinal humoral immunity in dermatitis herpetiformis without enteropathy and in coeliac disease. Lancet. 1990 Jun 23;335(8704):1487–1490. doi: 10.1016/0140-6736(90)93029-o. [DOI] [PubMed] [Google Scholar]
- Ramage J. K., Stanisz A., Scicchitano R., Hunt R. H., Perdue M. H. Effect of immunologic reactions on rat intestinal epithelium. Correlation of increased permeability to chromium 51-labeled ethylenediaminetetraacetic acid and ovalbumin during acute inflammation and anaphylaxis. Gastroenterology. 1988 Jun;94(6):1368–1375. [PubMed] [Google Scholar]
- Strobel S., Mowat A. M., Ferguson A. Prevention of oral tolerance induction to ovalbumin and enhanced antigen presentation during a graft-versus-host reaction in mice. Immunology. 1985 Sep;56(1):57–64. [PMC free article] [PubMed] [Google Scholar]
- Tosi R., Vismara D., Tanigaki N., Ferrara G. B., Cicimarra F., Buffolano W., Follo D., Auricchio S. Evidence that celiac disease is primarily associated with a DC locus allelic specificity. Clin Immunol Immunopathol. 1983 Sep;28(3):395–404. doi: 10.1016/0090-1229(83)90106-x. [DOI] [PubMed] [Google Scholar]
- Troncone R., Ferguson A. Gliadin presented via the gut induces oral tolerance in mice. Clin Exp Immunol. 1988 May;72(2):284–287. [PMC free article] [PubMed] [Google Scholar]
- Wagner J. D., Jerome C. P., Adams M. R. Gluten-sensitive enteropathy in a cynomolgus monkey. Lab Anim Sci. 1988 Oct;38(5):592–594. [PubMed] [Google Scholar]
- Weinstein W. M. Latent celiac sprue. Gastroenterology. 1974 Apr;66(4):489–493. [PubMed] [Google Scholar]


